Abstract
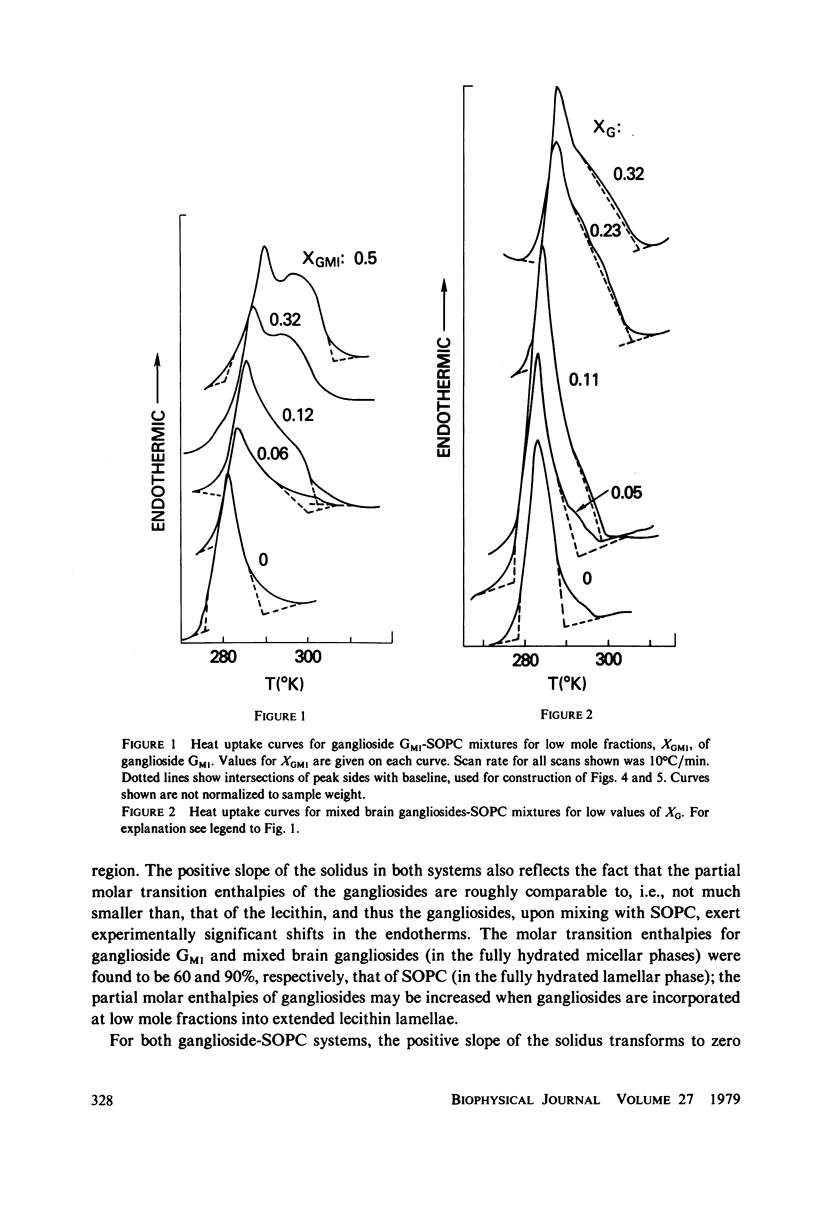
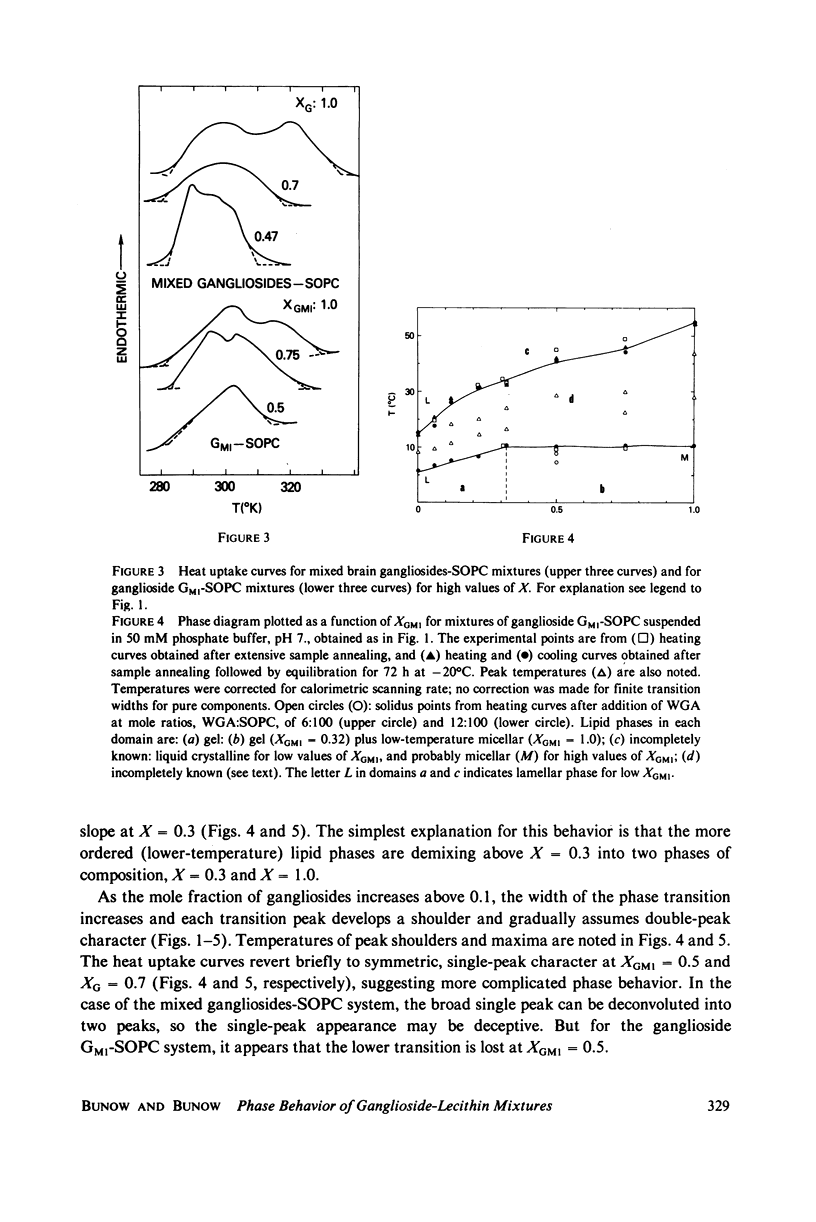
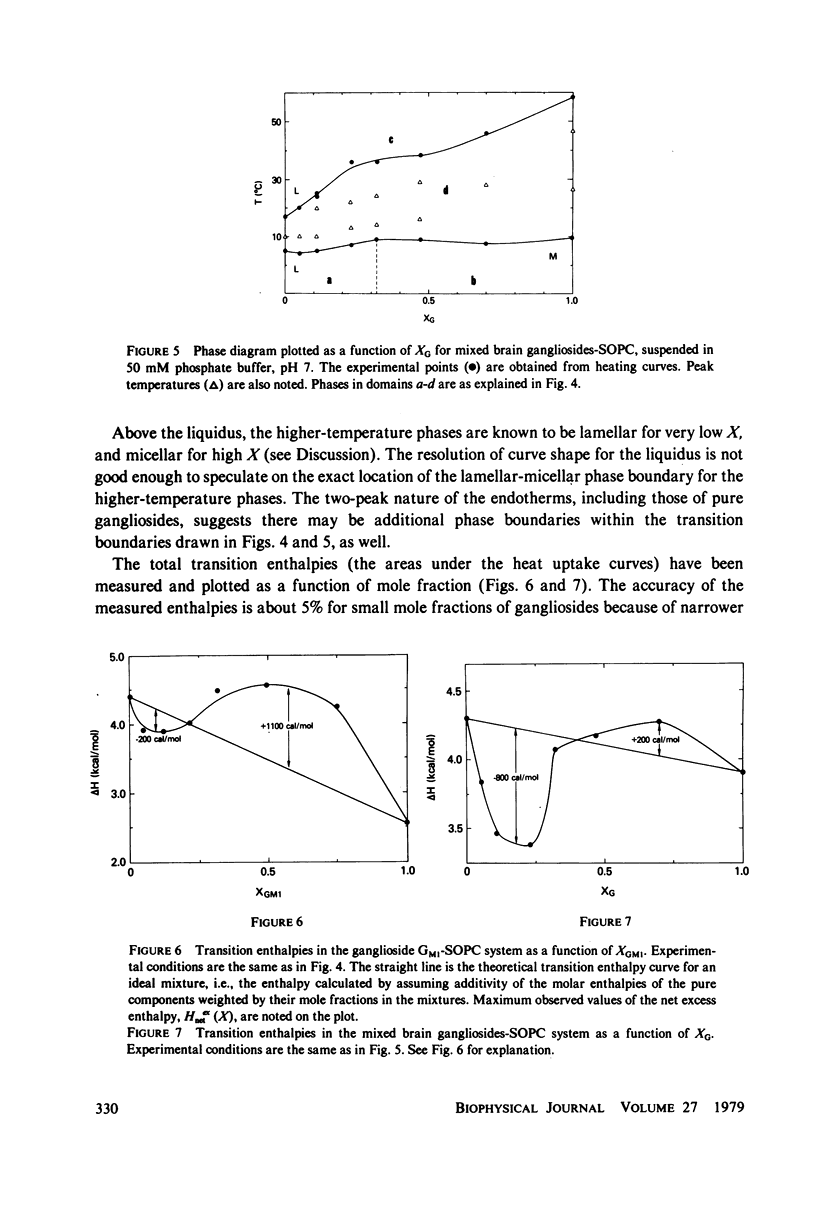
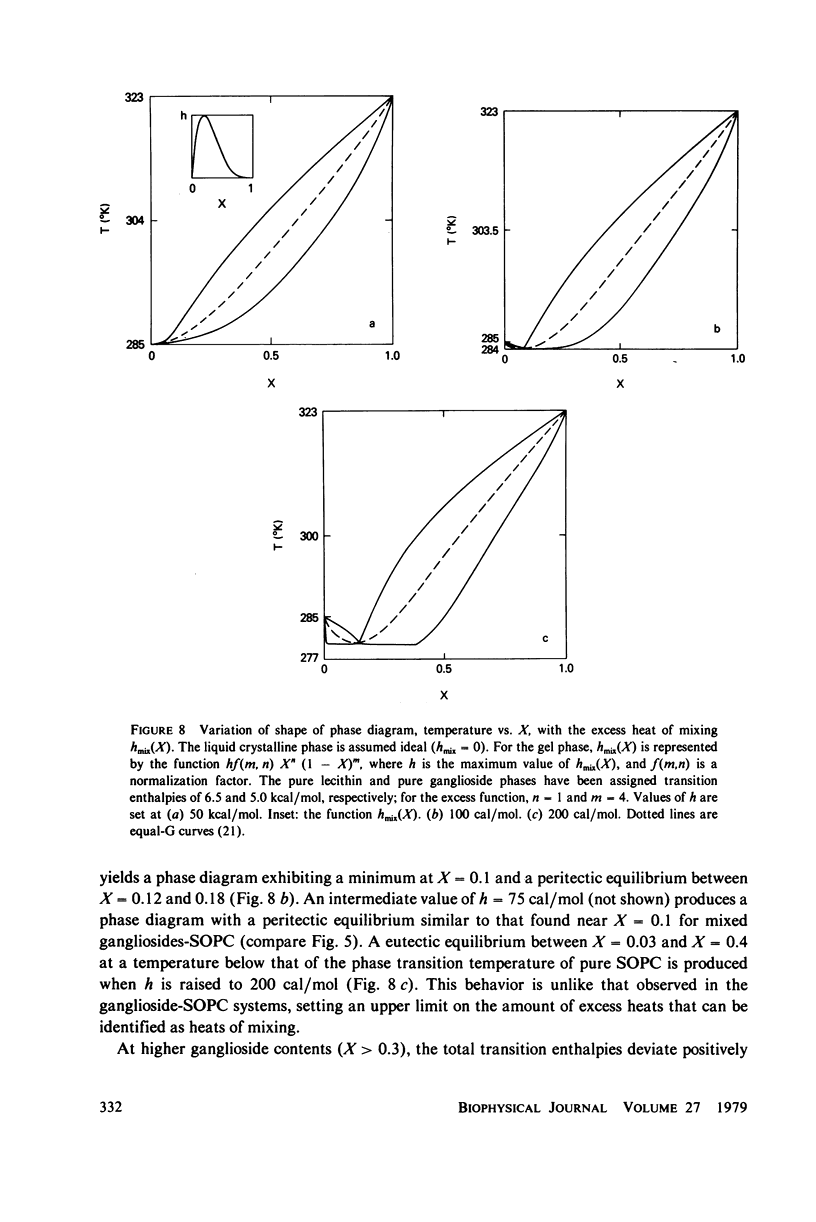
Ganglioside GM1 and mixed brain gangliosides were mixed with 1-stearoyl-2-oleoyl lecithin (SOPC) and examined by differential scanning calorimetry as a function of ganglioside content and temperature. Low mole fractions of ganglioside GM1 and of mixed brain gangliosides are shown to be miscible with SOPC in the gel phase up to X = 0.3, with the possible exception of a small region of immiscibility for the mixed brain gangliosides system centered around X = 0.05. Above X = 0.3, the low-temperature phases demix into a (gel) phase of composition X = 0.3 and a (micellar) phase of composition X = 1.0. Above the endothermic phase transition temperature, no phase boundaries are discerned. It is pointed out that phase structures need to be determined in each domain delineated in the phase diagrams, and that cylindrical phases may exist at higher temperatures and intermediate compositions. The effects of addition of wheat germ agglutinin, which binds to ganglioside GM1, on a ganglioside GM1-SOPC mixture (X = 0.5), are described and interpreted in terms of partial demixing of ganglioside and lecithin. Behavior of the ganglioside-SOPC system is discussed with respect to the kinetics of cholera toxin action in lymphocytes, as well as to other physiological roles of gangliosides in membranes.
Full text
PDF



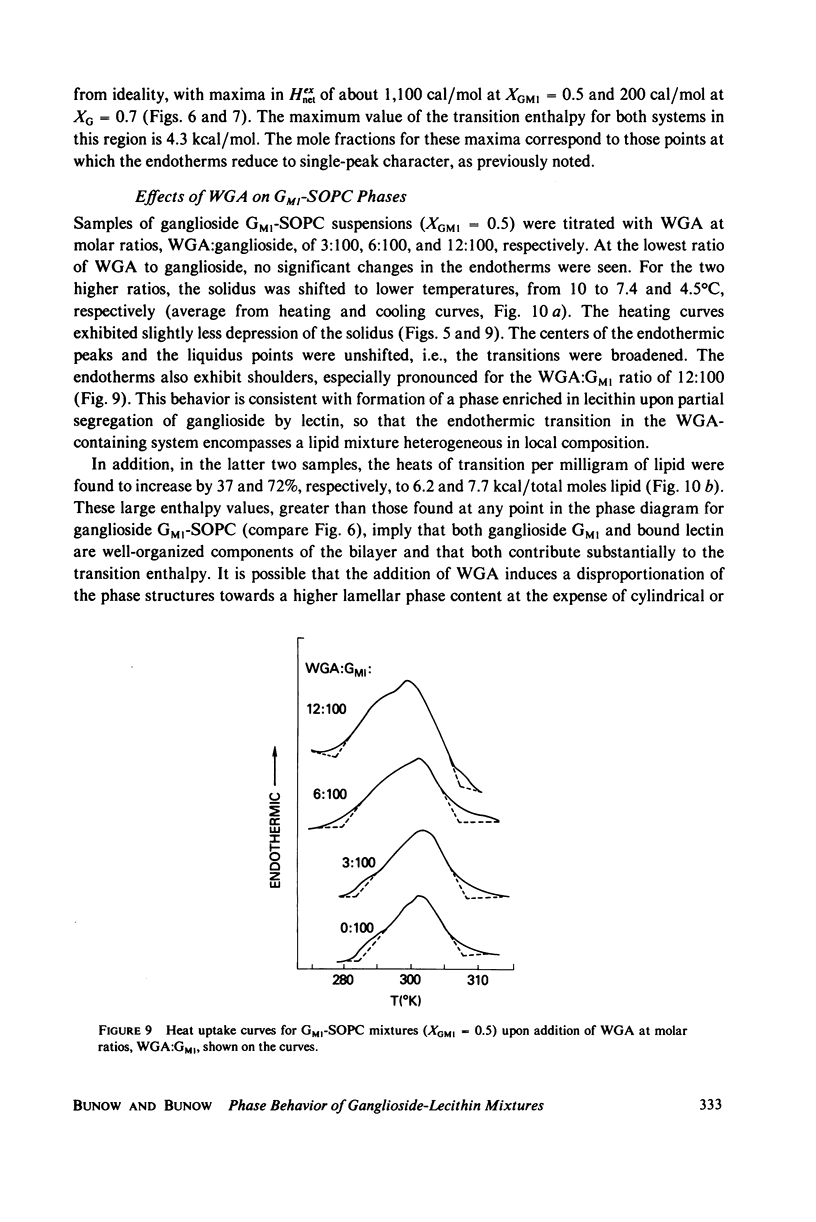



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton N. W., Rosenberg A. Action of Vibrio cholerae neuraminidase (sialidase) upon the surface of intact cells and their isolated sialolipid components. J Biol Chem. 1973 Nov 10;248(21):7353–7358. [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt D. H., Speckart S. F., Richards R. L., Alving C. R. Interactions of lectins with glycolipids in liposomes. Biochem Biophys Res Commun. 1977 Jan 10;74(1):208–214. doi: 10.1016/0006-291x(77)91395-x. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
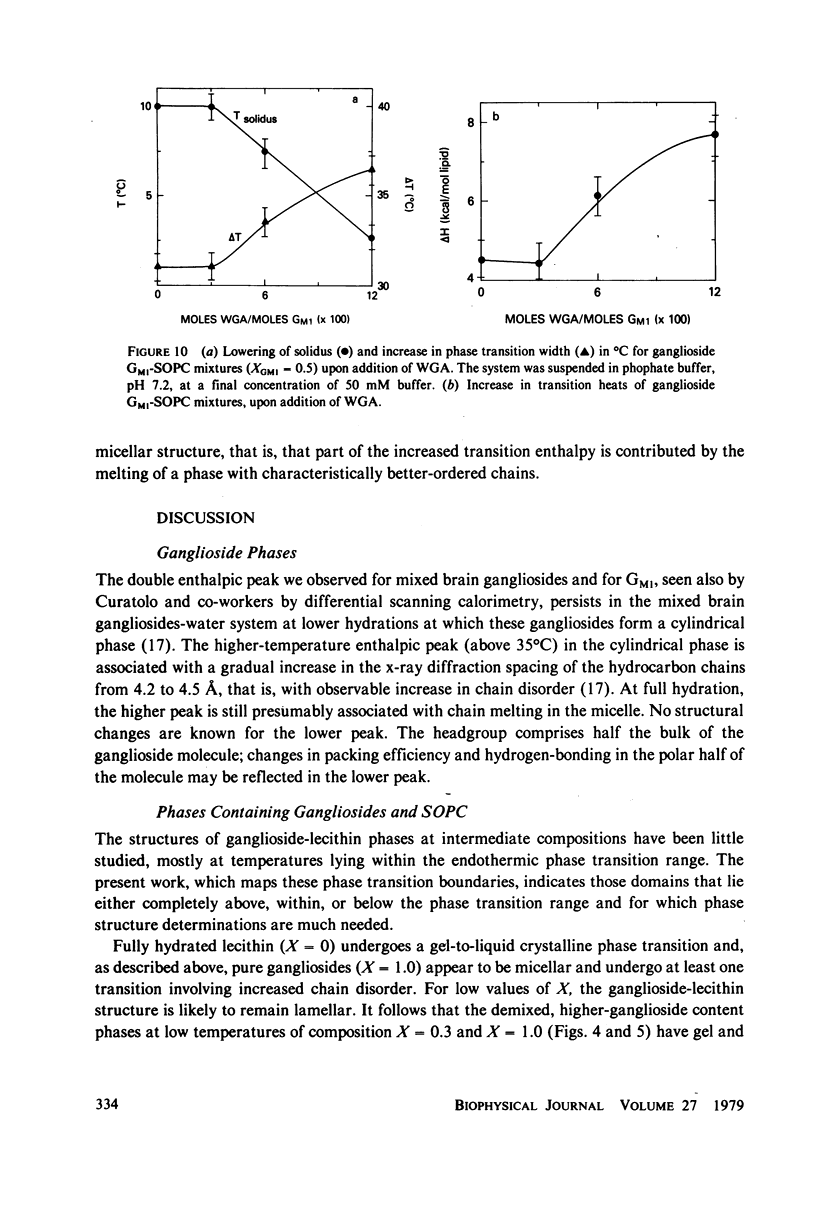
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Small D. M., Shipley G. G. Phase behavior and structural characteristics of hydrated bovine brain gangliosides. Biochim Biophys Acta. 1977 Jul 4;468(1):11–20. doi: 10.1016/0005-2736(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Moss J., Osborne J. C., Jr Interaction of choleragen with the oligosaccharide of ganglioside GM1: evidence for multiple oligosaccharide binding sites. Biochemistry. 1978 Feb 21;17(4):711–716. doi: 10.1021/bi00597a024. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Holmgren J., Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. W., Lester R. Mixtures of gangliosides and phosphatidylcholine in aqueous dispersions. Biochim Biophys Acta. 1972 Sep 1;282(1):18–30. doi: 10.1016/0005-2736(72)90307-0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R. The chemistry of gangliosides: a review. J Am Oil Chem Soc. 1966 Feb;43(2):57–66. doi: 10.1007/BF02641015. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta. 1977 Aug 9;472(2):237–281. doi: 10.1016/0304-4157(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. II. Mictures involving lipids. Biochim Biophys Acta. 1977 Nov 14;472(3-4):285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- McCabe P. J., Green C. The dispersion of cholesterol with phospholipids and glycolipids. Chem Phys Lipids. 1977 Dec;20(4):319–330. doi: 10.1016/0009-3084(77)90072-x. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Polefka T. G. Lectin-receptor interactions in liposomes. II. Interaction of wheat germ agglutinin with phosphatidylcholine liposomes containing incorporated monosialoganglioside. Biochim Biophys Acta. 1976 Dec 14;455(3):631–643. doi: 10.1016/0005-2736(76)90037-7. [DOI] [PubMed] [Google Scholar]
- Révész T., Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975 Sep 11;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- Sedlacek H. H., Stärk J., Seiler F. R., Ziegler W., Wiegandt H. Cholera toxin induced redistribution of sialoglycolipid receptor at the lymphocyte membrane. FEBS Lett. 1976 Jan 15;61(2):272–276. doi: 10.1016/0014-5793(76)81055-1. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Annese C., Slomiany B. L. The glycosphingolipids of rat sublingual and submaxillary glands. Biochim Biophys Acta. 1976 Aug 23;441(2):316–326. doi: 10.1016/0005-2760(76)90175-2. [DOI] [PubMed] [Google Scholar]
- Yohe H. C., Roark D. E., Rosenberg A. C20-sphingosine as a determining factor in aggregation of gangliosides. J Biol Chem. 1976 Nov 25;251(22):7083–7087. [PubMed] [Google Scholar]
- van Dijck P. W., Kaper A. J., Oonk H. A., de Gier J. Miscibility properties of binary phosphatidylcholine mixtures. A calorimetric study. Biochim Biophys Acta. 1977 Oct 3;470(1):58–69. doi: 10.1016/0005-2736(77)90061-x. [DOI] [PubMed] [Google Scholar]


