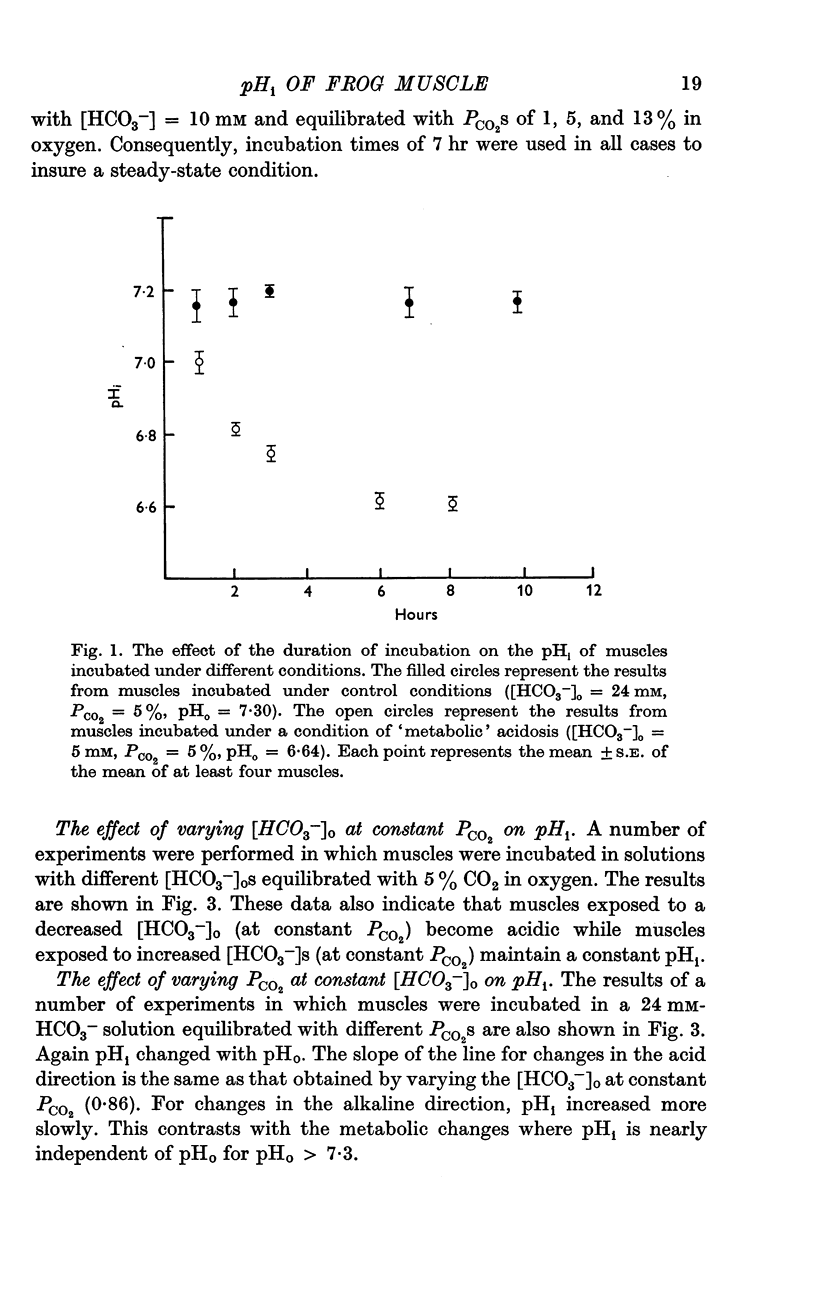
Abstract
1. The dimethyloxazolidinedione (DMO) technique was used to estimate intracellular pH (pHi) in bullfrog toe muscles incubated in vitro. The control value of pHi was 7·16 + ± 0·01 (S.D.).
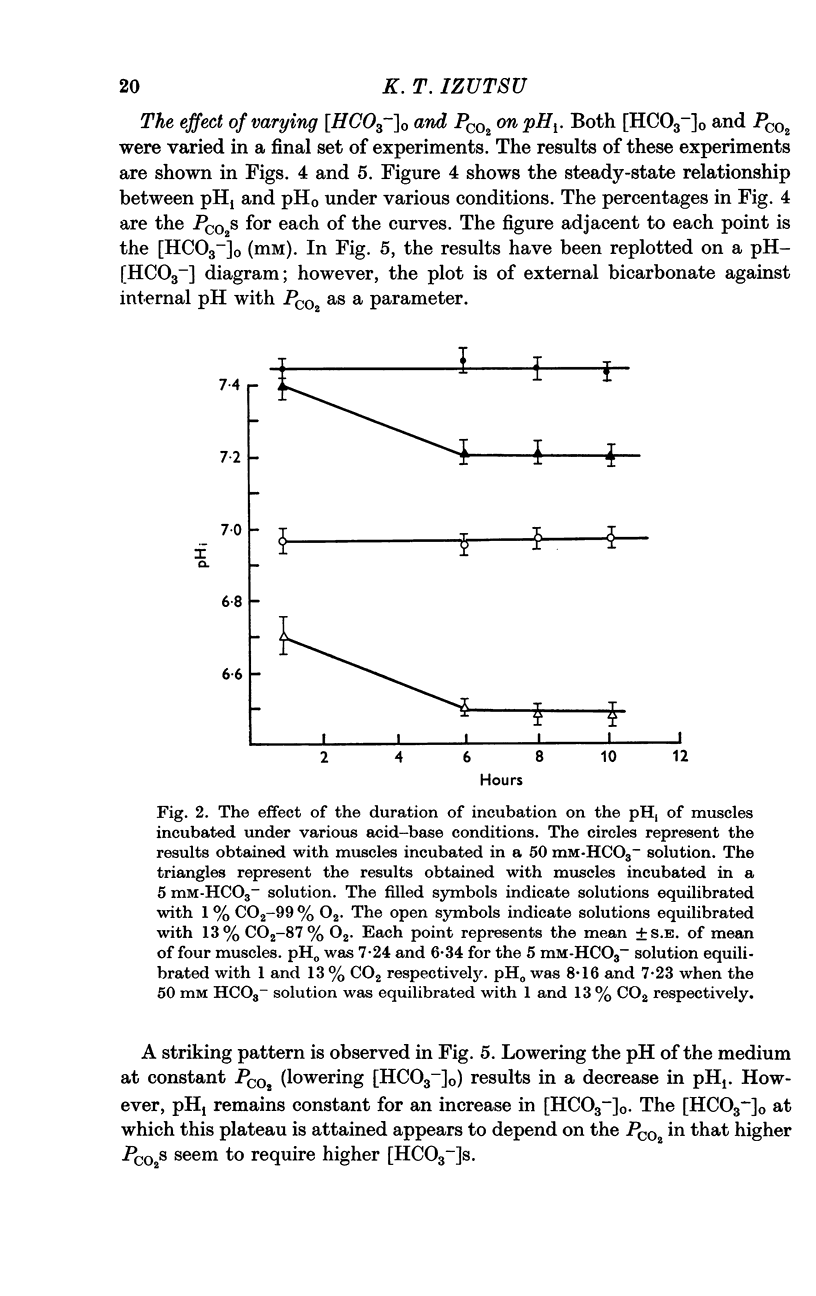
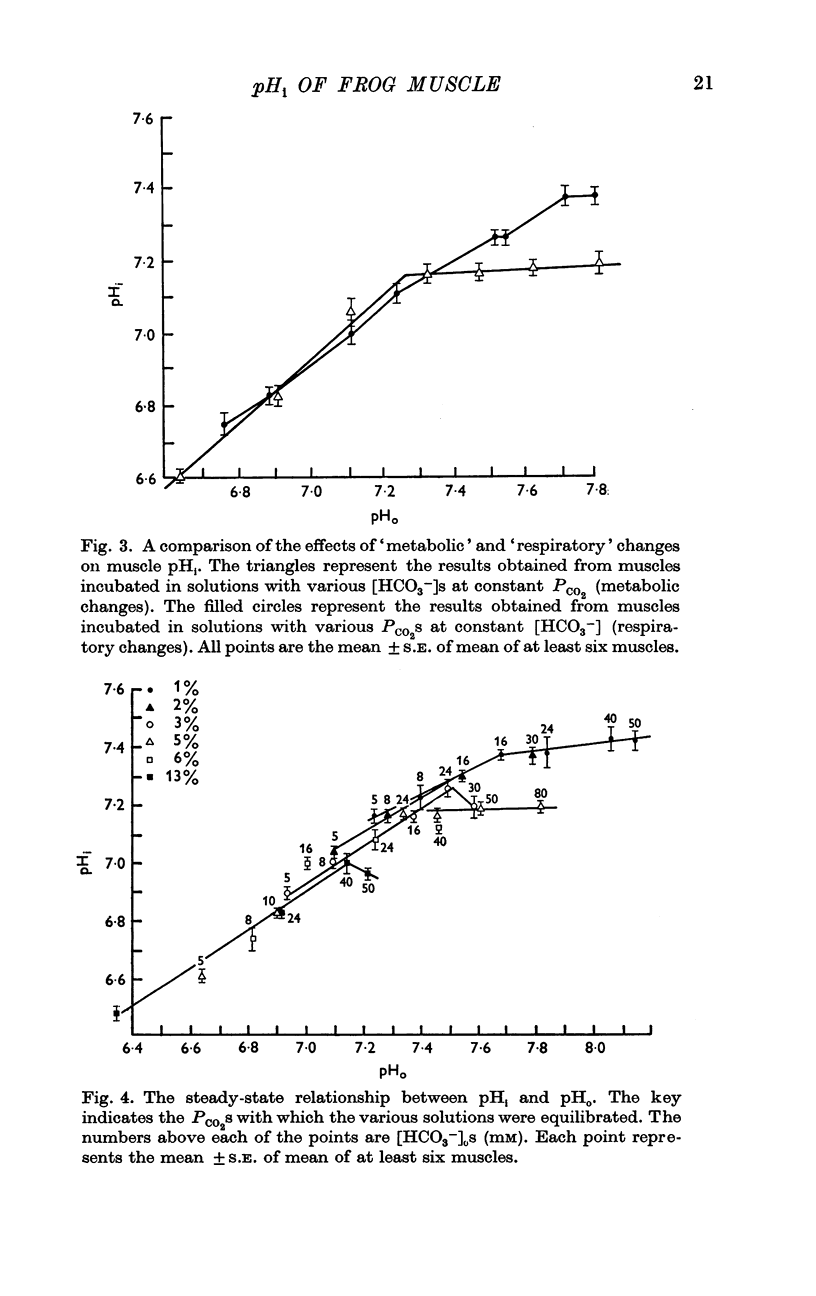
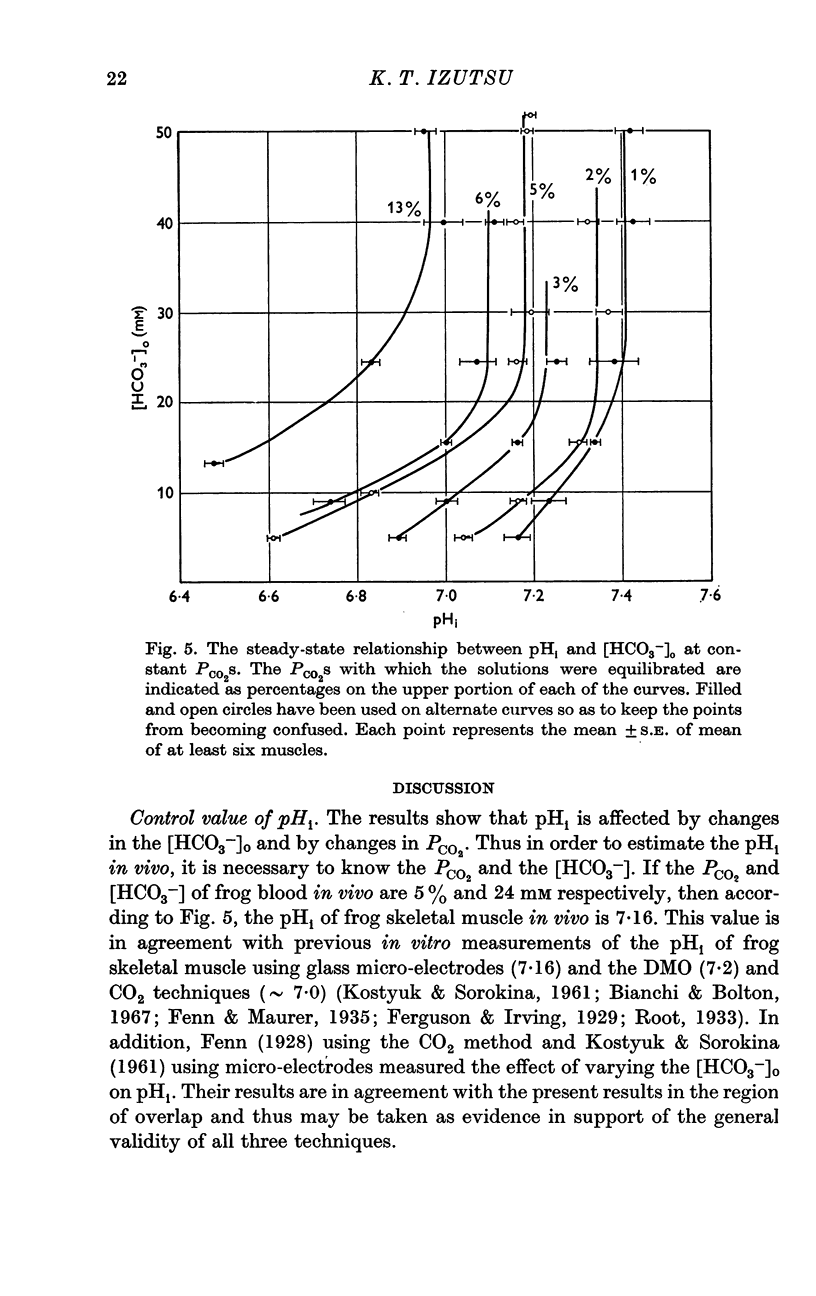
2. pHi was affected by changes in PCO2 and external bicarbonate ion concentration ([HCO3-]0). At a given PCO2, decreasing the external [HCO3-] was more effective in lowering pHi than increasing the external [HCO3-] was in increasing pHi.
3. On the assumption that the changes in pHi were due to hydrogen ion [H+) movements across the membrane, a H+ flux of 10-13 mole/cm2. sec was calculated. The corresponding H+ permeability coefficient was 10-3 cm/sec.
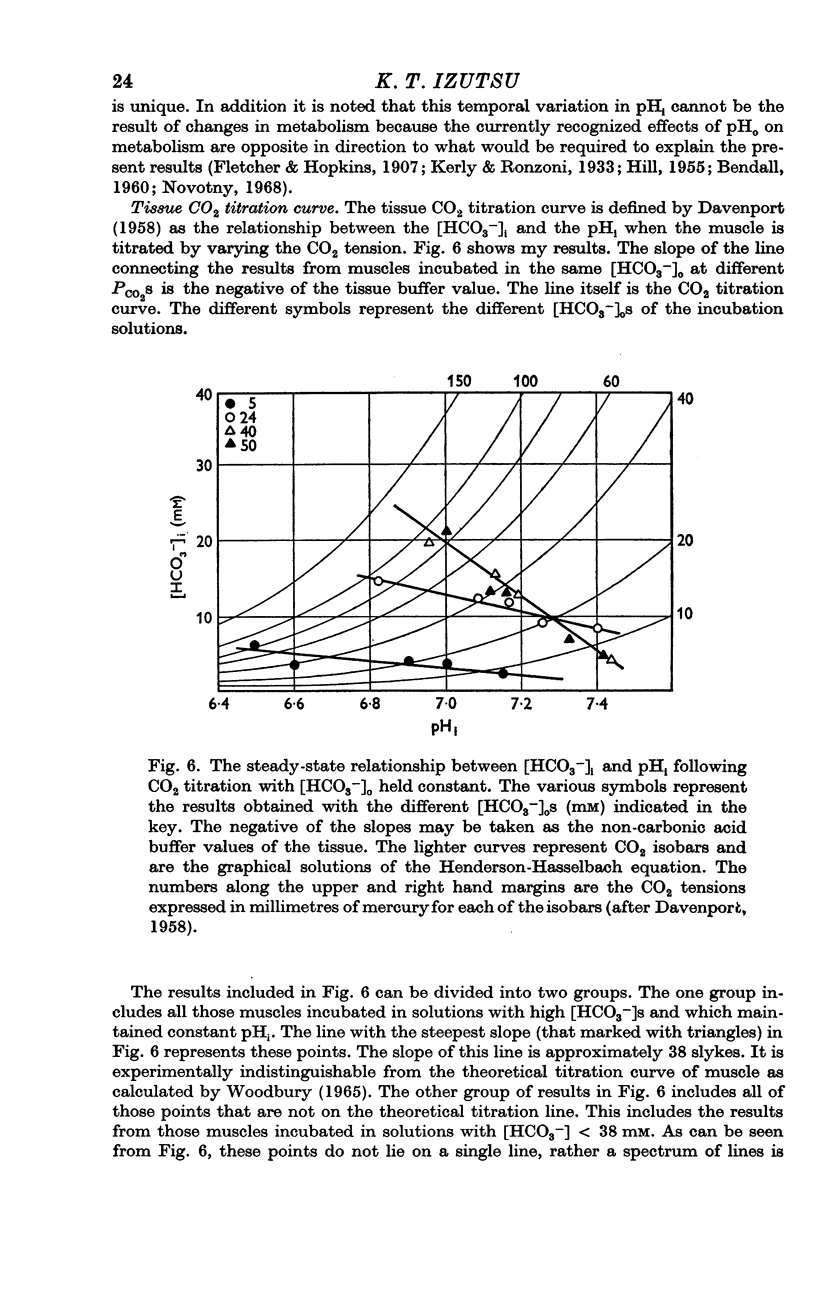
4. The variability of the tissue CO2 buffer value was examined.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi C. P., Bolton T. C. Action of local anesthetics on coupling systems in muscle. J Pharmacol Exp Ther. 1967 Aug;157(2):388–405. [PubMed] [Google Scholar]
- Butler T. C., Poole D. T., Waddell W. J. Acid-labile carbon dioxide in muscle: its nature and relationship to intracellular pH. Proc Soc Exp Biol Med. 1967 Jul;125(3):972–974. doi: 10.3181/00379727-125-32252. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH with glass microelectrodes. Fed Proc. 1967 Sep;26(5):1322–1326. [PubMed] [Google Scholar]
- Chance B., Lee C. P., Mela L. Control and conservation of energy in the cytochrome chain. Fed Proc. 1967 Sep;26(5):1341–1354. [PubMed] [Google Scholar]
- Fletcher W. M. Lactic acid in amphibian muscle. J Physiol. 1907 Mar 27;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERG R. J. Phosphorescence in liquid scintillation counting of proteins. Science. 1958 Jul 25;128(3317):199–200. doi: 10.1126/science.128.3317.199. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The influence of the external medium on the internal pH of muscle. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):1–22. doi: 10.1098/rspb.1955.0030. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVINE R. O., SAUNDERS S. J., MILNE M. D., CRAWFORD M. A. Gradients of potassium and hydrogen ion in potassiumdeficient voluntary muscle. Clin Sci. 1961 Feb;20:1–18. [PubMed] [Google Scholar]
- Novotný I. pH changes during splitting of ATP in skeletal and cardiac muscle extracts and microsomes. Physiol Bohemoslov. 1968;17(6):569–575. [PubMed] [Google Scholar]
- Paymaster N. J., Englesson S. Calculation of pH of human erythrocyte from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO). Acta Anaesthesiol Scand. 1966;10(4):219–224. doi: 10.1111/j.1399-6576.1966.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Reeves R. B. Role of body temperature in determining the acid-base state in vertebrates. Fed Proc. 1969 May-Jun;28(3):1204–1208. [PubMed] [Google Scholar]
- Steinmetz P. R. Acid-base relations in epithelium of turtle bladder: site of active step in acidification and role of metabolic CO2. J Clin Invest. 1969 Jul;48(7):1258–1265. doi: 10.1172/JCI106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN M., STEINBERG D., LOGAN J. Liquid scintillation counting of C14- and H3-labeled amino acids and proteins. Science. 1957 Sep 6;126(3271):446–447. doi: 10.1126/science.126.3271.446-a. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Wastl H., Seliskar A. Observations on the combination of CO(2) in the blood of the bull frog (Rana catesbiana). J Physiol. 1925 Sep 4;60(4):264–268. doi: 10.1113/jphysiol.1925.sp002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G. B. Actions of procaine on nicotine-induced effects in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):148–158. [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]
- Weiss G. B. The effect of pH on nicotine-induced contracture and Ca45 movements in frog sartorius muscle. J Pharmacol Exp Ther. 1966 Dec;154(3):605–612. [PubMed] [Google Scholar]


