Abstract
Osmotic shrinkage of giant egg-lecithin vesicles was observed by phase-contrast microscopy. The vesicles remained or became spherical when shrinking. Small and thick-walled vesicles formed visible fingers attached to the sphere. The water permeability of the single bilayer was found to be 41 micrometers/s. A variety of observations indicate that osmosis induces a parallel lipid flow between the monolayers of the bilayer, leading to a strong positive spontaneous curvature. They also suggest the formation of mostly submicroscopic daughter vesicles. The estimated coupling constant, 2 . 10(-6) mol/mol, is large enough to be biologically significant.
Full text
PDF

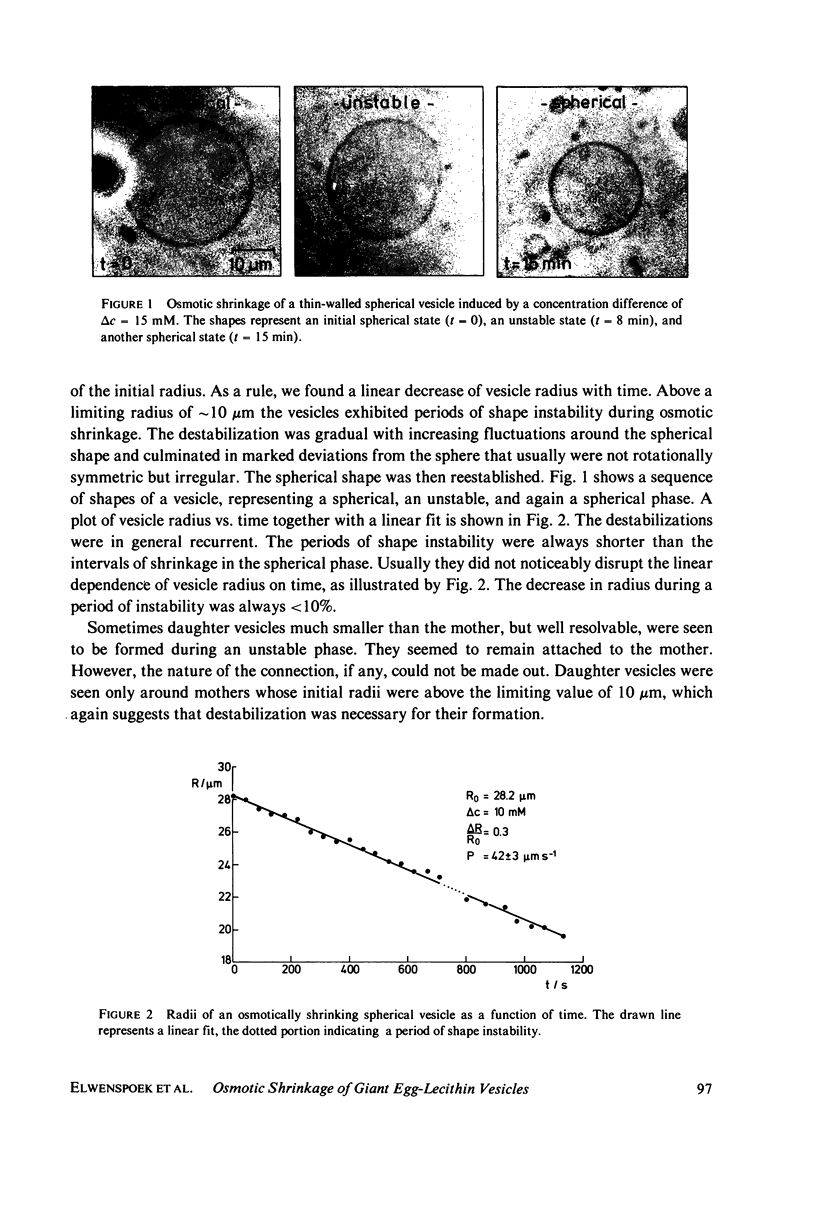
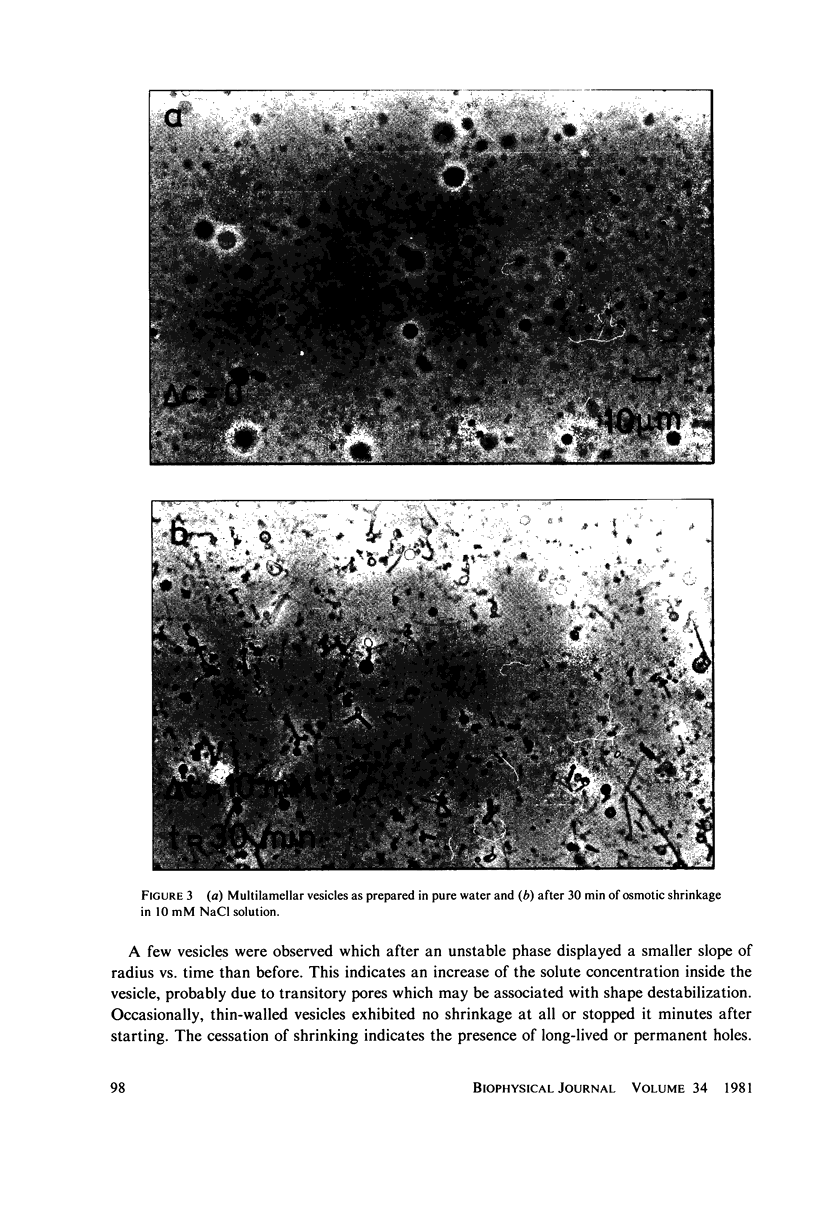

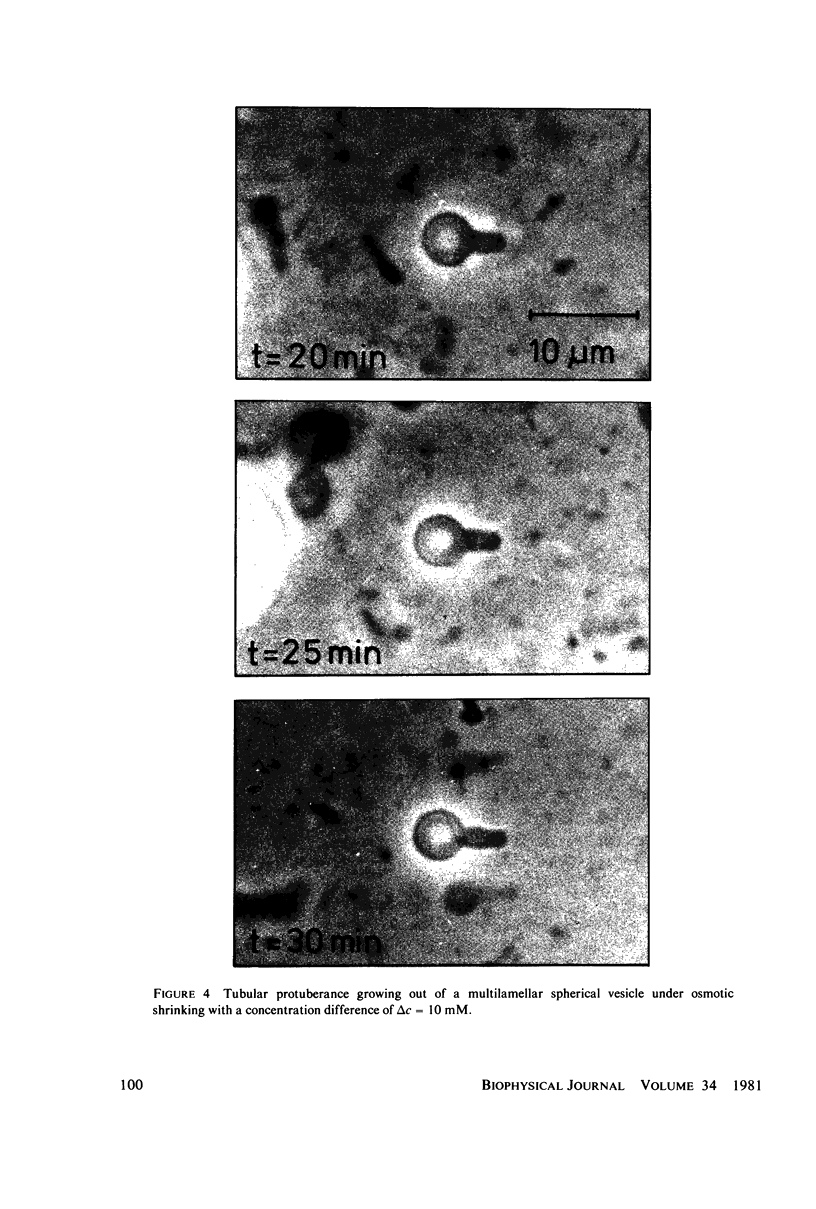









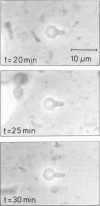
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. M., Haydon D. A. Electron microscope studies of lipid bilayer membranes. J Mol Biol. 1968 Feb 28;32(1):149–150. doi: 10.1016/0022-2836(68)90152-6. [DOI] [PubMed] [Google Scholar]
- Blok M. C., Van Deenen L. L., De Gier J. The effect of cholesterol incorporation on the temperature dependence of water permeation through liposomal membranes prepared from phosphatidylcholines. Biochim Biophys Acta. 1977 Feb 4;464(3):509–518. doi: 10.1016/0005-2736(77)90026-8. [DOI] [PubMed] [Google Scholar]
- De Kruijff B., Van Zoelen E. J. Effect of the phase transition on the transbilayer movement of dimyristoyl phosphatidylcholine in unilamellar vesicles. Biochim Biophys Acta. 1978 Jul 20;511(1):105–115. doi: 10.1016/0005-2736(78)90068-8. [DOI] [PubMed] [Google Scholar]
- De Kruijff B., Wirtz K. W. Induction of a relatively fast transbilayer movement of phosphatidylcholine in vesicles. A 13CNMR study. Biochim Biophys Acta. 1977 Jul 14;468(2):318–326. doi: 10.1016/0005-2736(77)90124-9. [DOI] [PubMed] [Google Scholar]
- Fettiplace R. The influence of the lipid on the water permeability of artificial membranes. Biochim Biophys Acta. 1978 Oct 19;513(1):1–10. doi: 10.1016/0005-2736(78)90106-2. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Blocked lipid exchange in bilayers and its possible influence on the shape of vesicles. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):510–515. doi: 10.1515/znc-1974-9-1010. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: composition studies. J Mol Biol. 1968 Jan 28;31(2):227–235. doi: 10.1016/0022-2836(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Servuss R. M., Harbich W., Helfrich W. Measurement of the curvature-elastic modulus of egg lecithin bilayers. Biochim Biophys Acta. 1976 Jul 15;436(4):900–903. doi: 10.1016/0005-2736(76)90422-3. [DOI] [PubMed] [Google Scholar]