Abstract
1. The chloride content and fluxes, and the membrane potential of L cells have been measured.
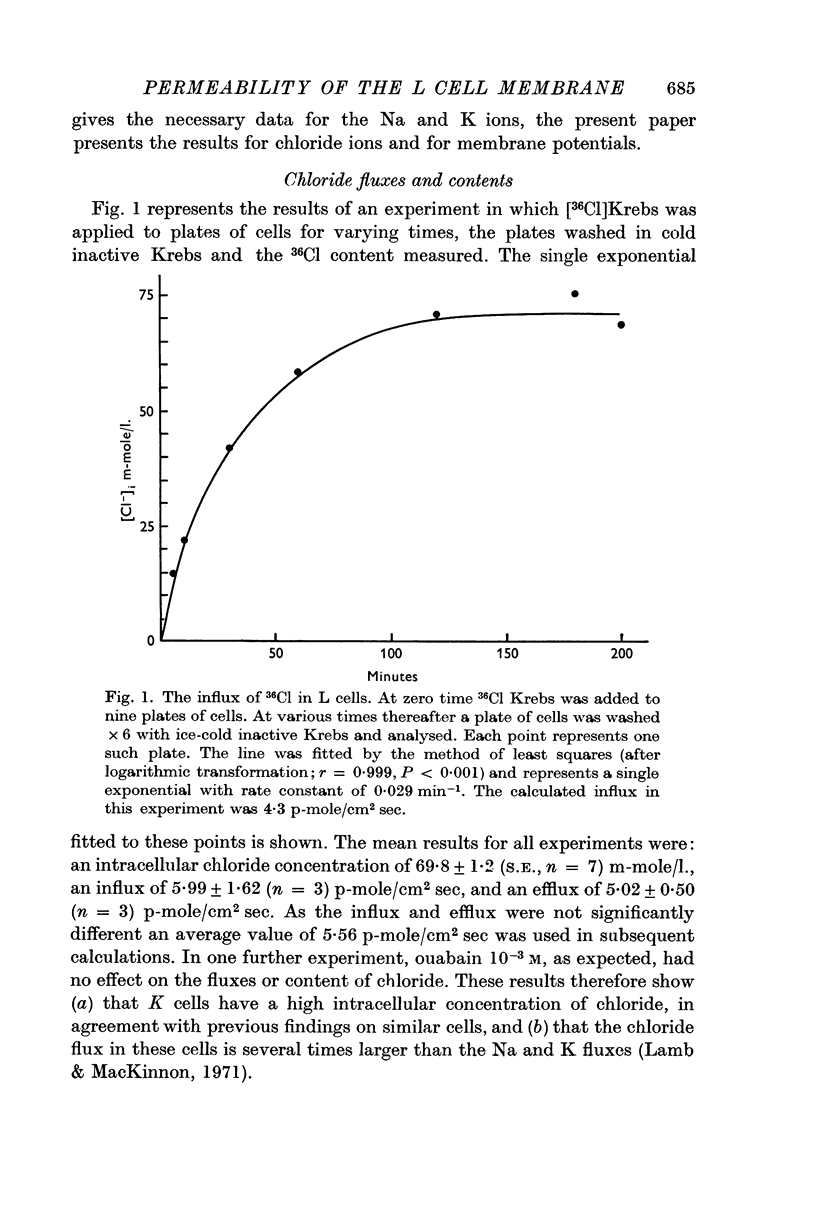
2. L cells contain chloride, 70 m-mole/l. intracellular water and have a flux of 5·5 p-mole/cm2 sec.
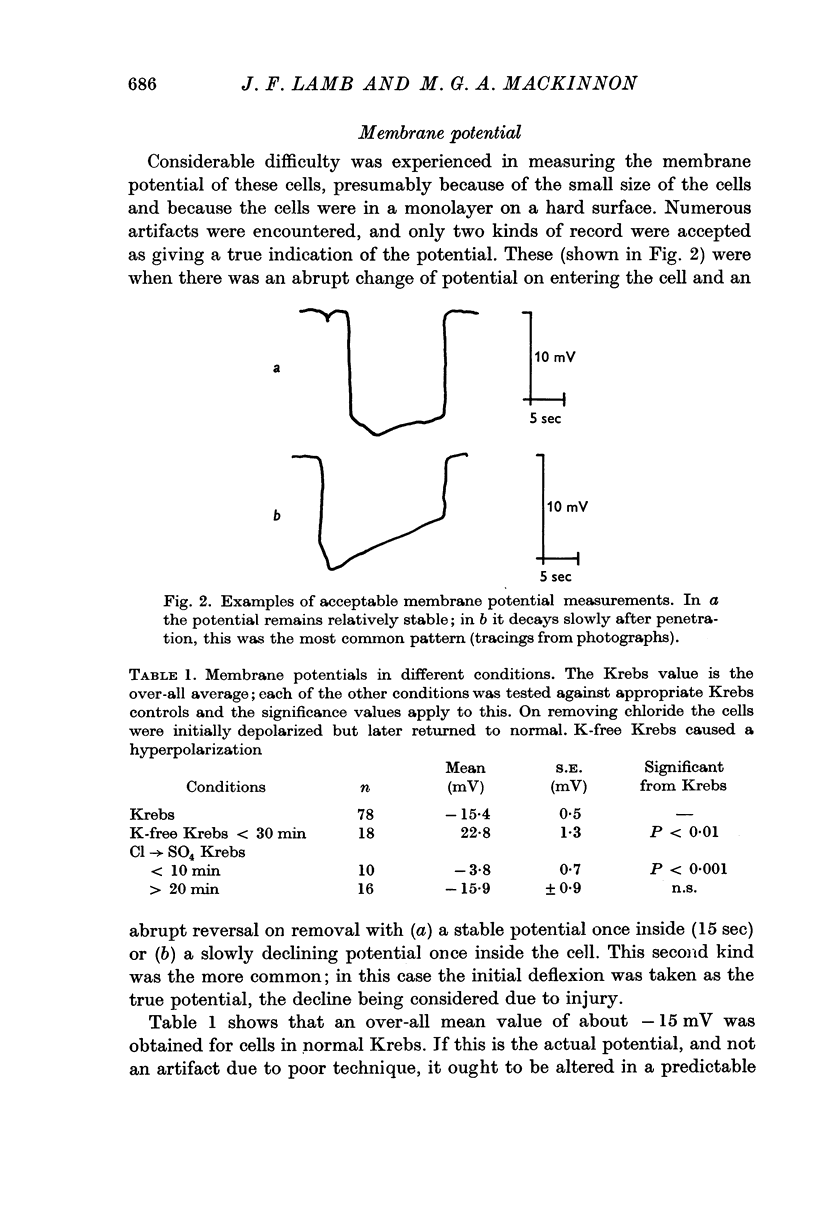
3. The membrane potential is -15 mV.K-free Krebs causes an increase in Em and replacing chloride with sulphate causes a temporary reduction in Em.
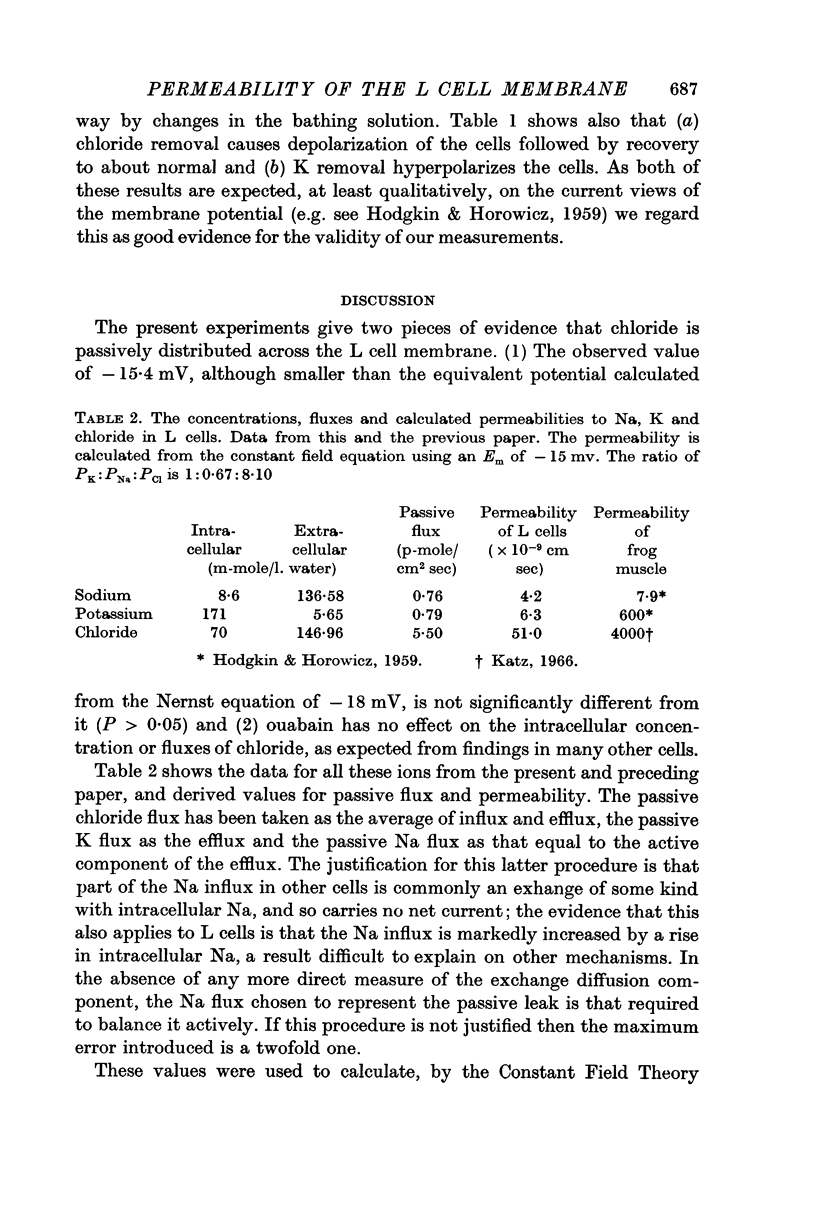
4. These values for Em and chloride, and previously obtained values for Na and K fluxes and contents were used to calculate the permeabilities of the various ions using the Goldman constant field theory. This gave permeabilities of 6·3, 4·2 and 51 × 10-9 cm/sec for K, Na and chloride respectively, a ratio of 1:0·67:8·10.
5. It is concluded that these cells have a low membrane potential because the PK is some 100 times lower than in skeletal muscle, therefore leading to a PK of the same order as PNa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Internal chloride concentration and chloride efflux of frog muscle. J Physiol. 1961 May;156:623–632. doi: 10.1113/jphysiol.1961.sp006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B., Loveday J. Effects of temperature, potassium, and calcium on the electrical potential difference in HeLa cells. Cancer Res. 1968 Dec;28(12):2401–2405. [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMPLING H. G. Potassium and sodium movements in the Ehrlich mouse ascites tumor cell. J Gen Physiol. 1958 Jan 20;41(3):565–583. doi: 10.1085/jgp.41.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. Effect of ouabain and metabolic inhibitors on the Na and K movements and nucleotide contents of L cells. J Physiol. 1971 Mar;213(3):665–682. doi: 10.1113/jphysiol.1971.sp009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanne O., Coraboeuf E. Potential and resistance measurements of rat liver cells in situ. Nature. 1966 Jun 25;210(5043):1390–1391. doi: 10.1038/2101390a0. [DOI] [PubMed] [Google Scholar]
- WICKSON-GINZBURG M., SOLOMON A. K. ELECTROLYTE METABOLISM IN HELA CELLS. J Gen Physiol. 1963 Jul;46:1303–1315. doi: 10.1085/jgp.46.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]


