Abstract
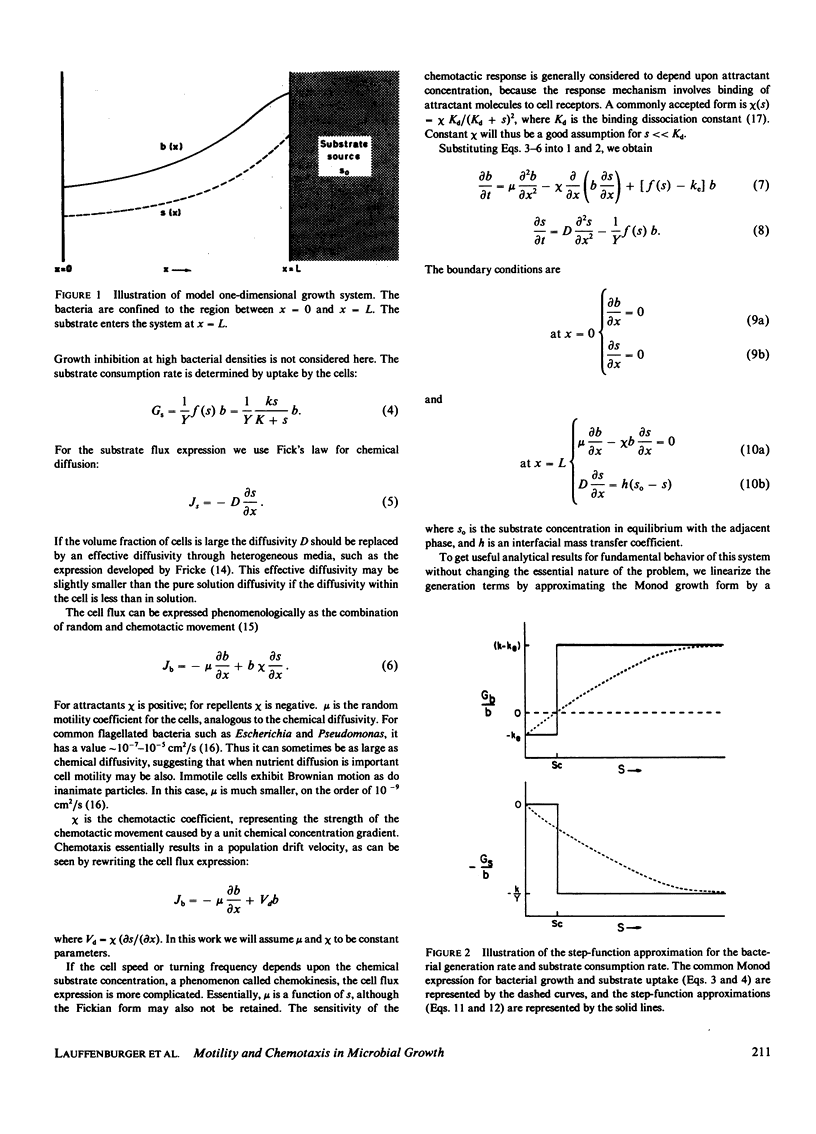
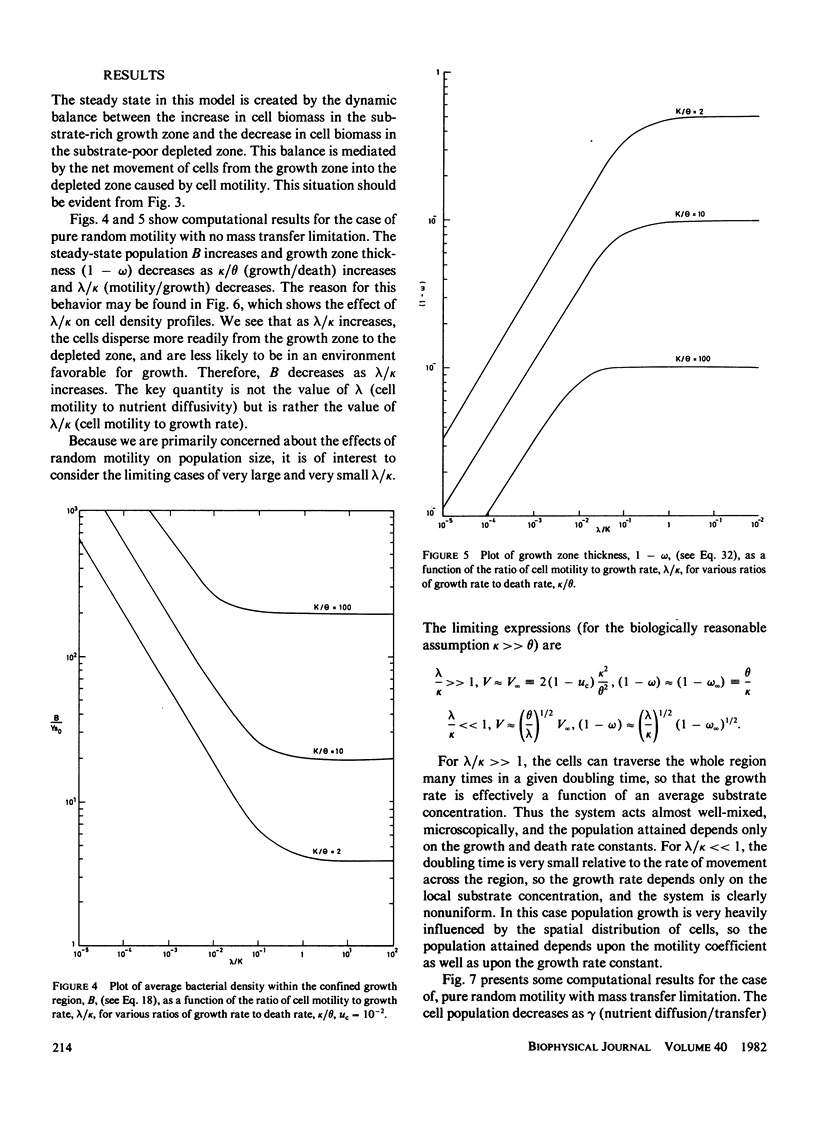
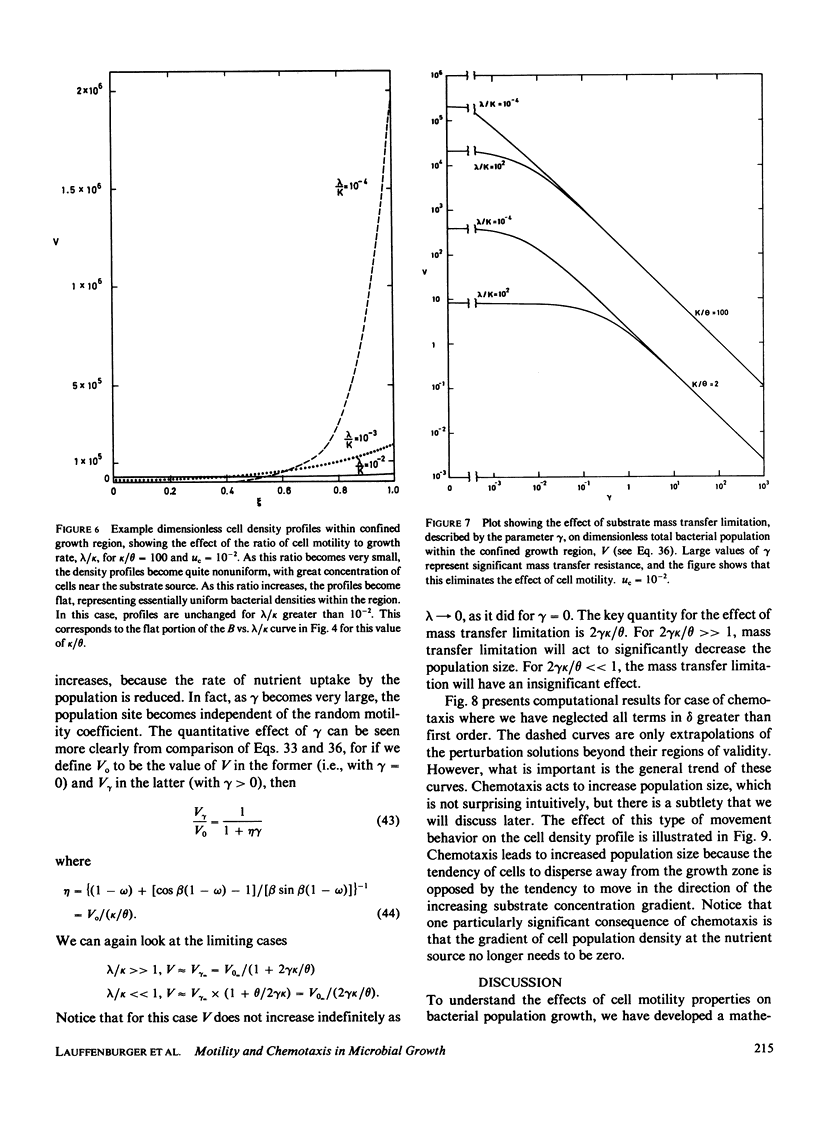
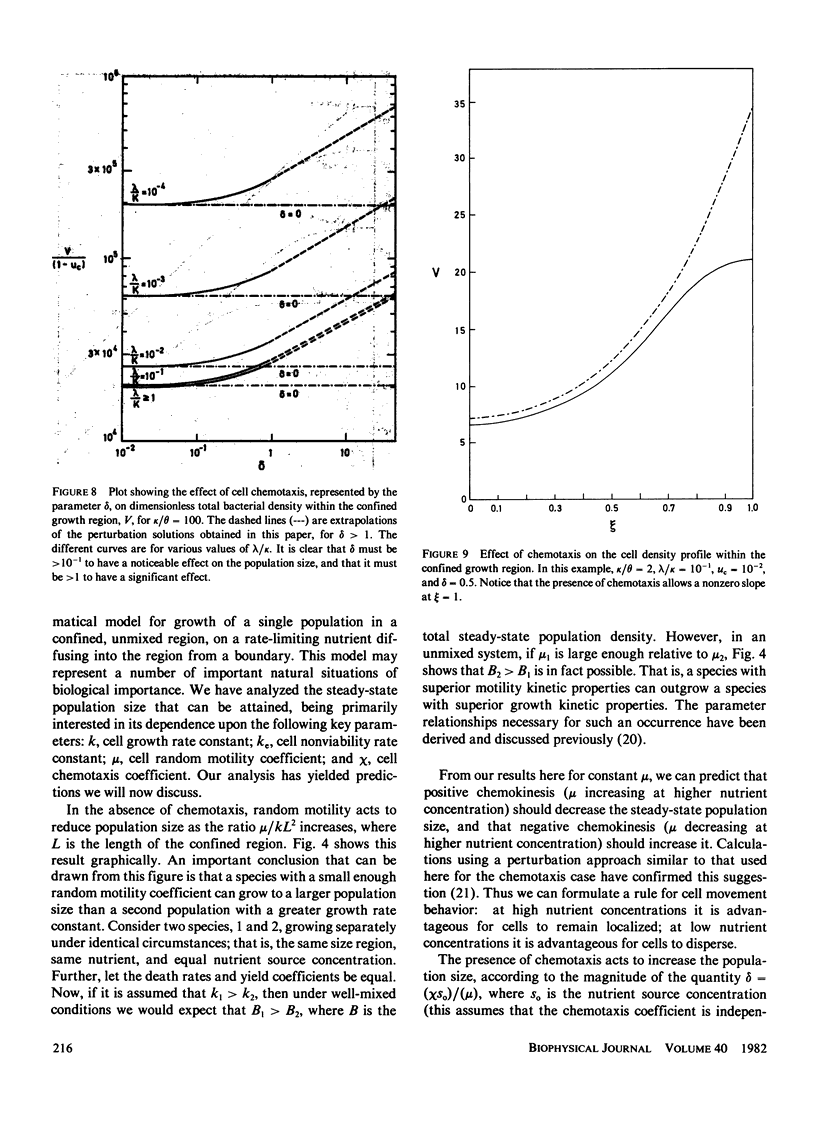
A mathematical model is developed to elucidate the effects of biophysical transport processes (nutrient diffusion, cell motility, and chemotaxis) along with biochemical reaction processes (cell growth and death, nutrient uptake) upon steady-state bacterial population growth in a finite one-dimensional region. The particular situation considered is that of growth limitation by a nutrient diffusing from an adjacent phase not accessible to the bacteria. It is demonstrated that the cell motility and chemotaxis properties can have great influence on steady-state population size. In fact, motility effects can be as significant as growth kinetic effects, in a manner analogous to diffusion- and reaction-limited regimes in chemically reacting systems. In particular, the following conclusions can be drawn from our analysis for bacterial populations growing at steady-state in a confined, unmixed region: (a) Random motility may lead to decreased population density; (b) chemotaxis can allow increased population density if the chemotactic response is large enough; (c) a species with superior motility properties can outgrow a species with superior growth kinetic properties; (d) motility effects become greater as the size of the confined growth region increases; and (e) motility effects are diminished by significant mass-transfer limitation of the nutrient from the adjacent source phase. The relationships of these results for populations to previous conclusions for individual cells is discussed, and implications for microbial competition are suggested.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARACCHINI O., SHERRIS J. C. The chemotactic effect of oxygen on bacteria. J Pathol Bacteriol. 1959 Apr;77(2):565–574. doi: 10.1002/path.1700770228. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Chemotaxis in bacteria. Annu Rev Biophys Bioeng. 1975;4(00):119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chet I., Mitchell R. Ecological aspects of microbial chemotactic behavior. Annu Rev Microbiol. 1976;30:221–239. doi: 10.1146/annurev.mi.30.100176.001253. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Chen S. H. Spatio-temporal structure of migrating chemotactic band of Escherichia coli. I. Traveling band profile. Biophys J. 1979 May;26(2):243–261. doi: 10.1016/S0006-3495(79)85248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. F., Segel L. A. Model for chemotaxis. J Theor Biol. 1971 Feb;30(2):225–234. doi: 10.1016/0022-5193(71)90050-6. [DOI] [PubMed] [Google Scholar]
- Kennedy C. R., Aris R. Traveling waves in a simple population model involving growth and death. Bull Math Biol. 1980;42(3):397–429. doi: 10.1007/BF02460793. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Lapidus I. R., Schiller R. Model for the chemotactic response of a bacterial population. Biophys J. 1976 Jul;16(7):779–789. doi: 10.1016/S0006-3495(76)85728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgram W. K., Williams F. D. Survival value of chemotaxis in mixed cultures. Can J Microbiol. 1976 Dec;22(12):1771–1773. doi: 10.1139/m76-262. [DOI] [PubMed] [Google Scholar]
- Segel L. A., Chet I., Henis Y. A simple quantitative assay for bacterial motility. J Gen Microbiol. 1977 Feb;98(2):329–337. doi: 10.1099/00221287-98-2-329. [DOI] [PubMed] [Google Scholar]
- Segel L. A., Jackson J. L. Theoretical analysis of chemotactic movement in bacteria. J Mechanochem Cell Motil. 1973 May;2(1):25–34. [PubMed] [Google Scholar]
- Seymour F. W., Doetsch R. N. Chemotactic responses by motile bacteria. J Gen Microbiol. 1973 Oct;78(2):287–296. doi: 10.1099/00221287-78-2-287. [DOI] [PubMed] [Google Scholar]
- Sinclair C. G., Topiwala H. H. Model for continuous culture which considers the viability concept. Biotechnol Bioeng. 1970 Nov;12(6):1069–1079. doi: 10.1002/bit.260120612. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Doetsch R. N. Studies on negative chemotaxis and the survival value of motility in Pseudomonas fluorescens. J Gen Microbiol. 1969 Mar;55(3):379–391. doi: 10.1099/00221287-55-3-379. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab R., Koshland D. E., Jr Common mechanism for repellents and attractants in bacterial chemotaxis. Science. 1973 Jul 6;181(4094):60–63. doi: 10.1126/science.181.4094.60. [DOI] [PubMed] [Google Scholar]


