Abstract
1. Sodium transport has been measured across in vitro preparations of pig ileum taken at birth or during the first 10 days of post-natal life.
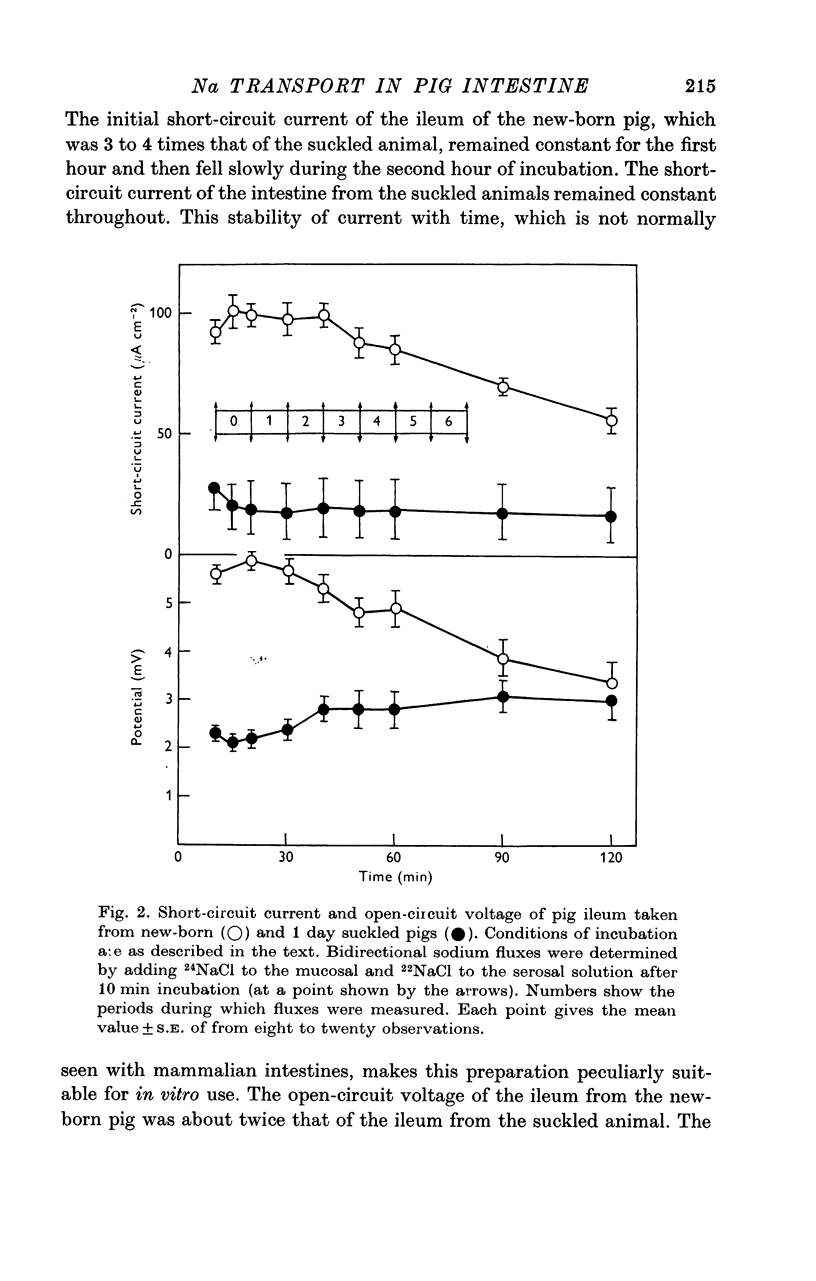
2. Both the unidirectional and net fluxes of sodium fell shortly after colostrum was ingested by the new-born piglet. These changes were associated with falls in short-circuit current, open-circuit voltage and tissue conductance and with the appearance of protein-filled vacuoles within the mucosal epithelium.
3. Overnight fasting of piglets following a period of suckling caused all sodium fluxes to revert to values similar to those found in the new-born animal. The short-circuit current also increased on fasting but the magnitude of this effect decreased with age.
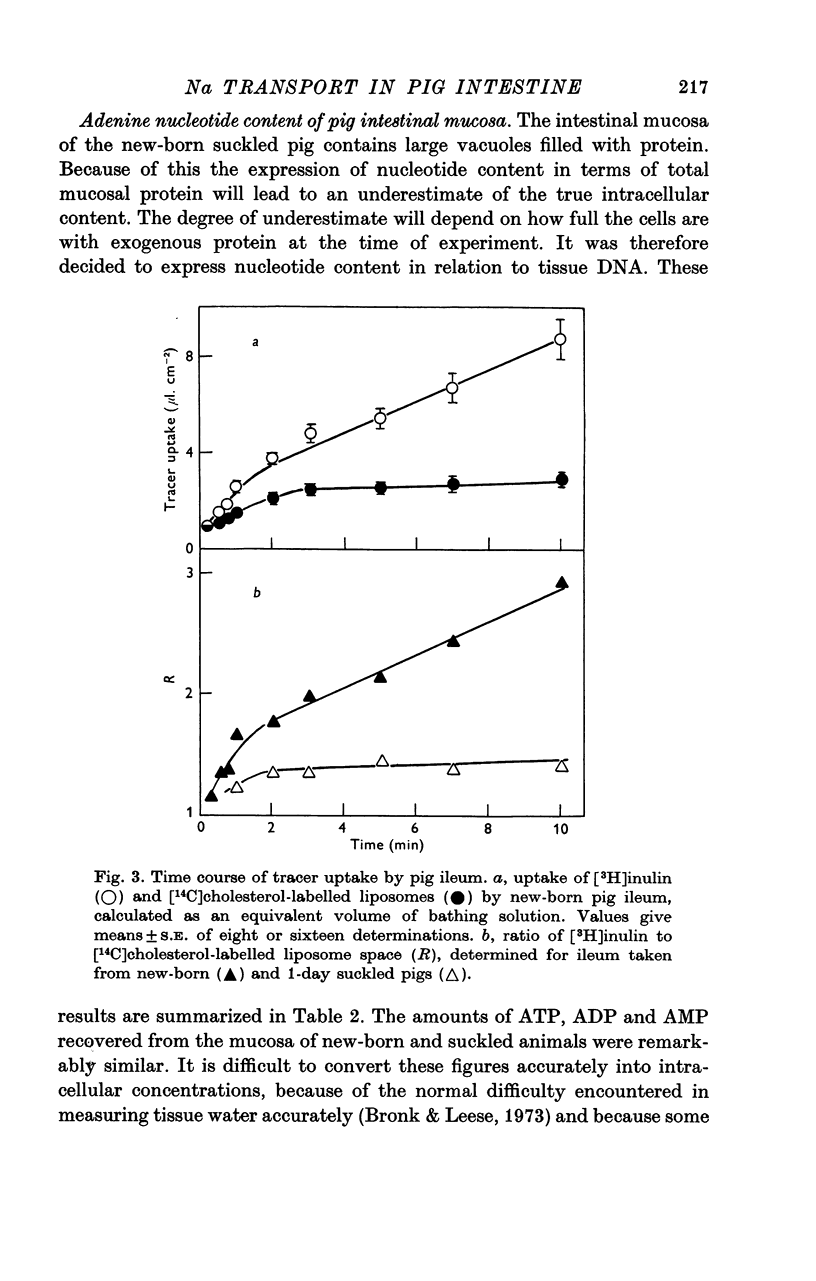
4. The suckling-dependent inhibition of sodium transport was not associated with any change in the level of adenine nucleotides within the mucosa. However the rate at which sodium entered the intestinal mucosa of suckled pigs was found to be only half that of the new-born.
5. It is suggested that pinocytosis might use up or chemically modify the microvillar membrane making it less permeable to sodium. Subsequent fasting would, by replenishment or further modification of existing membrane surface, allow the epithelium to recover its original permeability to sodium and possibly regain the ability to undergo a further cycle of pinocytotic activity.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Leese H. J. Changes in the adenine nucleotide content of preparations of the rat small intestine in vitro. J Physiol. 1973 Nov;235(1):183–196. doi: 10.1113/jphysiol.1973.sp010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Smith M. W., Witty R. Interdependence of albumin and sodium transport in the foetal and new-born pig intestine. J Physiol. 1968 Sep;198(2):365–381. doi: 10.1113/jphysiol.1968.sp008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M., Hardy R. N. Histological changes in the small intestine of the young pig and their relation to macromolecular uptake. J Anat. 1971 Jan;108(Pt 1):63–77. [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Nibelle J., Smith M. W. The effect of salt adaptation on the permeability and cation selectivity of the goldfish intestinal epithelium. J Physiol. 1973 May;231(1):105–115. doi: 10.1113/jphysiol.1973.sp010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques de Jesus C., Smith M. W. Protein and glucose-induced changes in sodium transport across the pig small intestine. J Physiol. 1974 Nov;243(1):225–242. doi: 10.1113/jphysiol.1974.sp010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Chez R. A., Fuisz R. E. Alanine and sodium fluxes across mucosal border of rabbit ileum. J Gen Physiol. 1967 May;50(5):1241–1260. doi: 10.1085/jgp.50.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Ionic dependence of protein transport across the new-born pig intestine. J Physiol. 1971 Apr;214(2):349–363. doi: 10.1113/jphysiol.1971.sp009436. [DOI] [PMC free article] [PubMed] [Google Scholar]


