Abstract
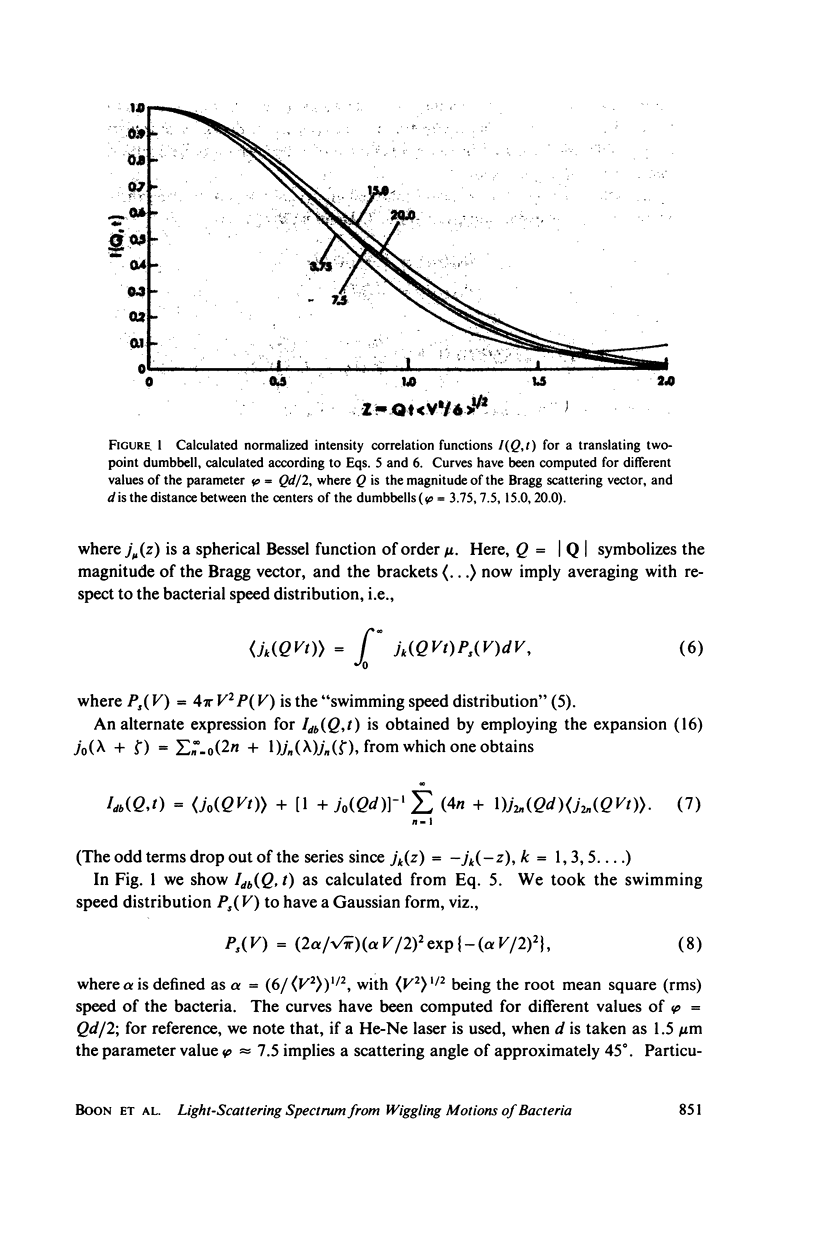
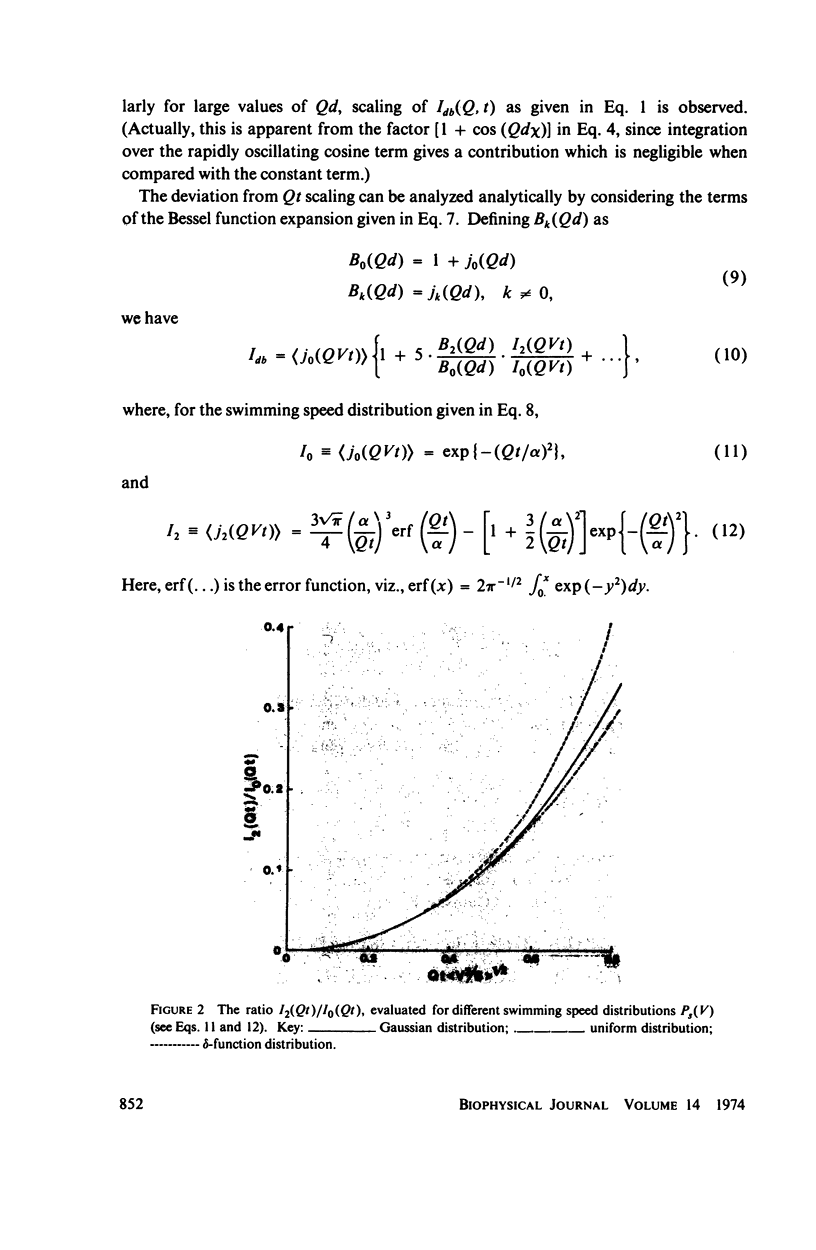
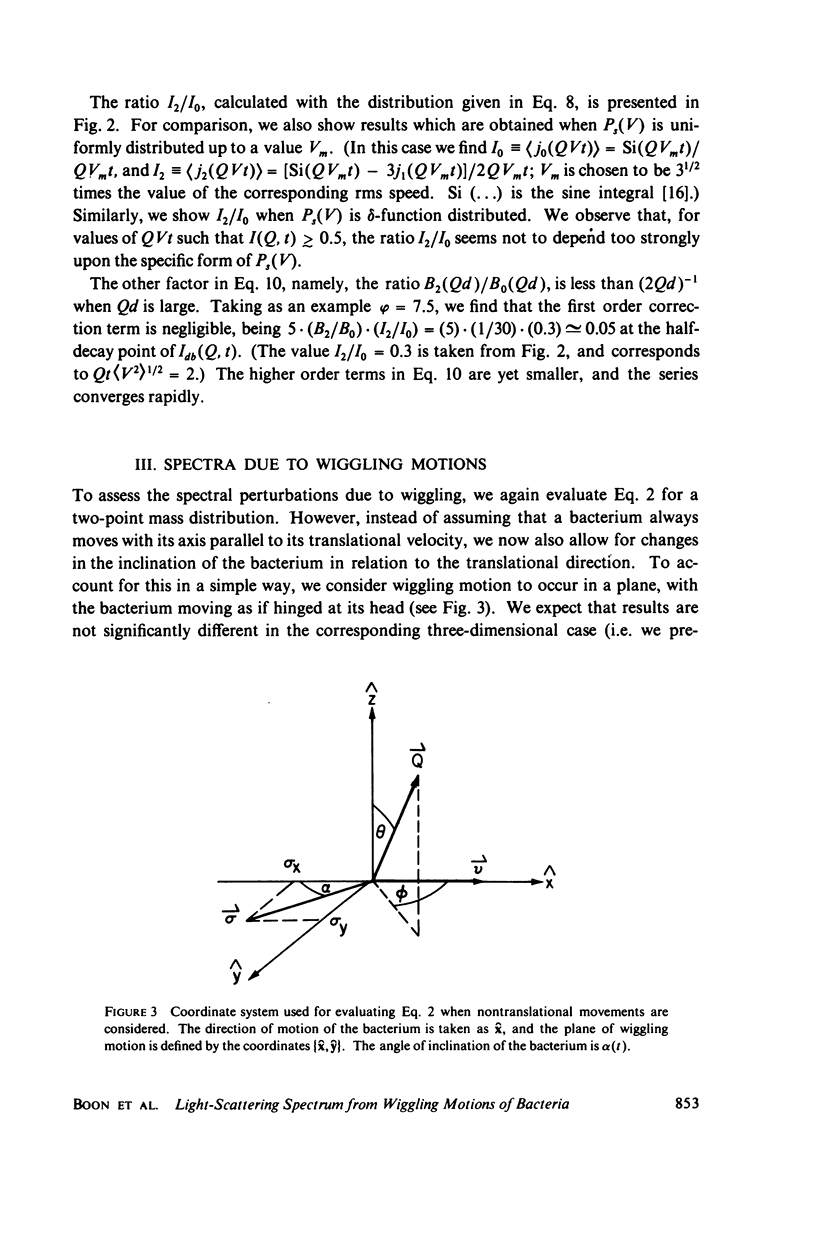
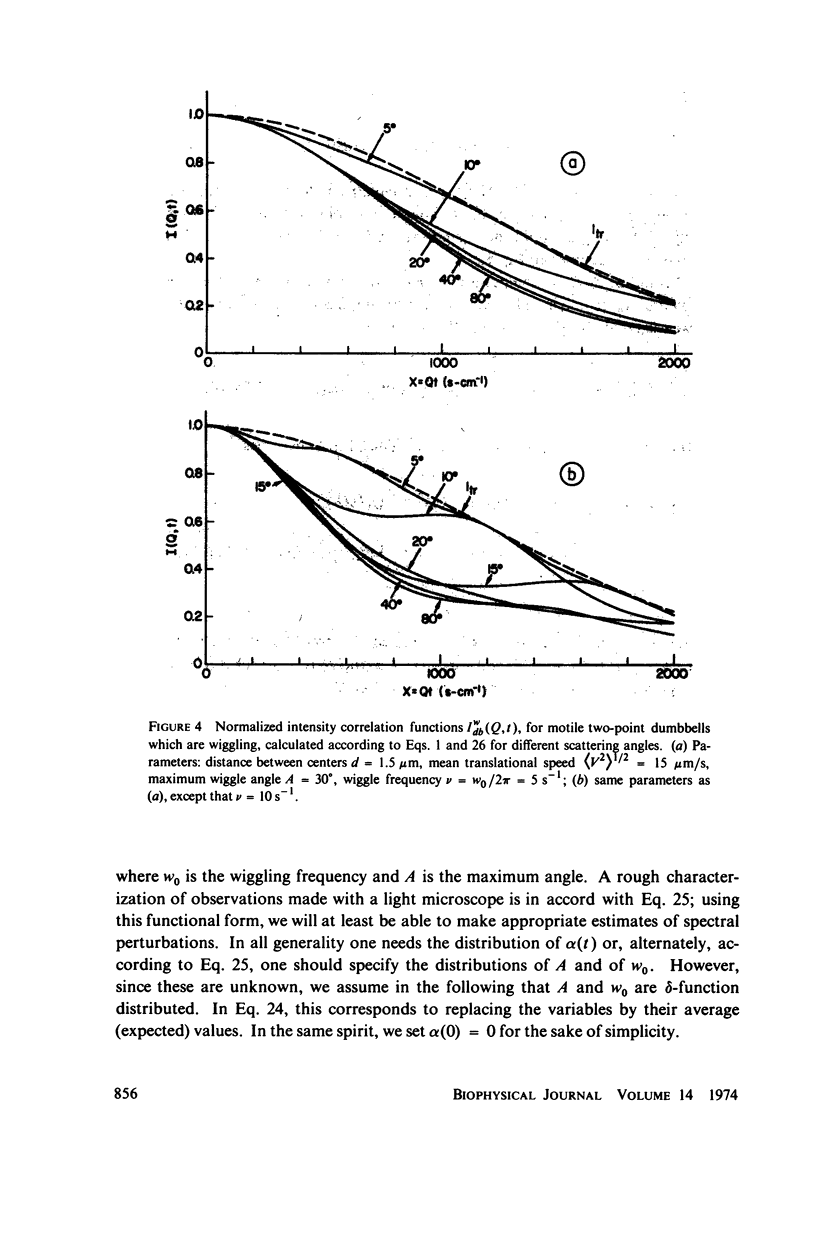
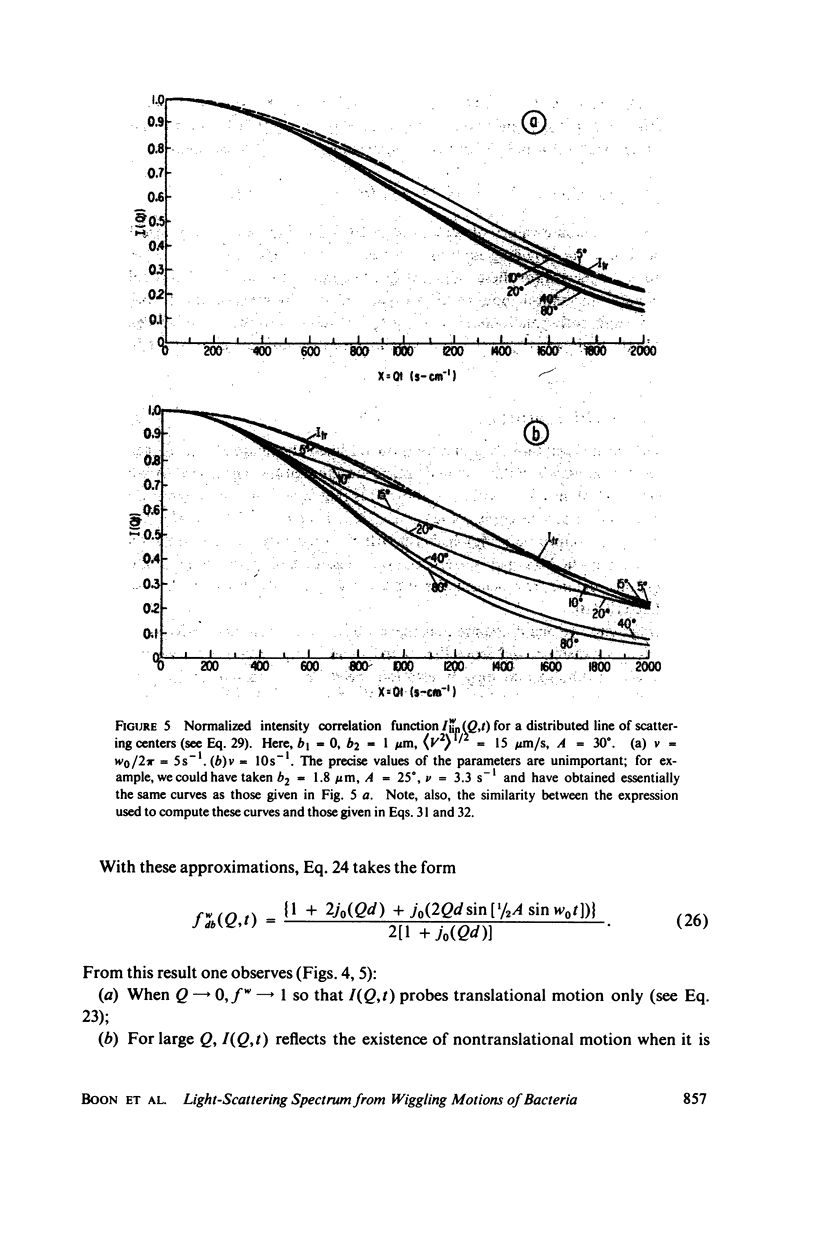
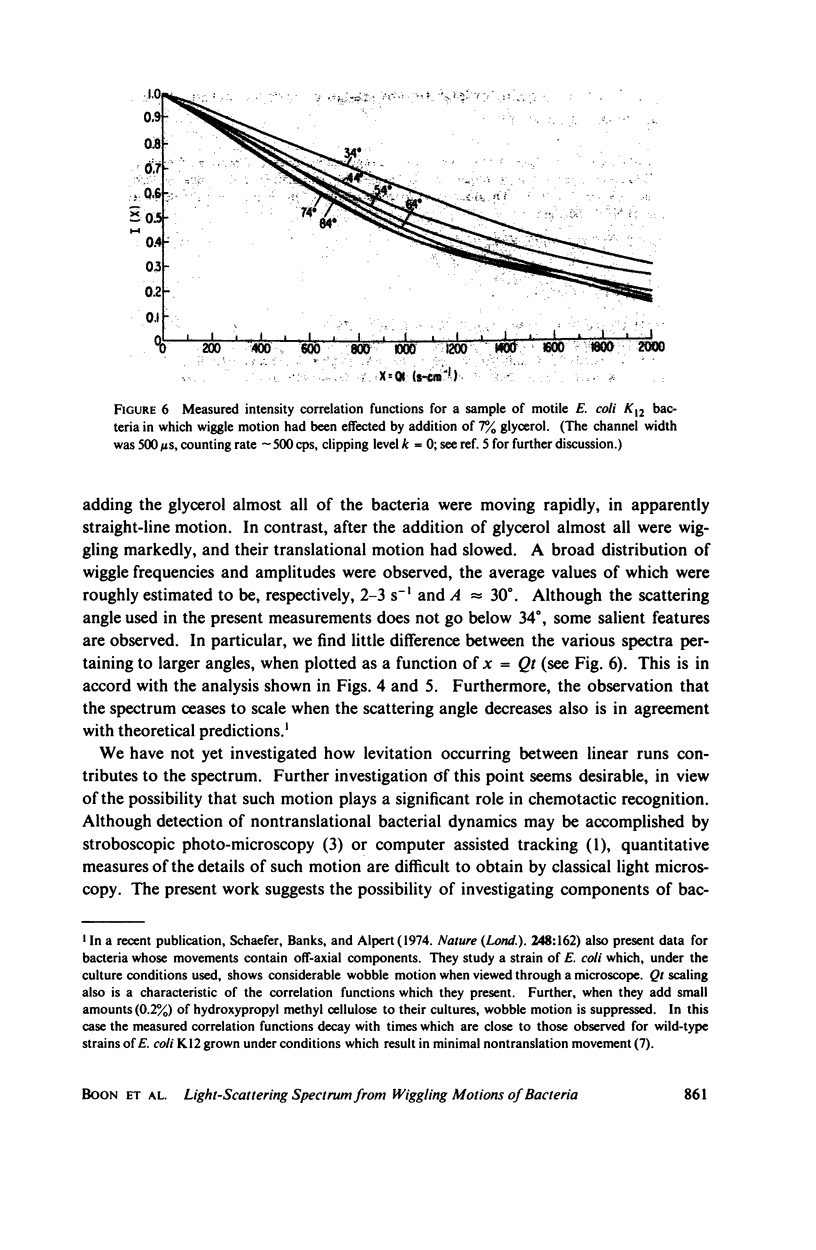
Simple models are used to calculate the inelastic light scattering spectrum of motile bacteria when wiggling motions are included in addition to translational displacement. Computations of spectra lead to the conclusion that nontranslational motions can be neglected when swimming speeds are deduced from light-scattering data for normal vigorously motile strains. On the other hand, for slowly translating bacteria, or for strains exhibiting noticeable wiggling motion when viewed in a microscope, additional spectral components may be significant. Such components are best distinguished when measurements are made at small and intermediate scattering angles; at large angles the spectra have approximately the same scaling properties (functionals of Qt, Q being the Bragg wave vector) as those associated with simple translational motility.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berne B. J., Nossal R. Inelastic light scattering by large structured particles. Biophys J. 1974 Nov;14(11):865–880. doi: 10.1016/S0006-3495(74)85955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Berg H. C. Temporal stimulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1388–1392. doi: 10.1073/pnas.71.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal R., Chen S. H. Effects of chemoattractants on the motility of Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):253–254. doi: 10.1038/newbio244253a0. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPEN H. Demonstration of the chromatinic bodies of Escherichia coli and Proteus vulgaris with the aid of the phase contrast microscope. J Bacteriol. 1950 Jul;60(1):81–87. doi: 10.1128/jb.60.1.81-87.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D. W., Banks G., Alpert S. S. Intensity fluctuation spectroscopy of motile microorganisms. Nature. 1974 Mar 8;248(5444):162–164. doi: 10.1038/248162a0. [DOI] [PubMed] [Google Scholar]
- Schaefer D. W. Dynamics of number fluctuations: motile microorganisms. Science. 1973 Jun 22;180(4092):1293–1295. doi: 10.1126/science.180.4092.1293. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]
- Zusman D. R., Carbonell A., Haga J. Y. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973 Sep;115(3):1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


