Abstract
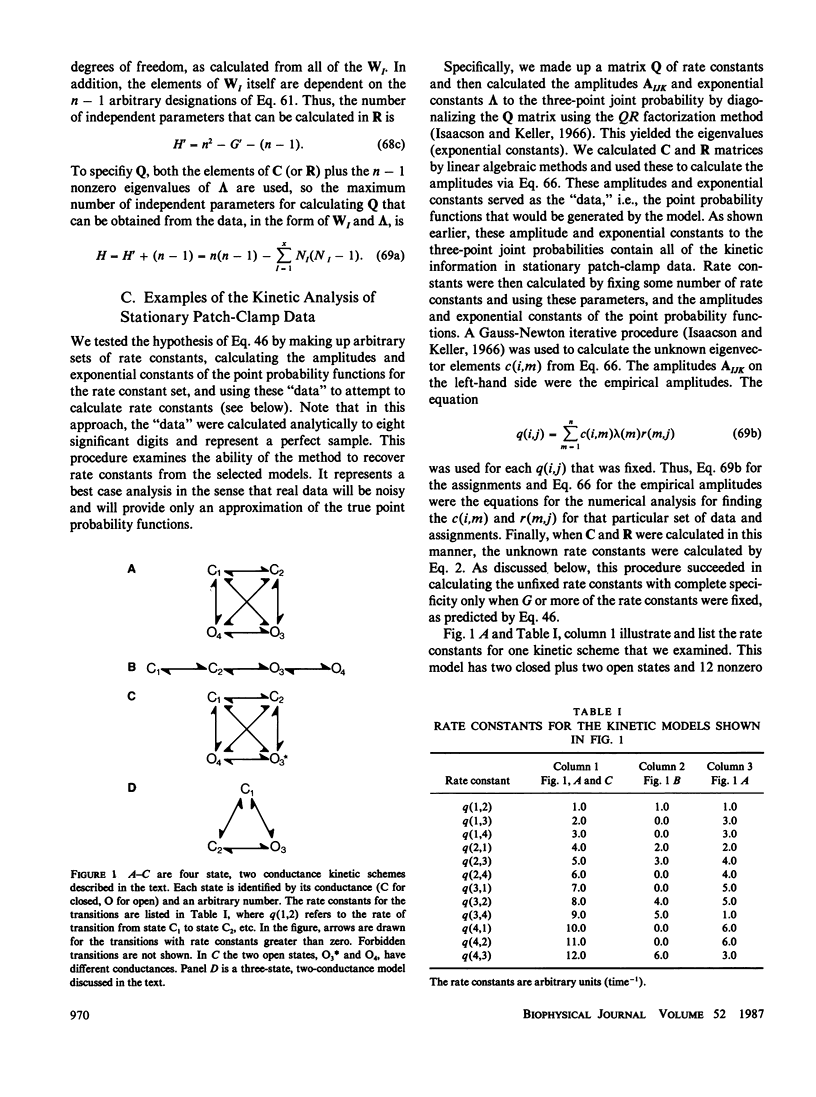
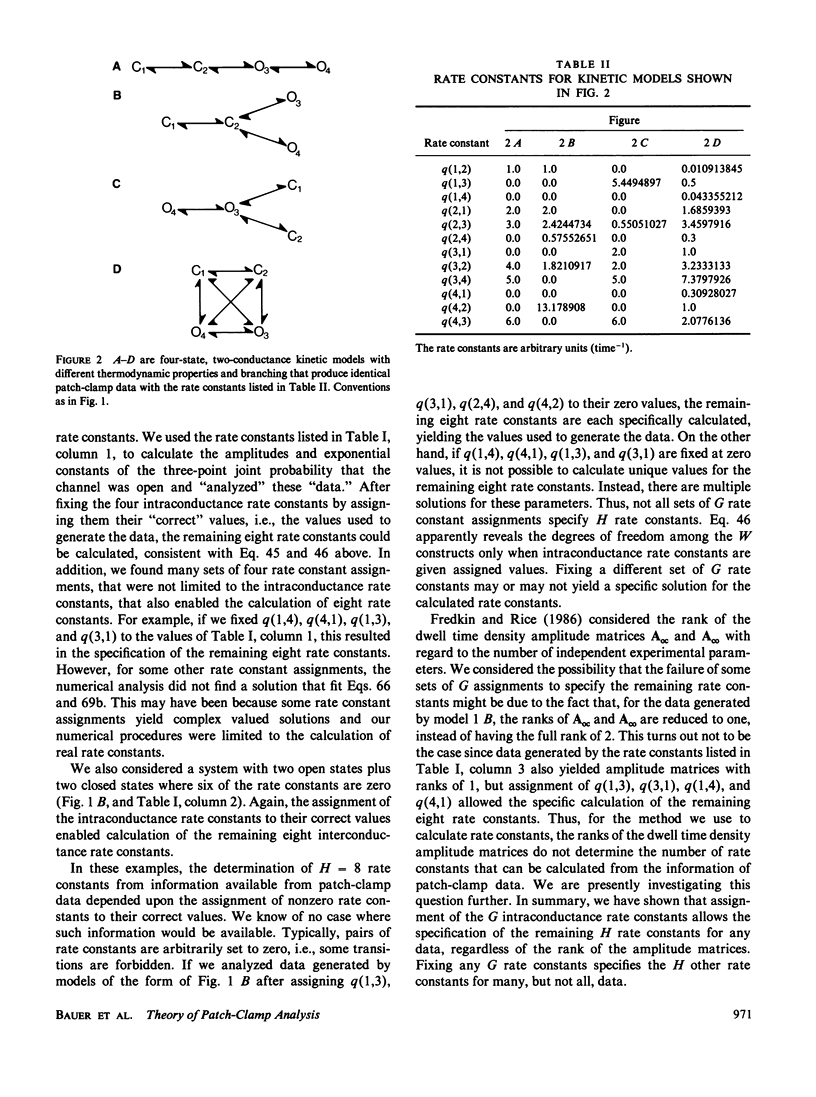
This paper describes a theory of the kinetic analysis of patch-clamp data. We assume that channel gating is a Markov process that can be described by a model consisting of n kinetic states and n(n - 1) rate constants at each voltage, and that patch-clamp data describe the occupancy of x different conductance levels over time. In general, all the kinetic information in a set of patch-clamp data is found in either two-dimensional dwell time histograms describing the frequency of observation of sequential dwell times of durations tau 1 and tau 2 (Fredkin, D. R., M. Montal, and J. A. Rice, 1985, Proceedings of the Berkeley Conference in Honor of Jerzy Neyman and Jack Kiefer, vol. 1, 269-289) or in three-point joint probability functions describing the probability that a channel is in a given conductance at time t, and at time t + tau 1, and at time t + tau 1 + tau 2. For the special case of channels with a single open state plus multiple closed states, one-dimensional analyses provide all of the kinetic information. Stationary patch-clamp data have information that can be used to determine H rate constants, where H = n(n - 1) - G and G is the number of intraconductance rate constants. Thus, to calculate H rate constants, G rate constants must be fixed. In general there are multiple sets of G rate constants that can be fixed to allow the calculation of H rate constants although not every set of G rate constants will work. Arbitrary assignment of the G intraconductance rate constants equal to zero always provides a solution and the calculation of H rate constants. Nonstationary patch-clamp data have information for the determination of H rate constants at a reference voltage plus n(n - 1) rate constants at all test voltages. Thus, nonstationary data have extra information about the voltage dependencies of rate constants that can be used to rule out kinetic models that cannot be disqualified on the basis of stationary data.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Hartshorne R. P., Talvenheimo J. A., Catterall W. A., Montal M. Sodium channels in planar lipid bilayers. Channel gating kinetics of purified sodium channels modified by batrachotoxin. J Gen Physiol. 1986 Jul;88(1):1–23. doi: 10.1085/jgp.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Kits K. S., Ramsey R. L., Sansom M. S., Usherwood P. N. Single channel kinetics of a glutamate receptor. Biophys J. 1986 Aug;50(2):367–374. doi: 10.1016/S0006-3495(86)83470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Lindstrom J., Montal M. The acetylcholine receptor channel from Torpedo californica has two open states. J Neurosci. 1984 Feb;4(2):502–507. doi: 10.1523/JNEUROSCI.04-02-00502.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Covariance of nonstationary sodium current fluctuations at the node of Ranvier. Biophys J. 1981 Apr;34(1):111–133. doi: 10.1016/S0006-3495(81)84840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeramian E., Trautmann A., Claverie P. Acetylcholine receptors are not functionally independent. Biophys J. 1986 Aug;50(2):253–263. doi: 10.1016/S0006-3495(86)83459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


