Abstract
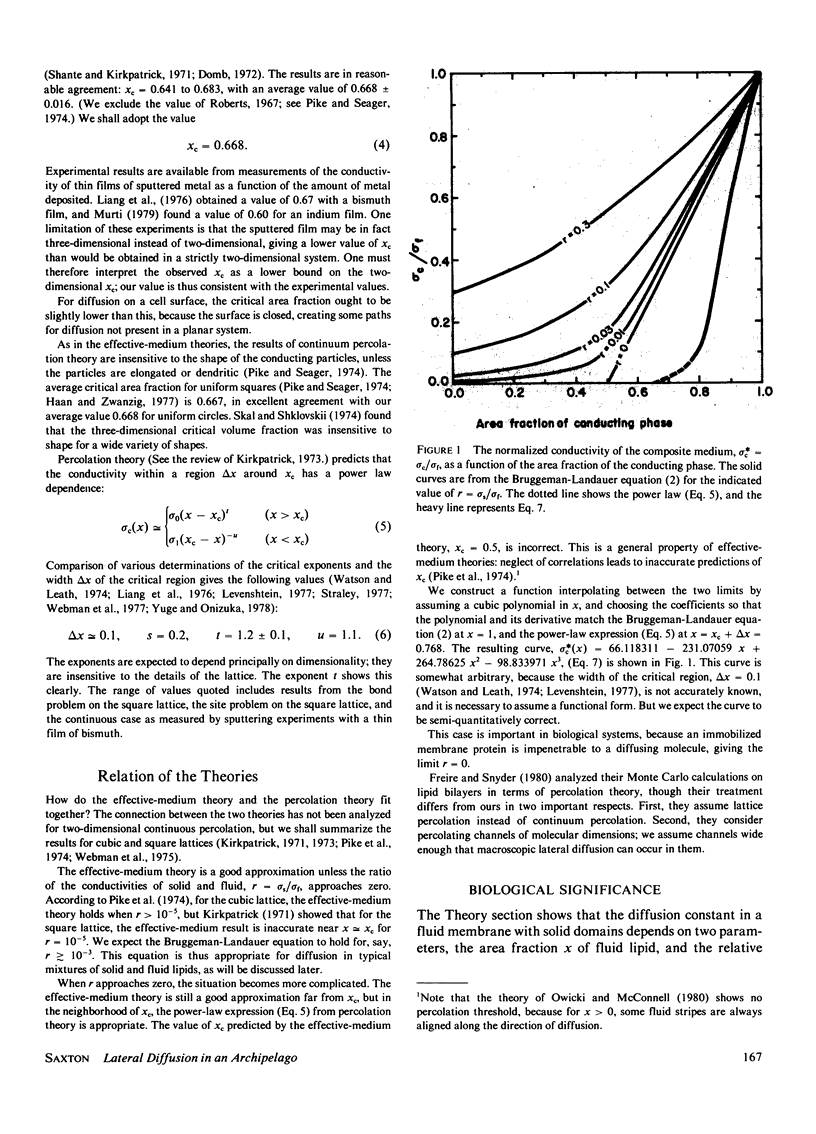
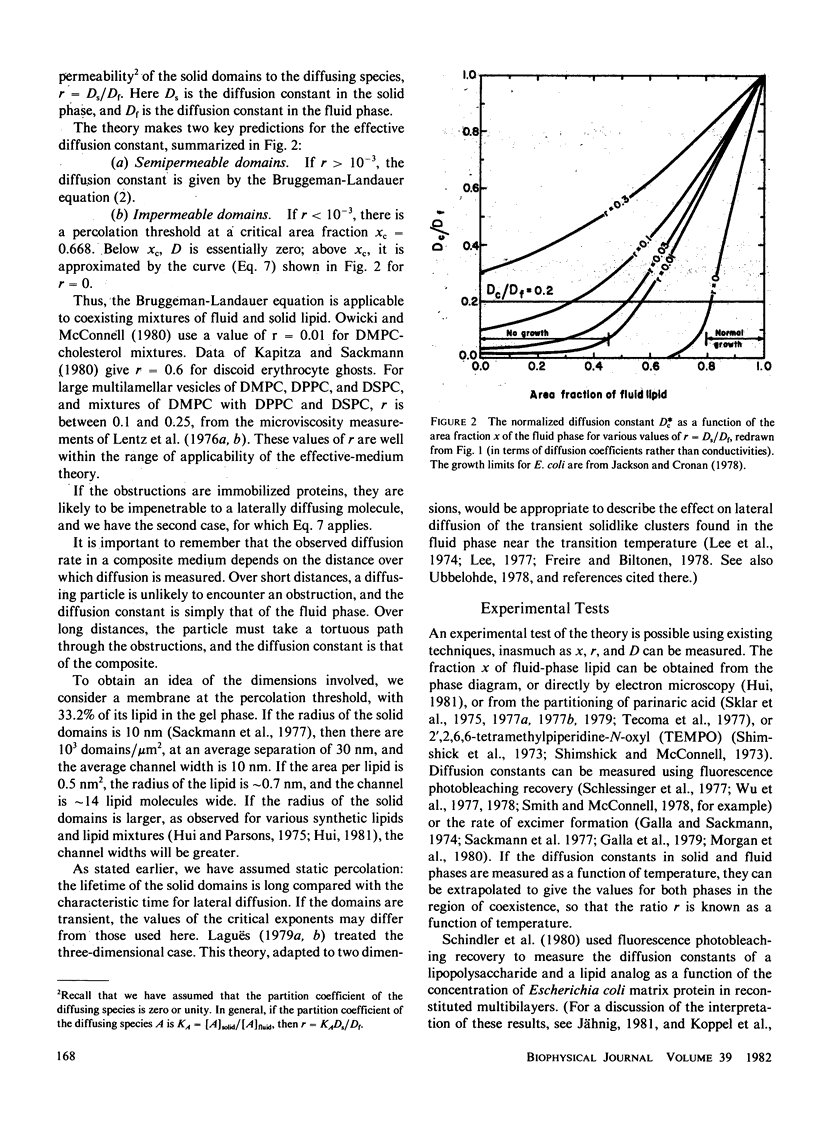
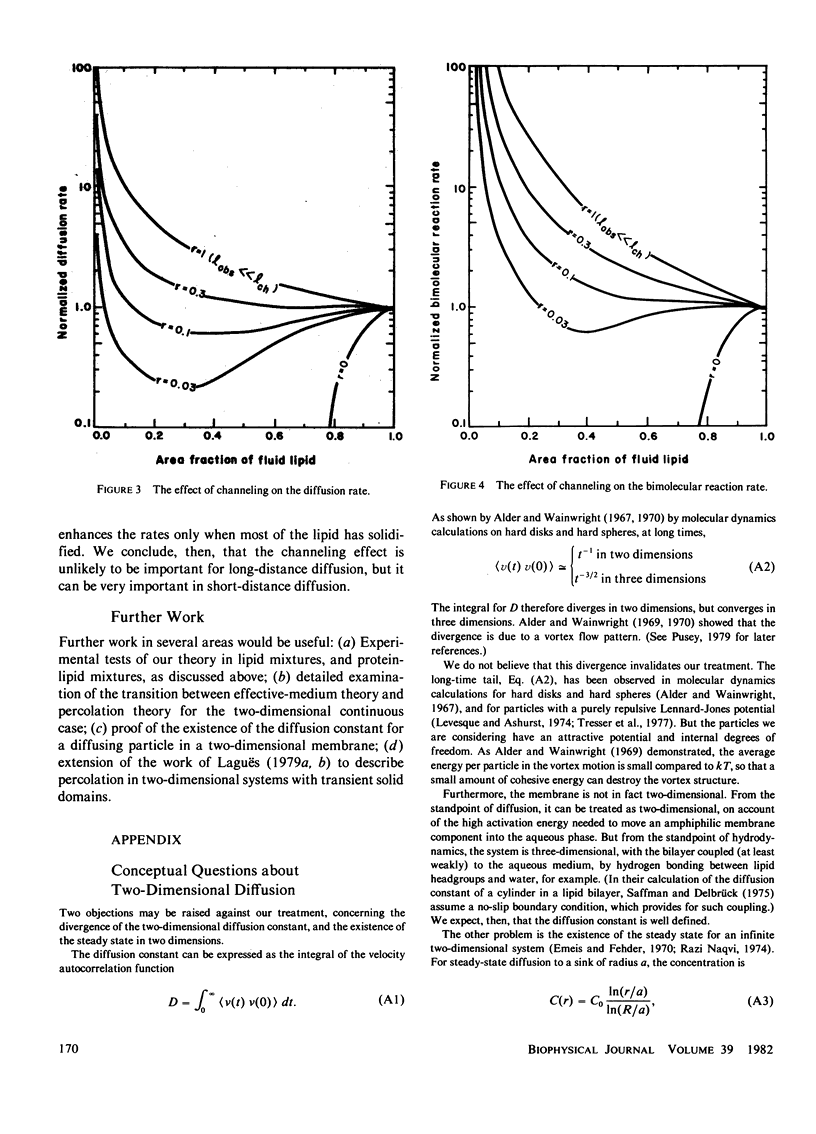
Lateral diffusion of molecules in lipid bilayer membranes can be hindered by the presence of impermeable domains of gel-phase lipid or of proteins. Effective-medium theory and percolation theory are used to evaluate the effective lateral diffusion constant as a function of the area fraction of fluid-phase lipid and the permeability of the obstructions to the diffusing species. Applications include the estimation of the minimum fraction of fluid lipid needed for bacterial growth, and the enhancement of diffusion-controlled reactions by the channeling effect of solid patches of lipid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freire E., Biltonen R. Estimation of molecular averages and equilibrium fluctuations in lipid bilayer systems from the excess heat capacity function. Biochim Biophys Acta. 1978 Dec 4;514(1):54–68. doi: 10.1016/0005-2736(78)90076-7. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Monte Carlo studies of the lateral organization of molecules in two-component lipid bilayers. Biochim Biophys Acta. 1980 Aug 14;600(3):643–654. doi: 10.1016/0005-2736(80)90468-x. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Geometry of phase-separated domains in phospholipid bilayers by diffraction-contrast electron microscopy. Biophys J. 1981 Jun;34(3):383–395. doi: 10.1016/S0006-3495(81)84857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Parsons D. F. Direct observation of domains in wet lipid bilayers. Science. 1975 Oct 24;190(4212):383–384. doi: 10.1126/science.1179216. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Cronan J. E., Jr An estimate of the minimum amount of fluid lipid required for the growth of Escherichia coli. Biochim Biophys Acta. 1978 Oct 4;512(3):472–479. doi: 10.1016/0005-2736(78)90157-8. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Sturtevant J. M. Phase behavior of lipids from Halobacterium halobium. Biochemistry. 1978 Oct 17;17(21):4470–4474. doi: 10.1021/bi00614a017. [DOI] [PubMed] [Google Scholar]
- Jähnig F. No need for a new membrane model. Nature. 1981 Feb 19;289(5799):694–696. doi: 10.1038/289694a0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitza H. G., Sackmann E. Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta. 1980;595(1):56–64. doi: 10.1016/0005-2736(80)90247-3. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Snipes W., Mehlhorn R. J., Gunter T. Factors restricting diffusion of water-soluble spin labels. Biophys J. 1977 Sep;19(3):205–218. doi: 10.1016/S0006-3495(77)85582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. Analysis of the defect structure of gel-phase lipid. Biochemistry. 1977 Mar 8;16(5):835–841. doi: 10.1021/bi00624a004. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Toon P. A., Warren G. B. Clusters in lipid bilayers and the interpretation of thermal effects in biological membranes. Biochemistry. 1974 Aug 27;13(18):3699–3705. doi: 10.1021/bi00715a013. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelja S., Wolfe J. Properties of bilayer membranes in the phase transition or phase separation region. Biochim Biophys Acta. 1979 Oct 19;557(1):24–31. doi: 10.1016/0005-2736(79)90086-5. [DOI] [PubMed] [Google Scholar]
- Morgan C. G., Hudson B., Wolber P. K. Photochemical dimerization of parinaric acid in lipid bilayers. Proc Natl Acad Sci U S A. 1980 Jan;77(1):26–30. doi: 10.1073/pnas.77.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Scott H. L., Jr Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta. 1978 Nov 2;513(2):236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Lateral diffusion in inhomogeneous membranes. Model membranes containing cholesterol. Biophys J. 1980 Jun;30(3):383–397. doi: 10.1016/S0006-3495(80)85103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Rimon G., Hanski E., Braun S., Levitzki A. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature. 1978 Nov 23;276(5686):394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- Roberts F. D. A Monte Carlo solution of a two-dimensional unstructured cluster problem. Biometrika. 1967 Dec;54(3):625–628. [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., Kleemann W., Hubbell W. L., McConnell H. M. Lateral phase separations in membranes. J Supramol Struct. 1973;1(4):285–294. doi: 10.1002/jss.400010406. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. A., Mingins J., Pethica B. A., Tan B. Y., Jackson C. M. Phase changes and mosaic formation in single and mixed phospholipid monolayers at the oil--water interface. Biochim Biophys Acta. 1973 Sep 27;323(1):157–160. doi: 10.1016/0005-2736(73)90439-2. [DOI] [PubMed] [Google Scholar]
- Tecoma E. S., Sklar L. A., Simoni R. D., Hudson B. S. Conjugated polyene fatty acids as fluorescent probes: biosynthetic incorporation of parinaric acid by Escherichia coli and studies of phase transitions. Biochemistry. 1977 Mar 8;16(5):829–835. doi: 10.1021/bi00624a003. [DOI] [PubMed] [Google Scholar]
- Wu E. S., Jacobson K., Szoka F., Portis A., Jr Lateral diffusion of a hydrophobic peptide, N-4-nitrobenz-2-oxa-1,3-diazole gramicidin S, in phospholipid multibilayers. Biochemistry. 1978 Dec 12;17(25):5543–5550. doi: 10.1021/bi00618a033. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., Kaper A. J., Oonk H. A., de Gier J. Miscibility properties of binary phosphatidylcholine mixtures. A calorimetric study. Biochim Biophys Acta. 1977 Oct 3;470(1):58–69. doi: 10.1016/0005-2736(77)90061-x. [DOI] [PubMed] [Google Scholar]


