Abstract
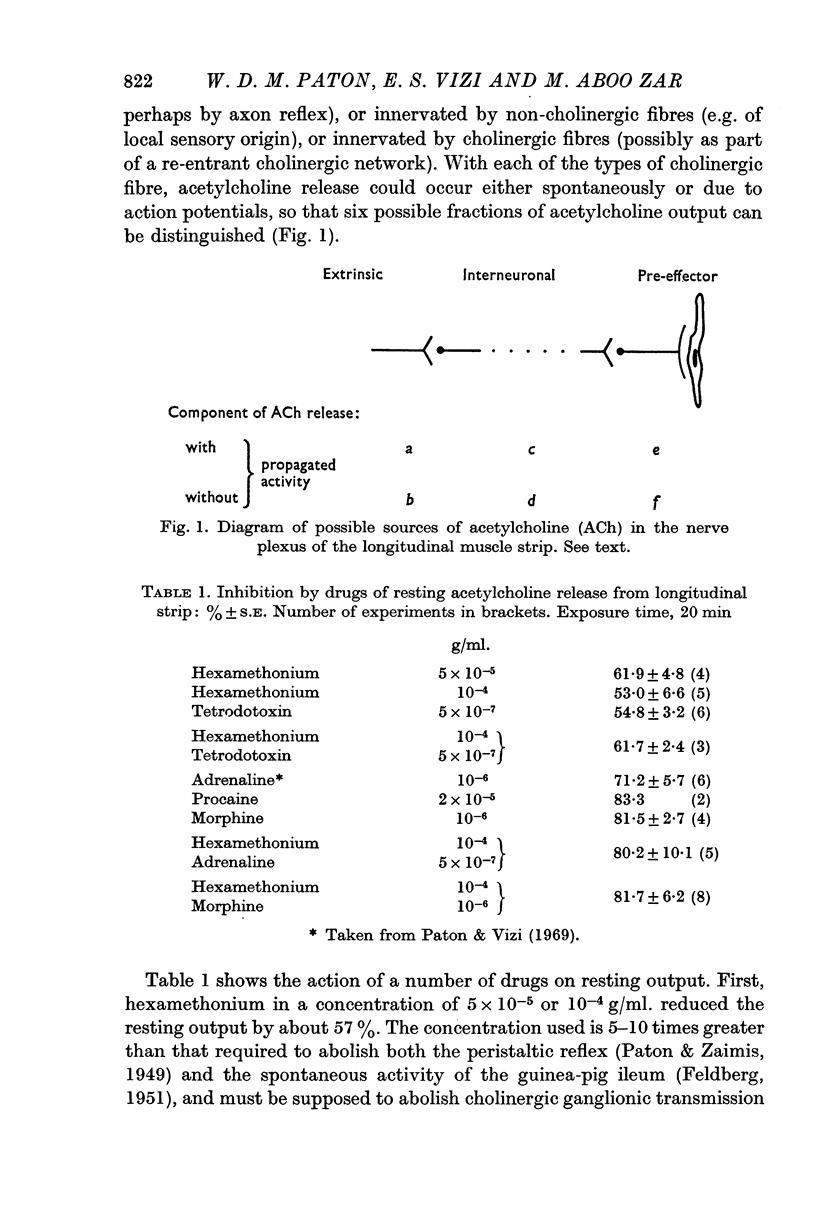
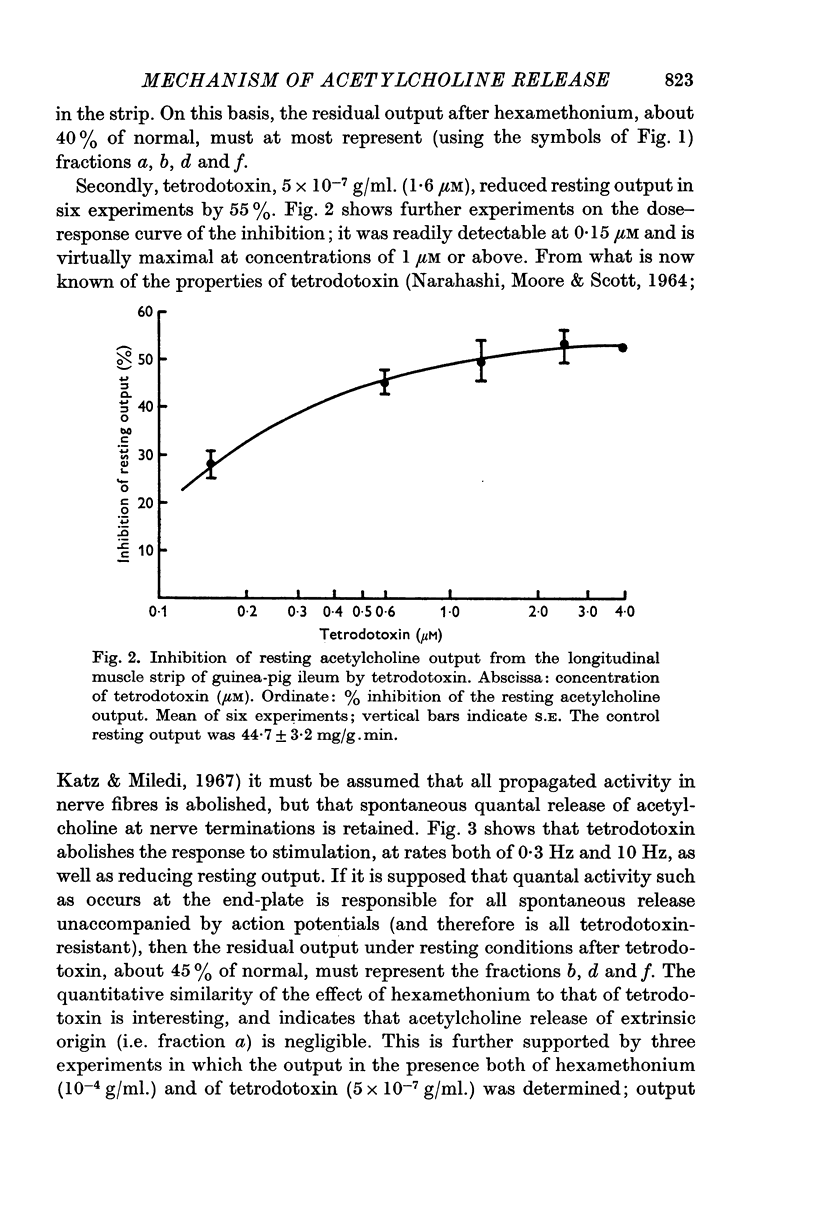
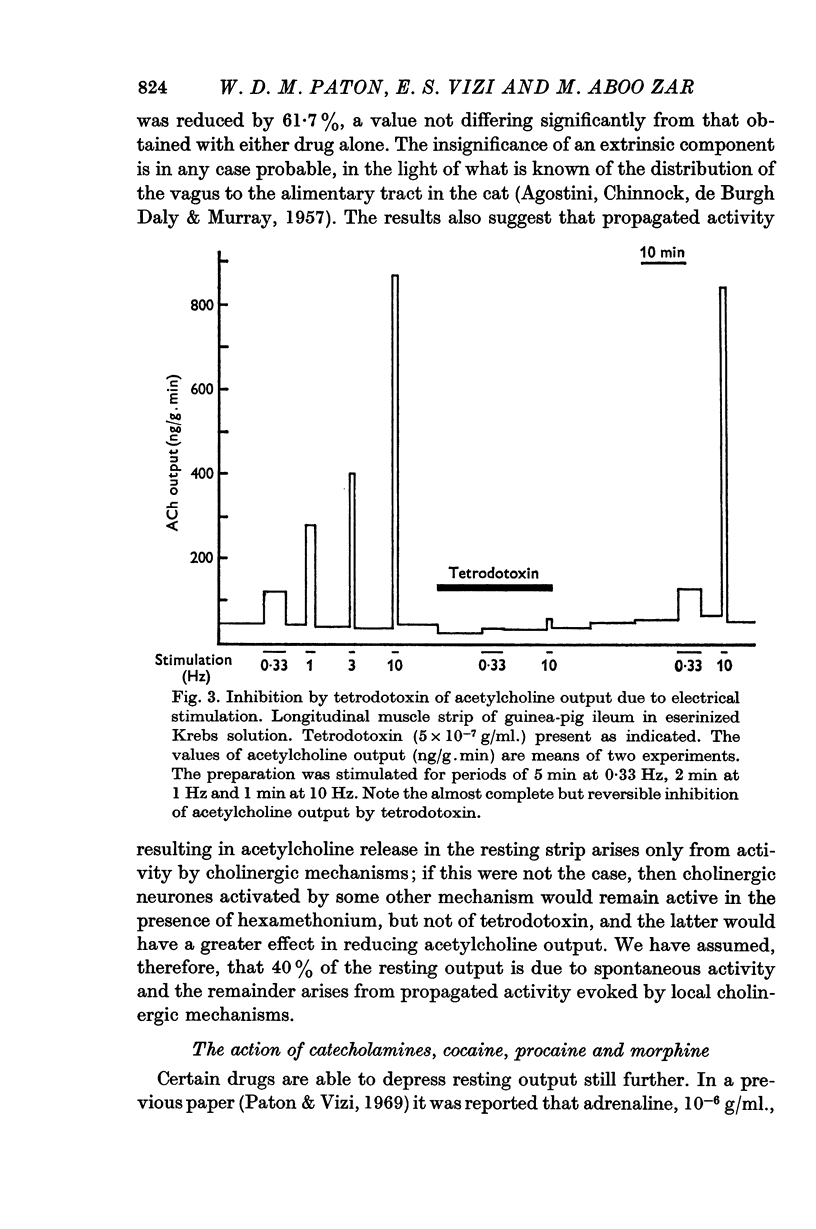
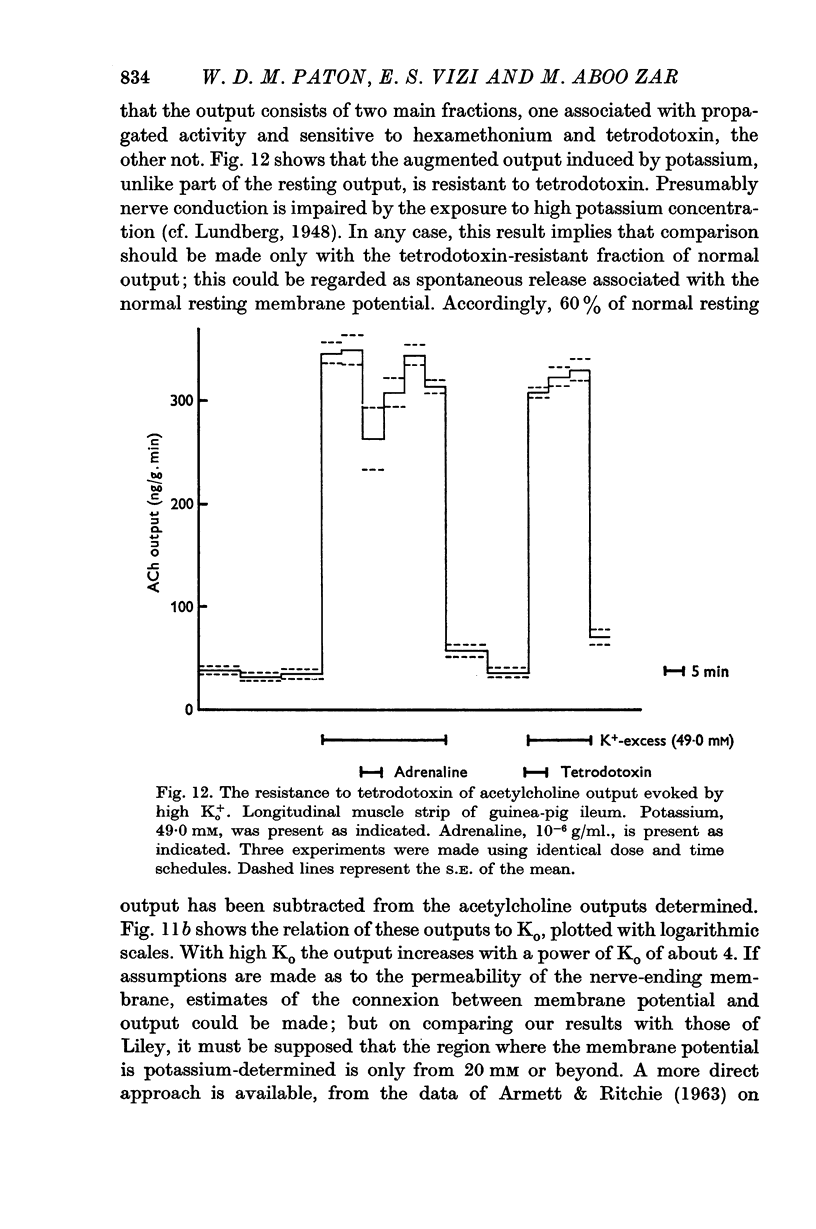
1. The output of acetylcholine from the plexus of the guinea-pig ileum longitudinal strip has been used to study the mechanism of acetylcholine release. From the effects of hexamethonium and tetrodotoxin, it was inferred that 60% of the normal resting output is due to propagated activity in the plexus, and 40% to spontaneous release. Tetrodotoxin virtually abolishes the increase in output in response to electrical stimulation.
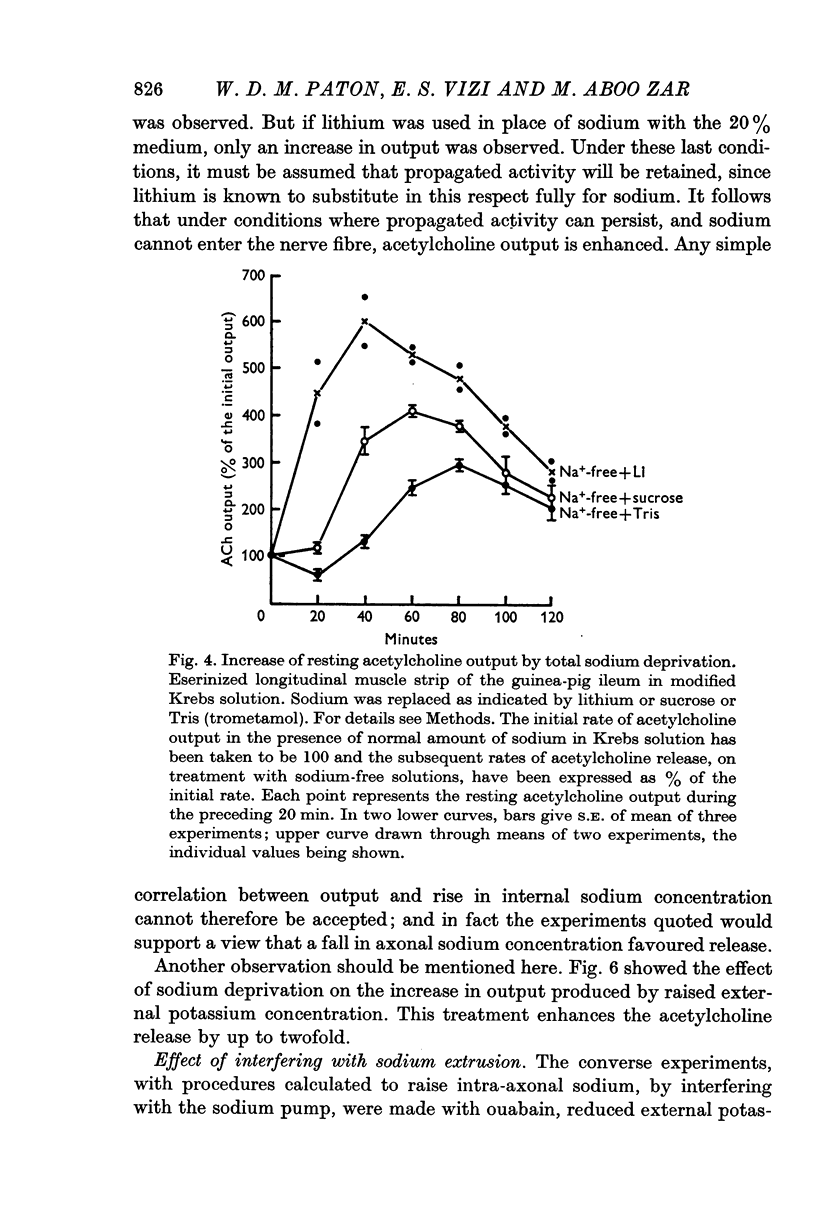
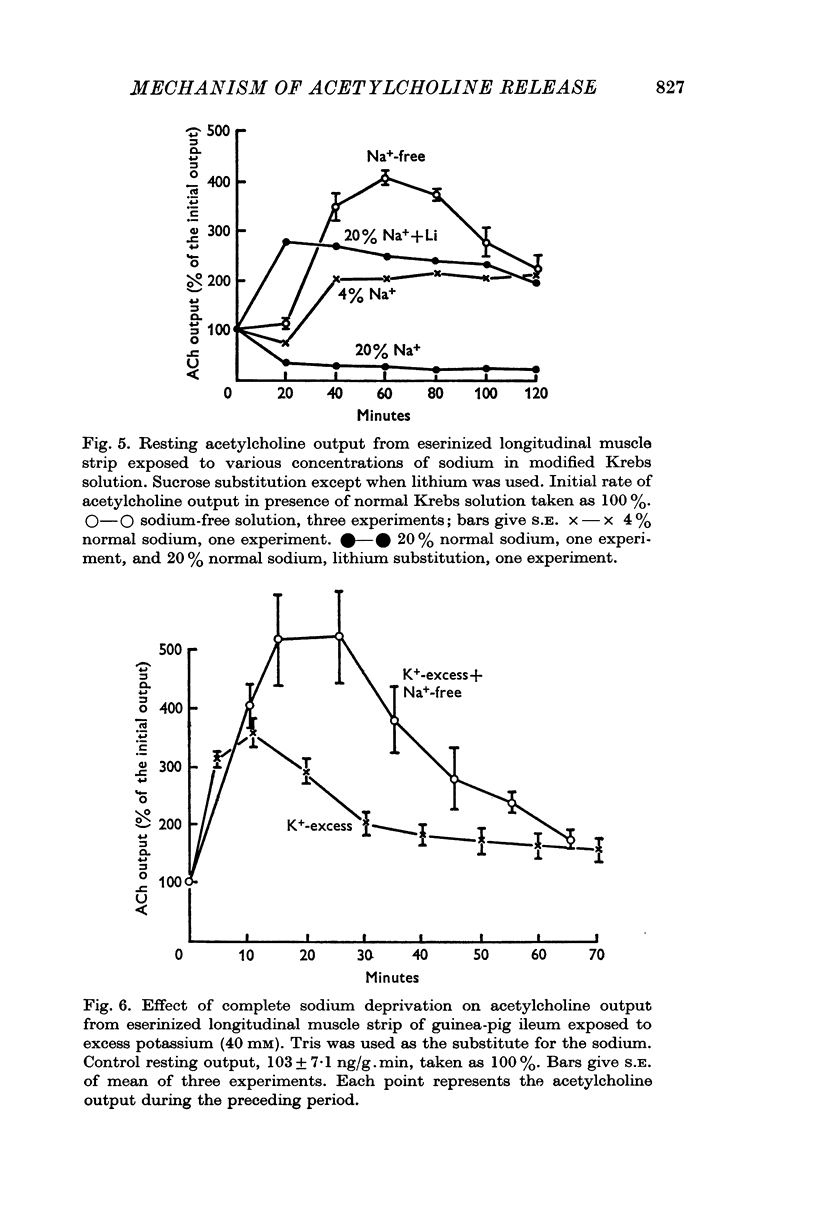
2. Resting acetylcholine output is increased when the bathing medium is changed in the following ways:
(a) sodium replacement by sucrose, trometamol or lithium;
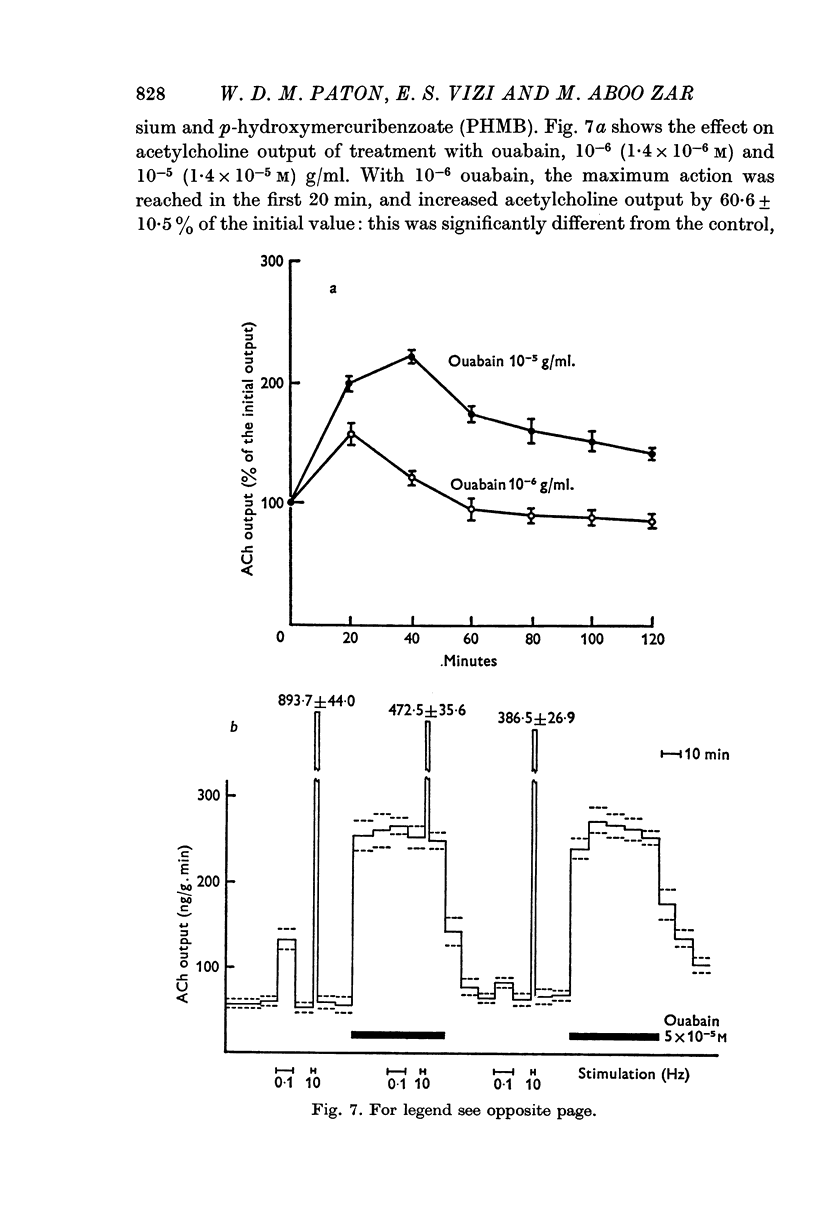
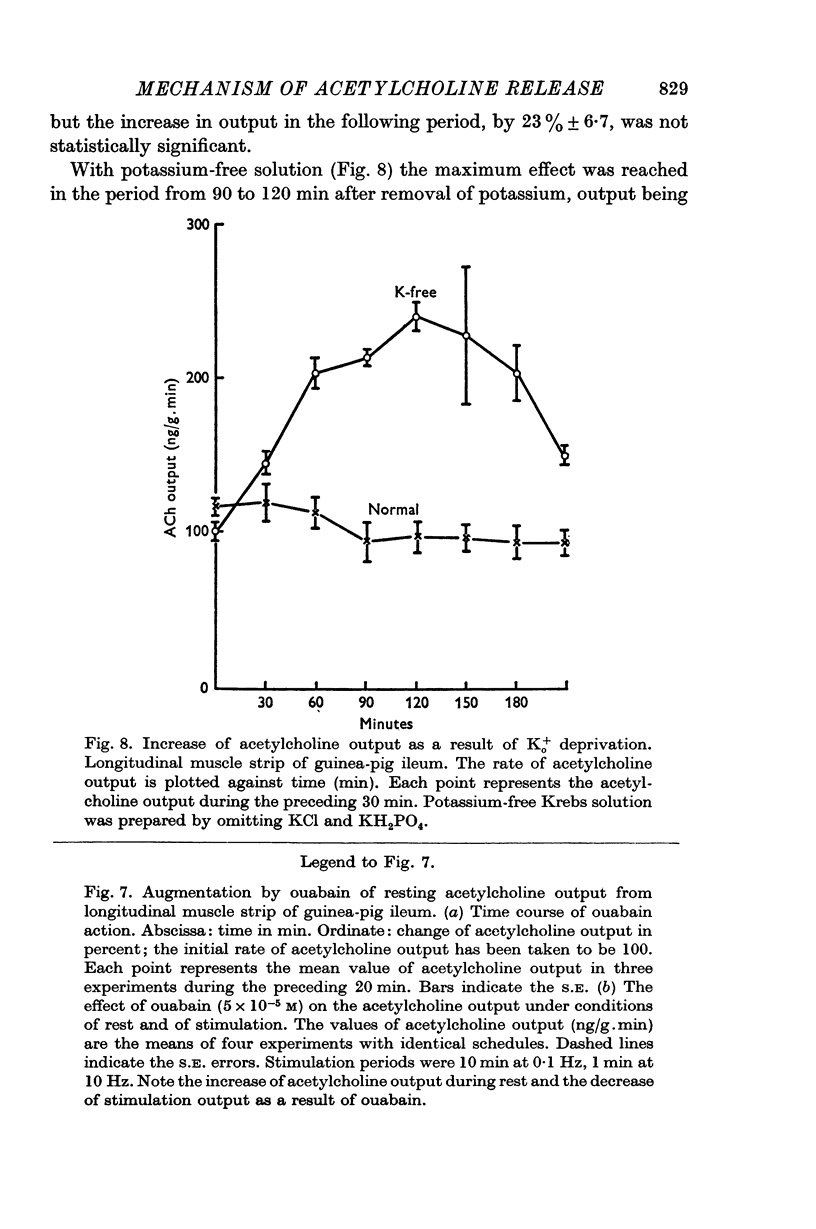
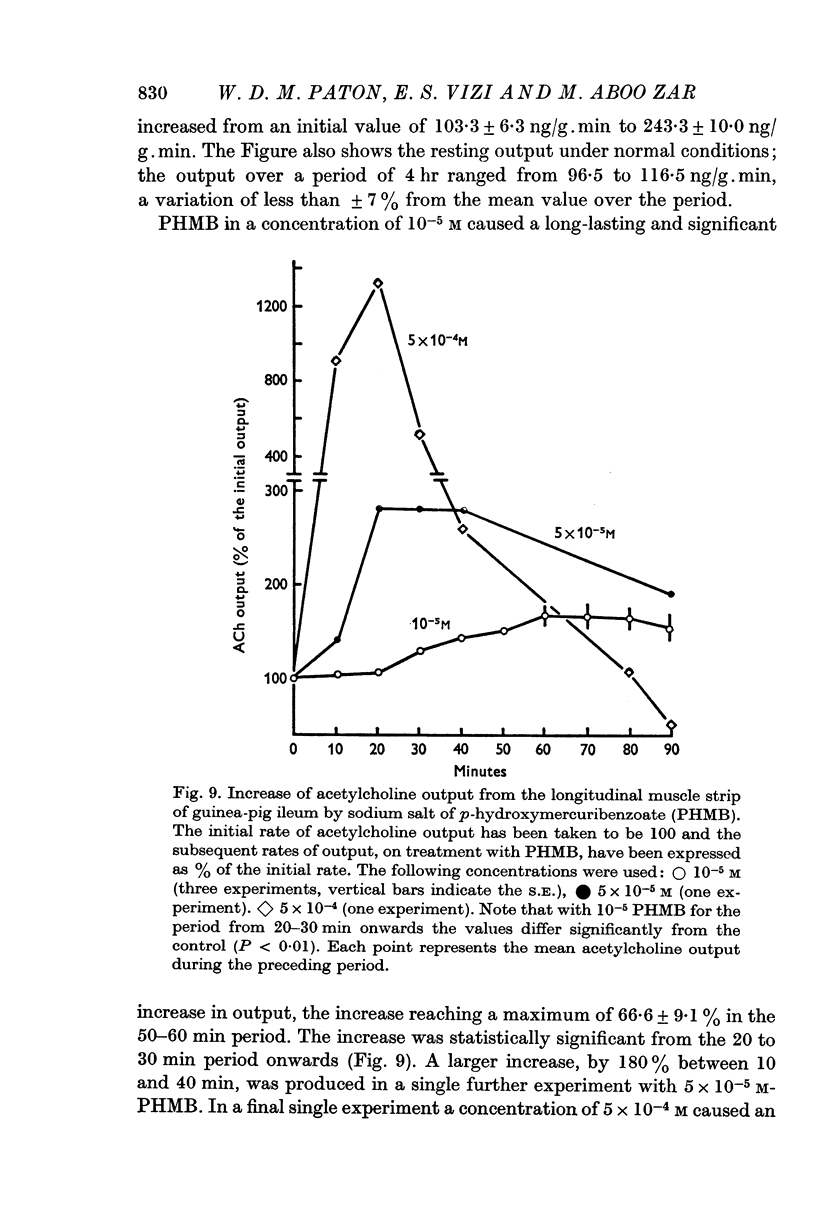
(b) addition of ouabain or p-hydroxymercuribenzoate (PHMB), or withdrawal of potassium;
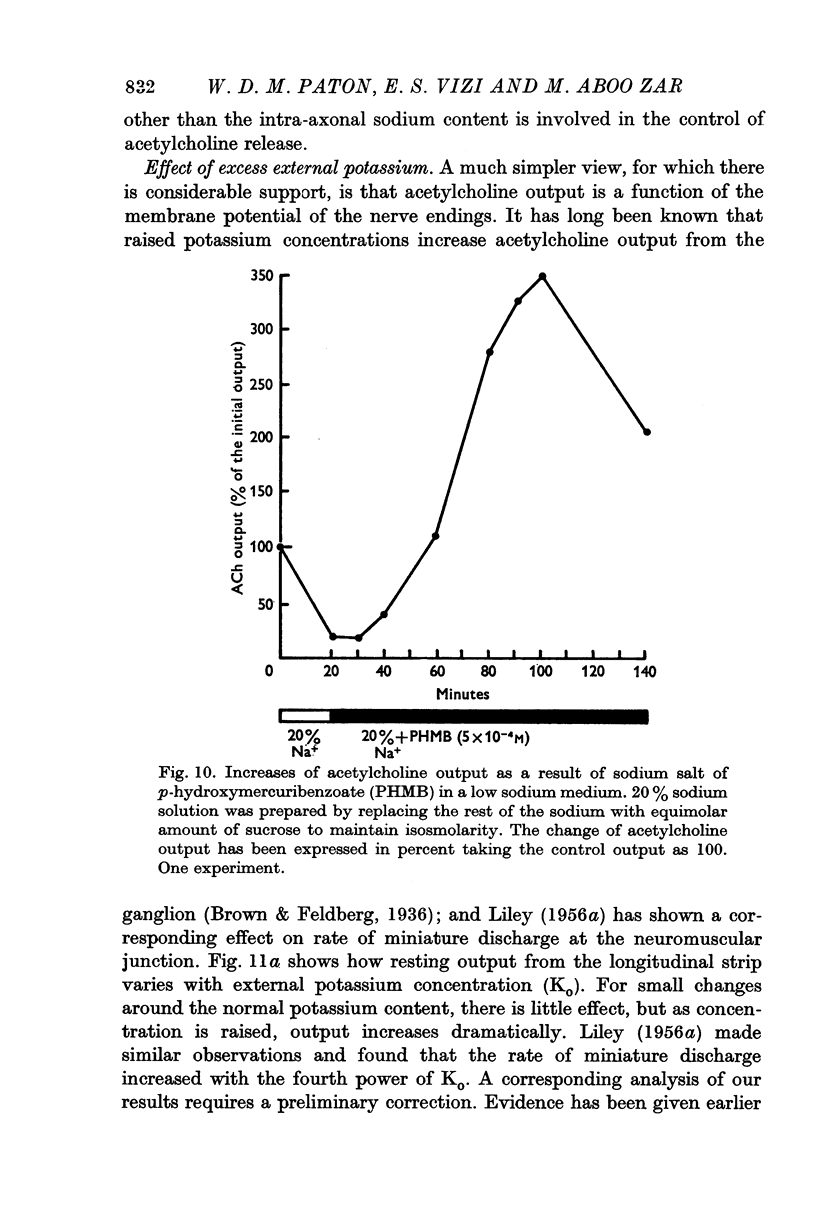
(c) the combination of PHMB and partial sodium replacement;
(d) addition of potassium; this increase in output becomes greater in the absence of sodium.
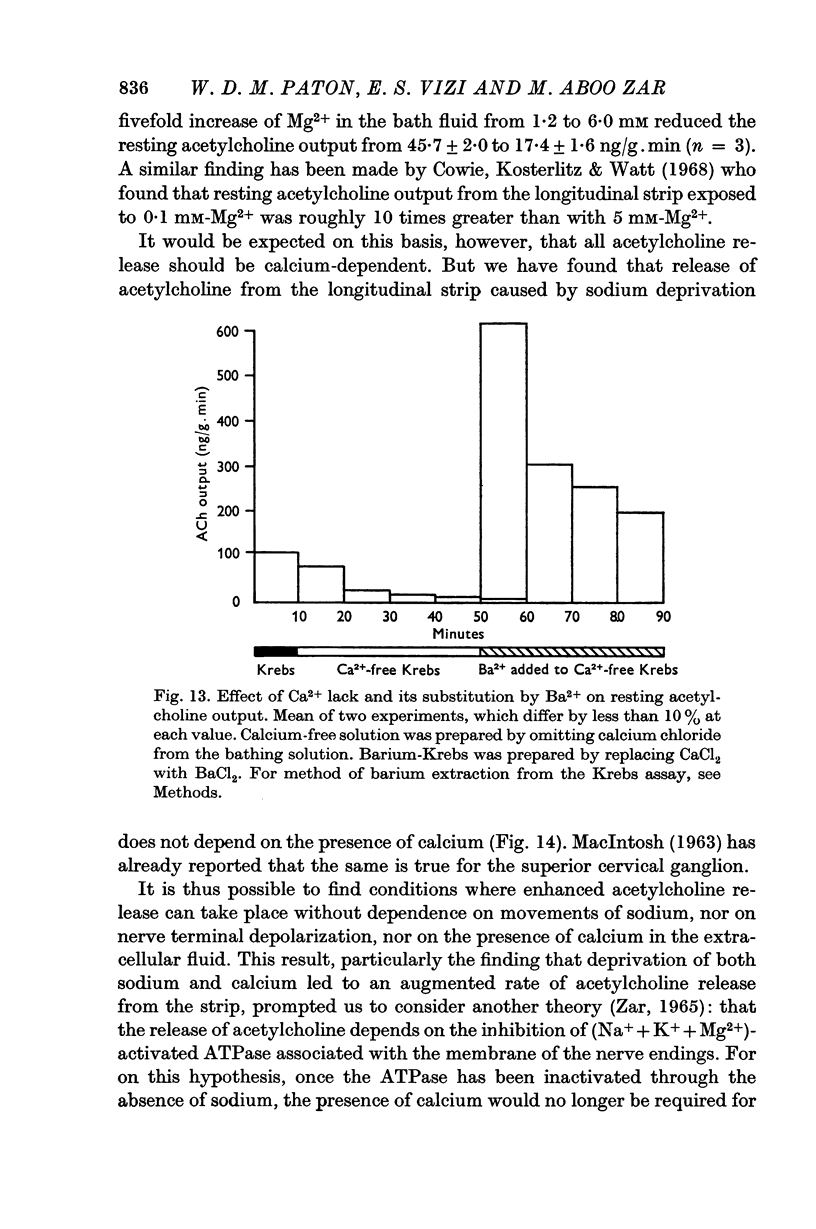
3. The resting output is virtually abolished by calcium withdrawal, and is restored by barium substitution for calcium. It is also reduced by raising the magnesium concentration.
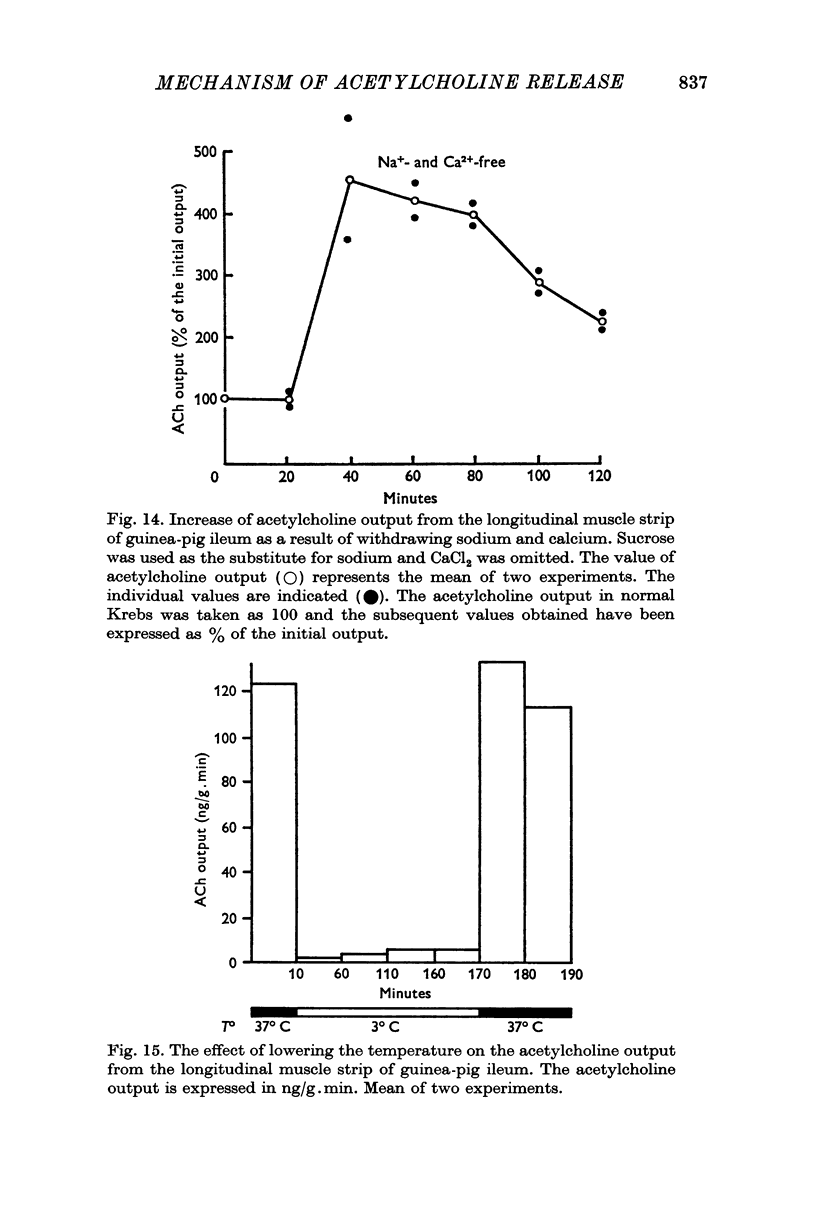
4. The enhanced resting output in response to sodium withdrawal also occurs in the absence of calcium.
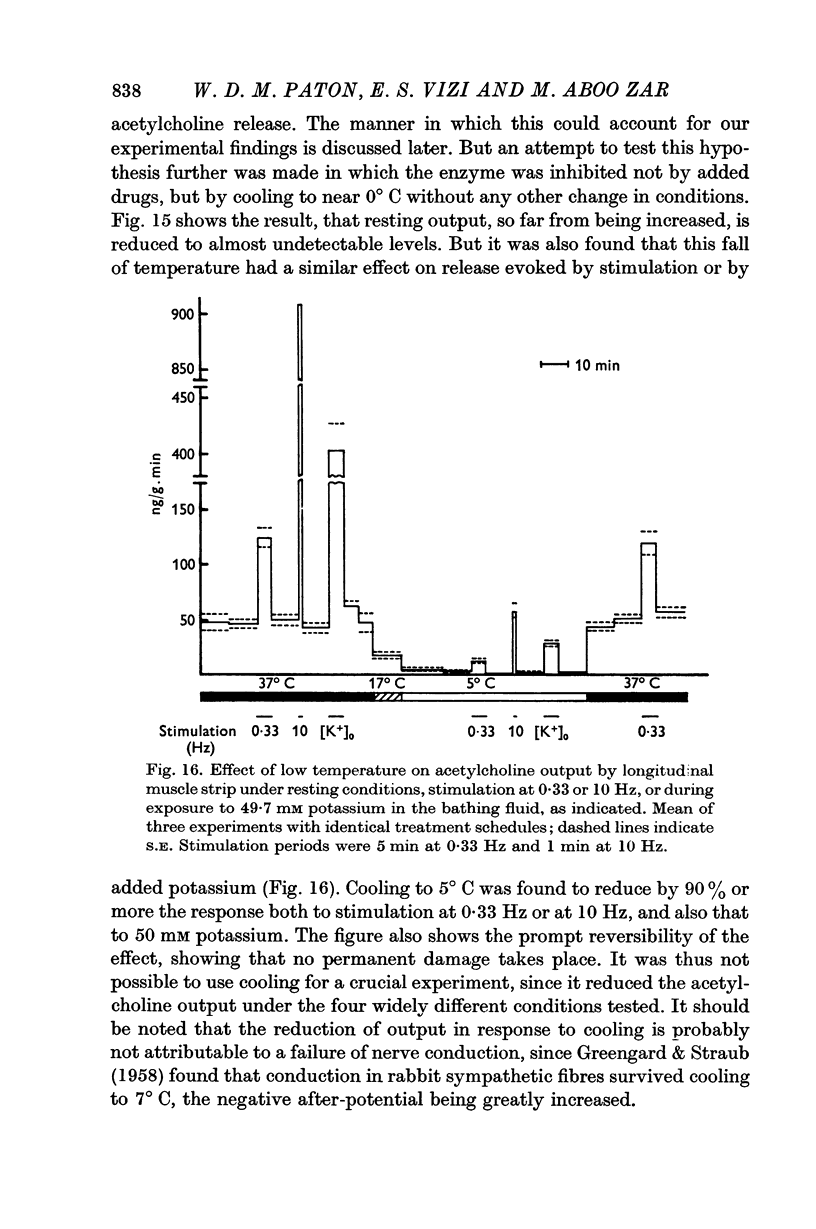
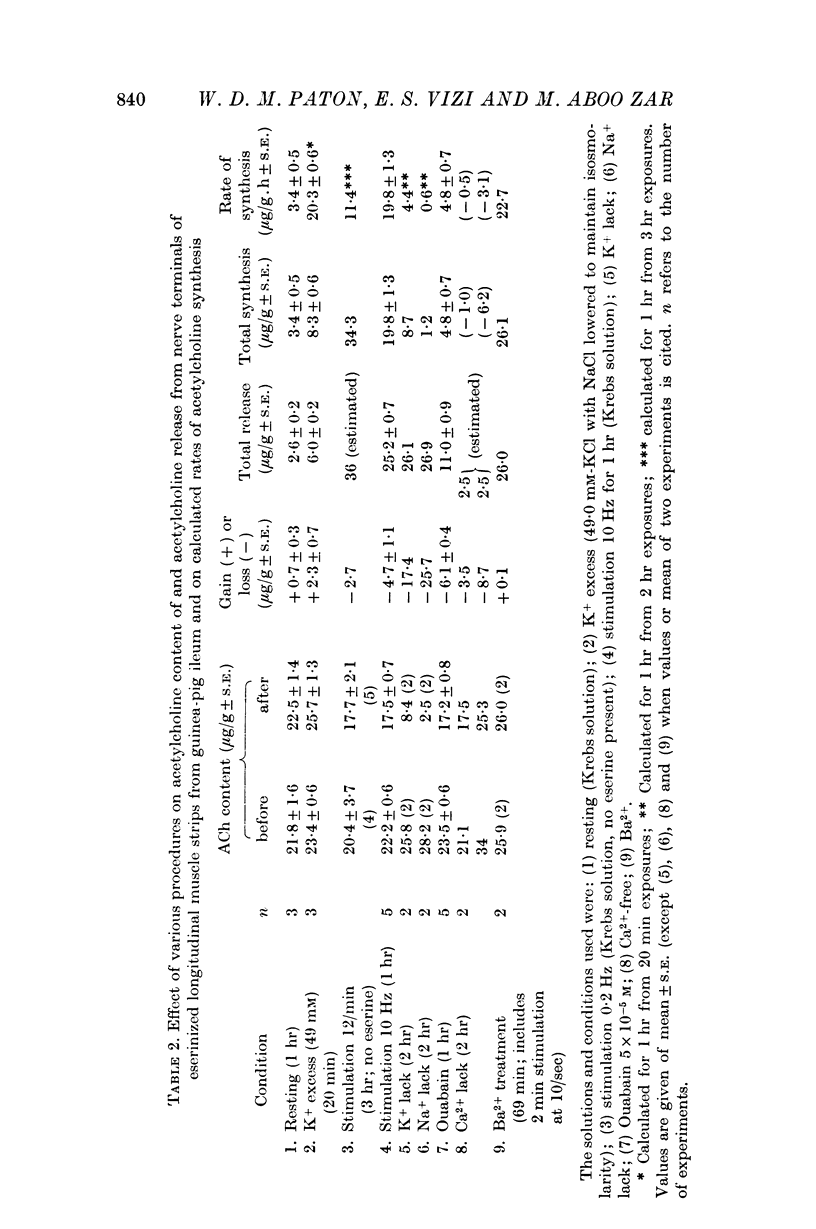
5. Cooling to 5° C greatly reduces both the resting output and the output in response to raised potassium concentration or to electrical stimulation.
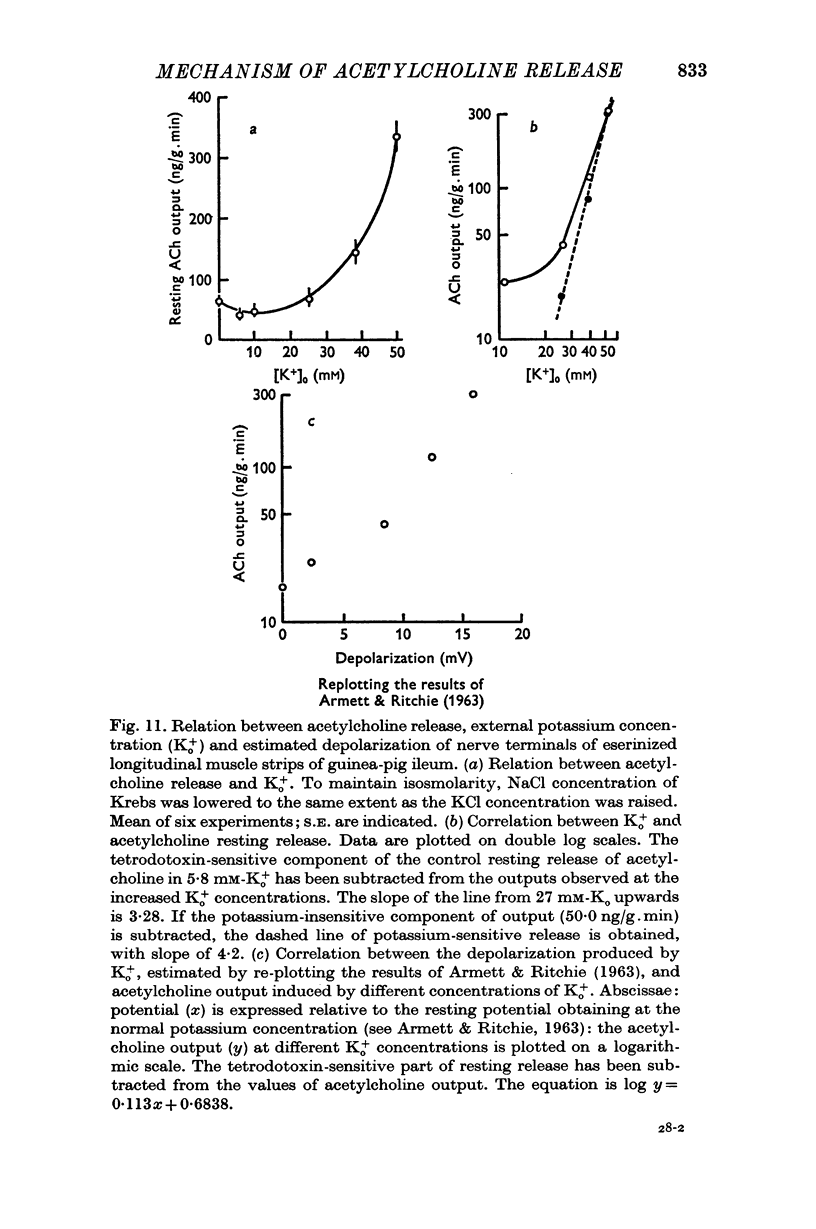
6. The increase in resting output due to potassium excess is slight up to 25 mM [K+]o, but increases thereafter with about the fourth power of the potassium concentration; it is resistant to tetrodotoxin.
7. Synthesis of acetylcholine by the longitudinal strip is increased when output is enhanced by electrical stimulation, by potassium excess or by addition of barium, so that the acetylcholine content of the strip is maintained approximately normal. Synthesis is reduced, in relation to output, by potassium lack or by treatment with ouabain, and is virtually abolished by sodium withdrawal.
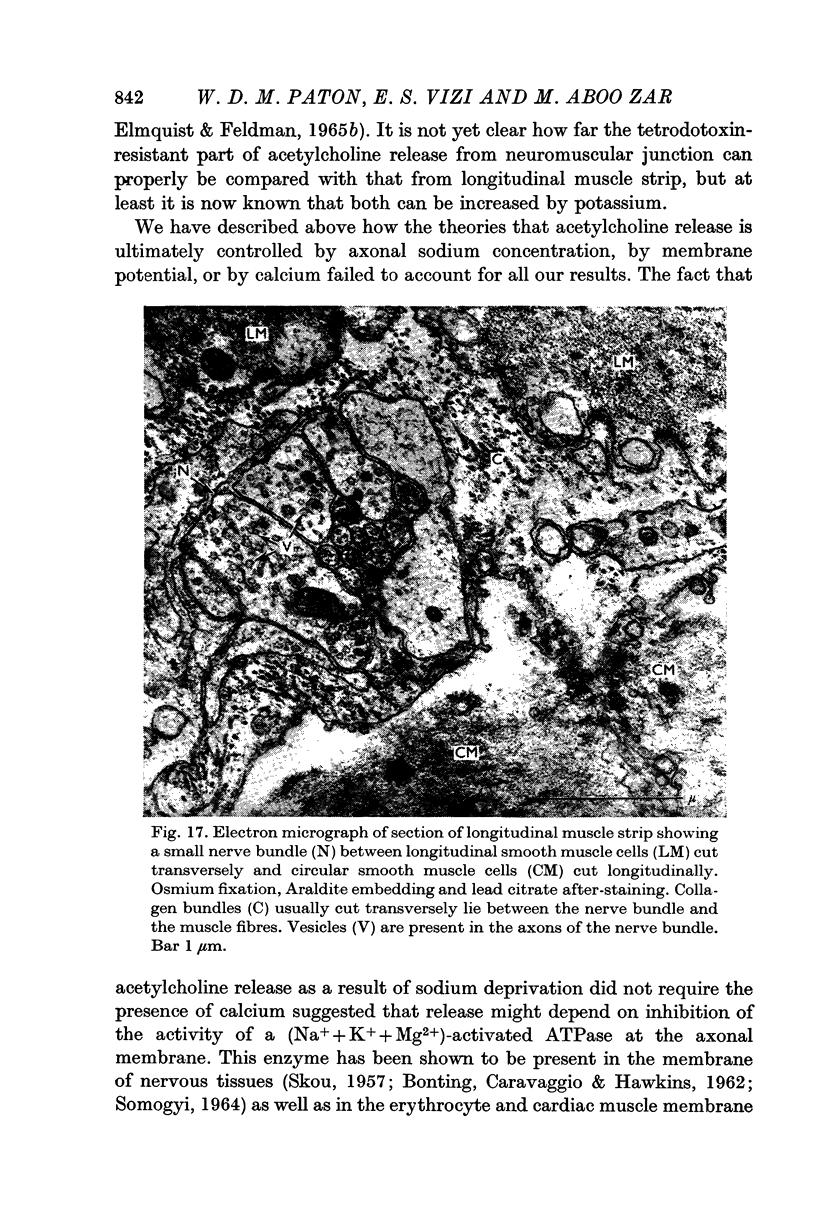
8. The theory is discussed that acetylcholine release depends on inhibition of the activity of a (Na+ + K+ + Mg2+)-activated ATPase at the axonal membrane.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI E., CHINNOCK J. E., DE DALY M. B., MURRAY J. G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957 Jan 23;135(1):182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett C. J., Ritchie J. M. On the permeability of mammalian non-myelinated fibres to sodium and to lithium ions. J Physiol. 1963 Jan;165(1):130–140. doi: 10.1113/jphysiol.1963.sp007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R. I. THE ROLE OF SODIUM IONS IN THE METABOLISM OF ACETYLCHOLINE. Can J Biochem Physiol. 1963 Dec;41:2573–2597. [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., HAWKINS N. M. Studies on sodium-potassium-activated adenosinetriphosphatase. IV. Correlation with cation transport sensitive to cardiac glycosides. Arch Biochem Biophys. 1962 Sep;98:413–419. doi: 10.1016/0003-9861(62)90206-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Feldberg W. The action of potassium on the superior cervical ganglion of the cat. J Physiol. 1936 Mar 9;86(3):290–305. doi: 10.1113/jphysiol.1936.sp003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomo F., Rahamimoff R. Interaction between sodium and calcium ions in the process of transmitter release at the neuromuscular junction. J Physiol. 1968 Sep;198(1):203–218. doi: 10.1113/jphysiol.1968.sp008602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A. L., Kosterlitz H. W., Watt A. J. Mode of action of morphine-like drugs on autonomic neuro-effectors. Nature. 1968 Dec 7;220(5171):1040–1042. doi: 10.1038/2201040a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., LYWOOD D. W., STRAUB R. W. The stimulant effect of barium on the release of acetylcholine from the superior cervical ganglion. J Physiol. 1961 May;156:515–522. doi: 10.1113/jphysiol.1961.sp006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELIN N., MUREN A. Acetylcholine release at parasympathetic synapses. Acta Physiol Scand. 1950 Feb 20;20(1):13–32. doi: 10.1111/j.1748-1716.1950.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of sodium ions on neuromuscular transmission. J Physiol. 1952 Sep;118(1):73–87. doi: 10.1113/jphysiol.1952.sp004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W. Effects of ganglion-blocking substances on the small intestine. J Physiol. 1951 May;113(4):483–505. doi: 10.1113/jphysiol.1951.sp004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. S. THE ORIGIN OF THE ACETYLCHOLINE RELEASED SPONTANEOUSLY FROM THE GUINEA-PIG ISOLATED ILEUM. Br J Pharmacol Chemother. 1963 Dec;21:555–568. doi: 10.1111/j.1476-5381.1963.tb02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Knoll J., Vizi E. S. Presynaptic inhibition of acetylcholine release by endogenous and exogenous noradrenaline at high rate of stimulation. Br J Pharmacol. 1970 Nov;40(3):554P–555P. [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lydon R. J., Watt A. J. The effects of adrenaline, noradrenaline and isoprenaline on inhibitory alpha- and beta-adrenoceptors in the longitudinal muscle of the guinea-pig ileum. Br J Pharmacol. 1970 Jun;39(2):398–413. doi: 10.1111/j.1476-5381.1970.tb12903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACINTOSH F. C. SYNTHESIS AND STORAGE OF ACETYLCHOLINE IN NERVOUS TISSUE. Can J Biochem Physiol. 1963 Dec;41:2555–2571. [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. J. The pharmacological actions of polymethylene bistrimethyl-ammonium salts. Br J Pharmacol Chemother. 1949 Dec;4(4):381–400. doi: 10.1111/j.1476-5381.1949.tb00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., ROTHSCHILD A. M. THE CHANGES IN RESPONSE AND IN IONIC CONTENT OF SMOOTH MUSCLE PRODUCED BY ACETYLCHOLINE ACTON AND BY CALCIUM DEFICIENCY. Br J Pharmacol Chemother. 1965 Apr;24:437–448. doi: 10.1111/j.1476-5381.1965.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY W. L. M. Acetylcholine release in the cat's superior cervical ganglion. J Physiol. 1953 Mar;119(4):439–454. doi: 10.1113/jphysiol.1953.sp004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDI R., UHR M. L. SODIUM, POTASSIUM-REQUIRING ADENOSINETRIPHOSPHATASE ACTIVITY. I. PURIFICATION AND PROPERTIES. Biochim Biophys Acta. 1964 Sep 18;89:520–531. doi: 10.1016/0926-6569(64)90078-1. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Studies on the Na ion and K ion activated ATP hydrolysing enzyme system. The role of SH groups. Biochem Biophys Res Commun. 1963 Jan 18;10:79–84. doi: 10.1016/0006-291x(63)90272-9. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Somogyi J. Ueber die bindung der Ca-ionen an das Na+-K+-aktivierbare Adenosintriphosphatase-System des Gehirns. Experientia. 1964 Jan 15;20(1):28–29. doi: 10.1007/BF02146025. [DOI] [PubMed] [Google Scholar]
- Stahl W. L., Swanson P. D. Uptake of calcium by subcellular fractions isolated from ouabain-treated cerebral tissues. J Neurochem. 1969 Dec;16(12):1553–1563. doi: 10.1111/j.1471-4159.1969.tb10354.x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespi H. H. Active transport and passive fluxes of K, Na, and Li in mammalian non-myelinated nerve fibres. Pflugers Arch. 1969;306(3):262–280. doi: 10.1007/BF00592437. [DOI] [PubMed] [Google Scholar]



