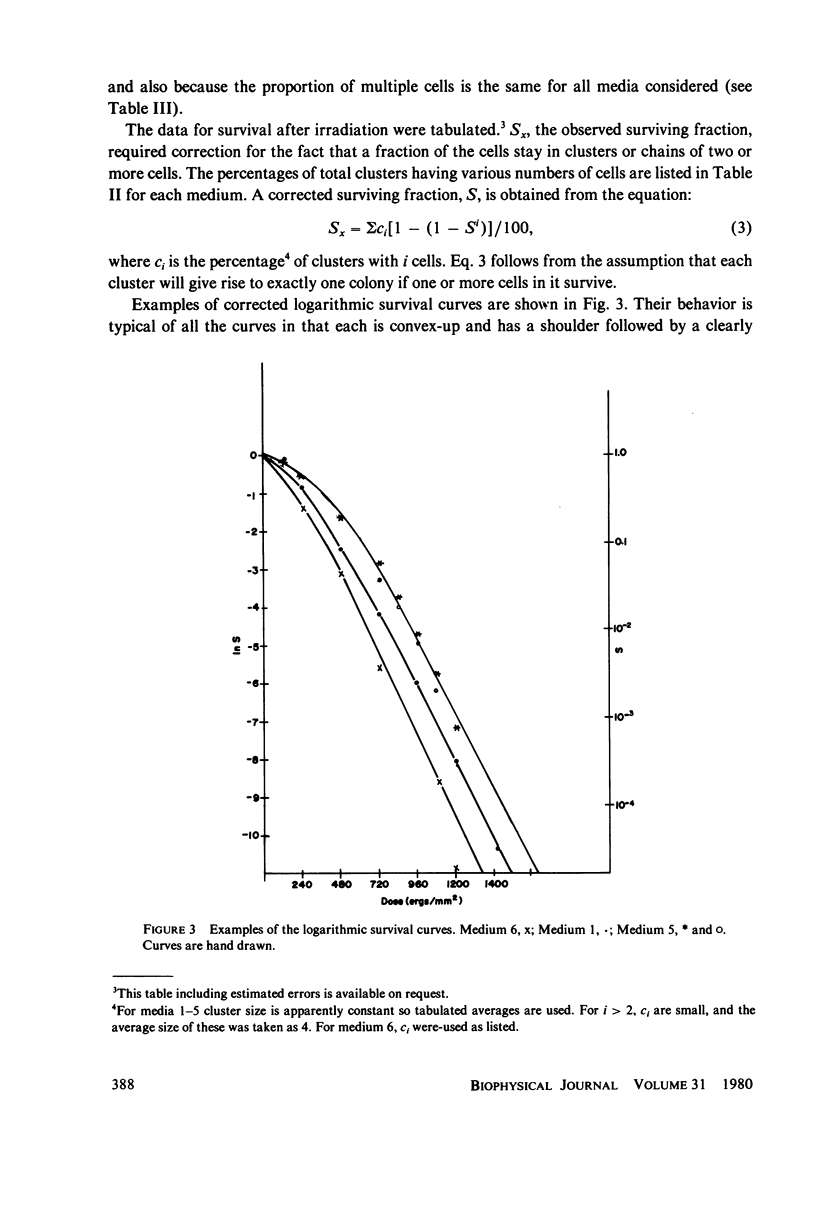
Abstract
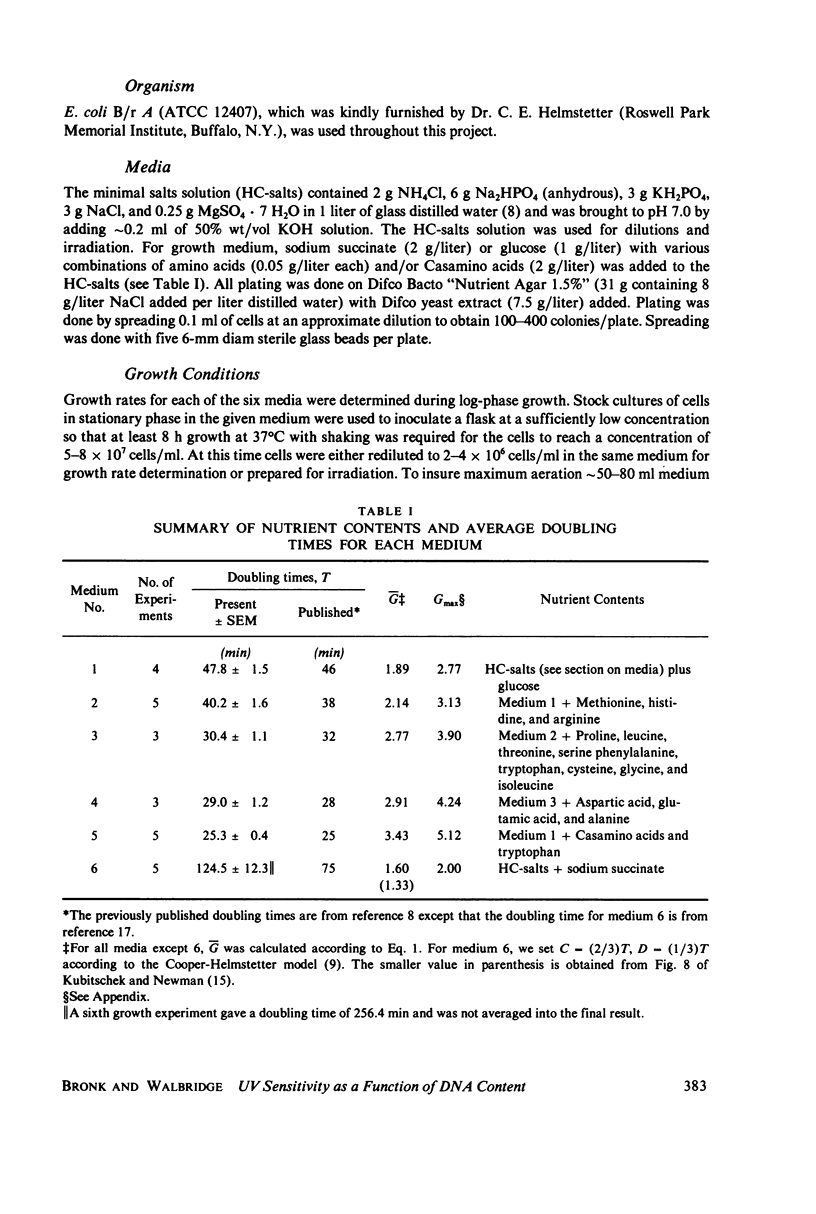
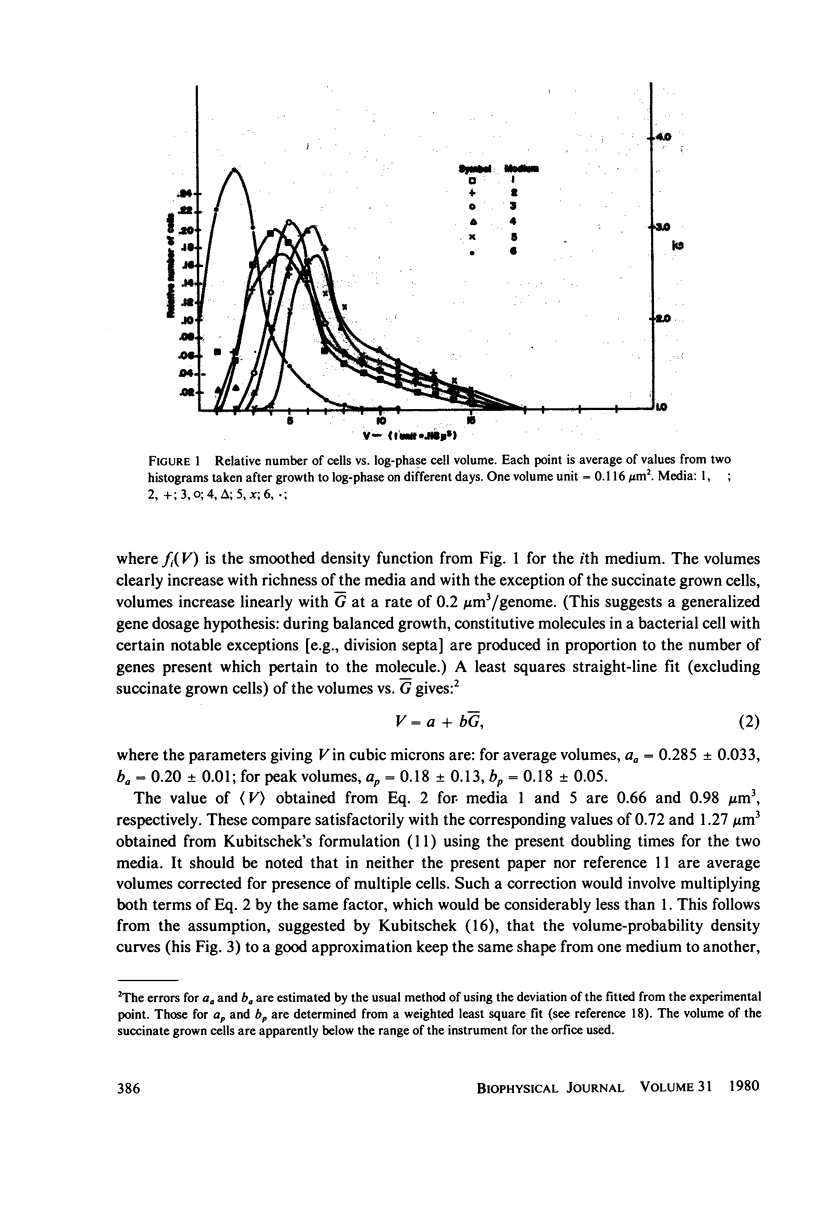
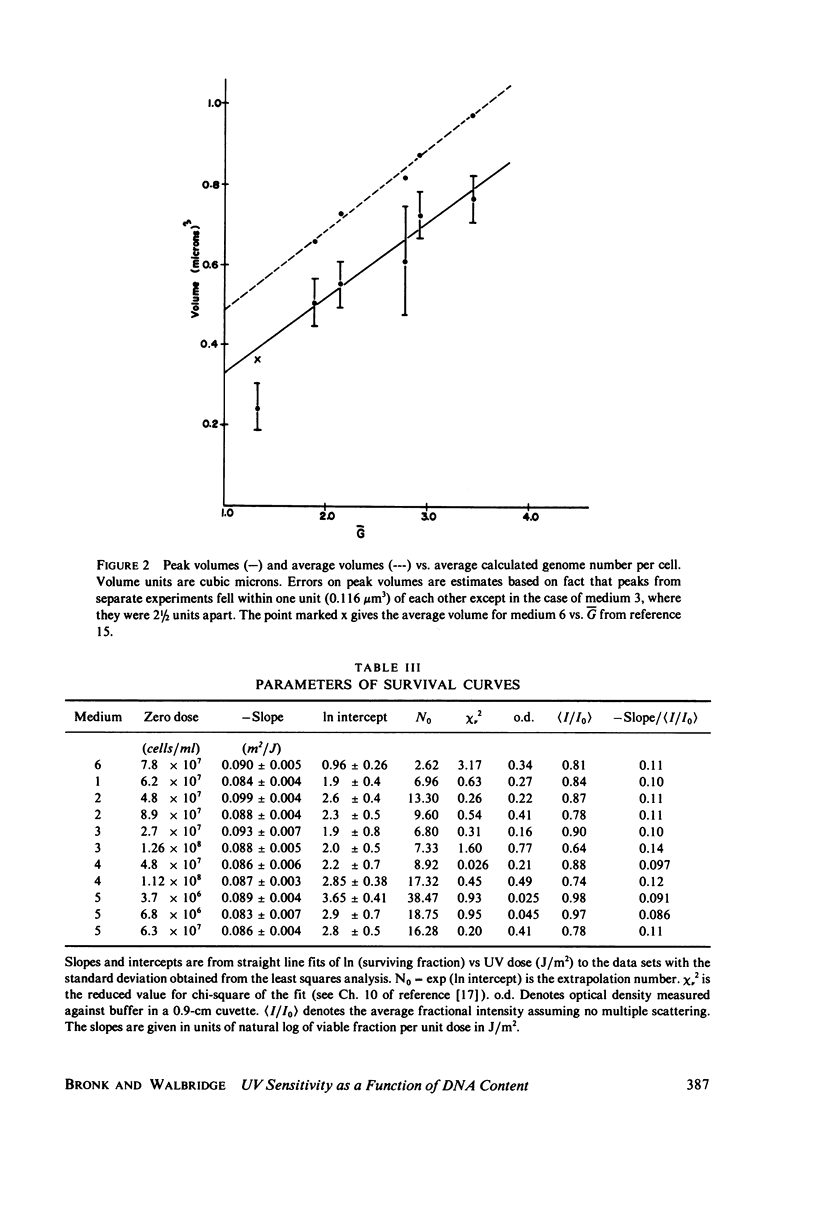
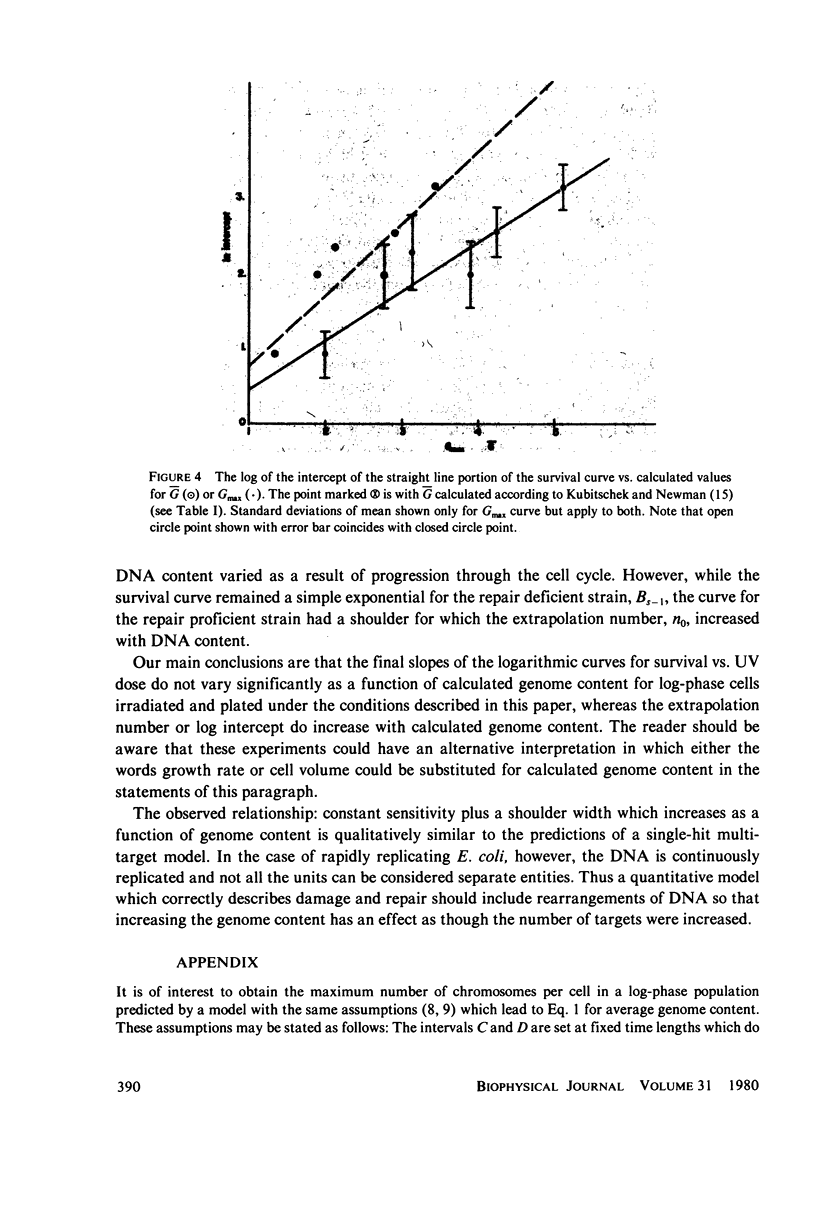
Populations of Escherichia coli B/r A were grown to log phase at various growth rates determined by the richness of the medium. The genome content, G, was calculated from log phase doubling times by means of the Cooper-Helmstetter formula. Cell volumes were measured and found to vary linearly with this genome content. Cells with various DNA contents were prepared for ultraviolet irradiation and plated for dark repair under similar conditions. The resulting logarithmic survival curves were all similar in shape: convex up, with straight line portions having approximately the same slope (D0 = 11.4 +/- 0.2 J/m2). The shoulders however increase in width with calculated DNA content giving an extrapolation number which varies roughly as exp(G) or exp (0.6 Gmax).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk B. V., Dienes G. J., Paskin A. The stochastic theory of cell proliferation. Biophys J. 1968 Nov;8(11):1353–1398. doi: 10.1016/S0006-3495(68)86561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk B. V., Dienes G. J., Schindler R., Gautschi J. R. Kinetic cell-cycle analysis of a cultured mammalian cell population. Biophys J. 1974 Aug;14(8):607–624. doi: 10.1016/S0006-3495(74)85938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. Effects of ionizing radiation on synchronous cultures of Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1150–1158. doi: 10.1128/jb.96.4.1150-1158.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- DEERING R. A. Radiation sensitivity of filamentous Escherichia coli. Biochim Biophys Acta. 1959 Jan;31(1):11–19. doi: 10.1016/0006-3002(59)90433-0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Hucul J. A., Helmstetter C. E. A comparison of DNA content between two substrains of Escherichia coli B/r. Biochem Biophys Res Commun. 1978 Feb 28;80(4):970–974. doi: 10.1016/0006-291x(78)91340-2. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Constancy of the ratio of DNA to cell volume in steady-state cultures of Escherichia coli B-r. Biophys J. 1974 Feb;14(2):119–123. doi: 10.1016/S0006-3495(74)70003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Finney A. C., Krisch R. E. Constancy of the UV sensitivity of E. coli during the cell cycle. Photochem Photobiol. 1973 Nov;18(5):365–370. doi: 10.1111/j.1751-1097.1973.tb06436.x. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Growth during the bacterial cell cycle: analysis of cell size distribution. Biophys J. 1969 Jun;9(6):792–809. doi: 10.1016/S0006-3495(69)86418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Newman C. N. Chromosome replication during the division cycle in slowly growing, steady-state cultures of three Escherichia coli B/r strains. J Bacteriol. 1978 Oct;136(1):179–190. doi: 10.1128/jb.136.1.179-190.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- MORTIMER R. K. Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae. Radiat Res. 1958 Sep;9(3):312–326. [PubMed] [Google Scholar]
- MORTIMER R. K., VONBORSTEL R. C. RADIATION-INDUCED DOMINANT LETHALITY IN HAPLOID AND DIPLOID SPERM OF THE WASP MORMONIELLA. Genetics. 1963 Nov;48:1545–1549. doi: 10.1093/genetics/48.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORMAN A. The nuclear role in the ultraviolet inactivation of Neurospora conidia. J Cell Physiol. 1954 Aug;44(1):1–10. doi: 10.1002/jcp.1030440102. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SPARROW A. H., EVANS H. J. Nuclear factors affecting radiosensitivity. I. The influence of nuclear size and structure, chromosome complement, and DNA content. Brookhaven Symp Biol. 1961 Nov;14:76–100. [PubMed] [Google Scholar]
- TILL J. E. Radiosensitivity and chromosome numbers in strain L mouse cells in tissue culture. Radiat Res. 1961 Sep;15:400–409. [PubMed] [Google Scholar]


