Abstract
Many growth factors and hormones modulate the reproductive status in mammals. Among these, insulin and insulin-like growth factor I (IGF-I) regulate the development of gonadal tissues. SH2-B has been shown to interact with insulin and IGF-I receptors, although the role of SH2-B in these signals has not been clarified. To investigate the role of SH2-B, we generated mice with a targeted disruption of the SH2-B gene. Both male and female SH2-B−/− mice showed slight retardation in growth and impaired fertility. Female knockout mice possess small, anovulatory ovaries with reduced numbers of follicles and male SH2-B−/− mice have small testes with a reduced number of sperm. SH2-B−/− cumulus cells do not respond to either follicle-stimulating hormone or IGF-I. These data suggest that SH2-B plays a critical role in the IGF-I-mediated reproductive pathway in mice.
Cytokine and growth factor receptors trigger multiple signaling cascades that regulate cell growth and differentiation. Many growth factor receptors have a protein tyrosine kinase domain in their cytoplasmic region (receptor tyrosine kinase [RTK]). In contrast, cytokine receptors, such as those for interleukins, interferons, and colony-stimulating factors, do not have an intrinsic kinase domain but instead constitutively associate with Janus tyrosine kinases (JAKs). Binding of growth factors and cytokines to their cognate receptors induces the homo- and heterodimerization of the receptors, an event which positions the kinase domains close to each other. This leads to transphosphorylation and thereby activation of RTKs and receptor-associated JAKs. The activated kinases further phosphorylate other tyrosine residues in the cytoplasmic region, where various signaling molecules containing Src homology 2 (SH2) or phosphotyrosine binding domains are recruited. As a consequence, these recruited adaptor molecules contribute to specification and amplification of signaling downstream of the receptors. Lnk family proteins, including Lnk, APS, and SH2-B, are some of these adaptor molecules (39).
SH2-B was originally identified by using a yeast trihybrid system as a protein associated with an immunoreceptor tyrosine-based activation motif in the high-affinity immunoglobulin E (IgE) receptor Fcɛ-RI (29). SH2-B contains a proline-rich domain, a Pleckstrin homology (PH) domain, and an SH2 domain. APS was initially cloned from a B-cell cDNA library using a yeast two-hybrid screening with the c-Kit RTK as bait, and it was shown to associate with a B-cell receptor (44). Lnk was cloned from a rat lymph node cDNA library and was shown to participate in T-cell signaling (20, 38, 39). In Lnk−/− mice, T-cell development was unaffected, but pre-B and immature B cells accumulated in the spleen and in the bone marrow, thereby indicating that the Lnk protein negatively regulates the production of pro-B cells and c-Kit (39, 40).
Recently, SH2-B was reported to mediate signaling through many cytokine and growth factor receptors, including growth hormone (GH), insulin, insulin-like growth factor I (IGF-I), platelet-derived growth factor (PDGF), and nerve growth factor (NGF) receptors (23, 31-33, 36, 37, 47). SH2-B has been shown to mediate mitogenic signals as well as ERK activation through these receptors (44, 45). A variant form of SH2-B, SH2-Bβ, was reported to be a substrate of the tyrosine kinase JAK2 and to potentiate JAK2 kinase activity (34, 35). However, these studies were performed using an in vitro cultured cell system, and the conclusions were obtained from the overexpression of wild-type or domain negative forms. To clarify the physiological role of SH2-B adaptor molecules, we used gene targeting to acquire mice lacking the SH2-B gene. SH2-B−/− mice displayed normal development of lymphoid organs but decreased body weight and developmental defects in gonadal organs similar to the phenotype seen in mice with IGF-I or follicle-stimulating hormone receptor (FSH-R) deficiencies (24, 26). We propose that while SH2-B is dispensable for JAK2 activation, it does play an important role in the IGF-I pathway that up-regulates FSH-R levels in vivo.
MATERIALS AND METHODS
Generation of SH2-B−/− mice.
Genomic clones of the SH2-B locus, including all exons, were isolated from a 129sv mouse strain genomic library (Stratagene). The targeting vector was constructed by replacing the second through the eighth exons of the SH2-B gene with a PGK-NEO cassette, preserving 8.0-kb (left arm) and 3.8-kb (right arm) flanks of homologous sequences (see Fig. 1). The diphtheria toxin A gene was inserted for negative selection. Homologous recombination in murine embryonic stem cells was performed as described previously (19) and was confirmed by Southern blot analysis (probes are shown below in Fig. 1). Chimeric mice were mated with wild-type C57BL/6 mice to generate heterozygous F1 progeny. The F1 progeny were intercrossed to acquire F2 progeny for analysis.
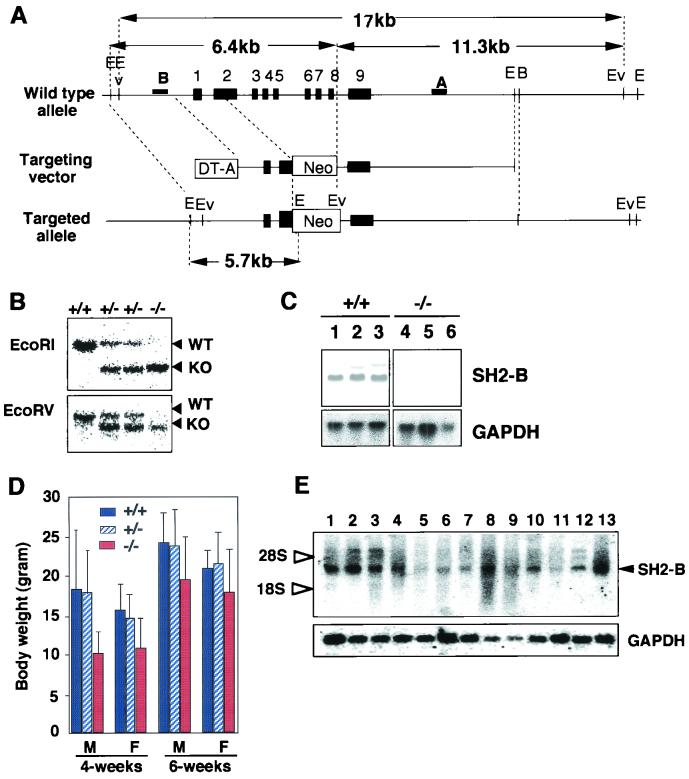
FIG. 1.
Generation of SH2-B-deficient mice. (A) Schematic representations of wild-type and mutant loci of the SH2-B gene together with the targeting vector. Exons for genes encoding SH2-B are represented by black boxes. The neomycin resistance gene (Neo) driven by the phosphoglycerate kinase promoter and the gene coding for diphtheria toxin fragment A (DT-A) driven by an MC1 promoter are indicated by white boxes. The 5′ and 3′ probes used for Southern blotting are indicated by a solid bar. The EcoRI- and EcoRV-digested genomic DNA fragments were detected by probe B and probe A, respectively. Restriction sites: E, EcoRI; Ev, EcoRV; B, BamHI. (B) Representative Southern blot analysis with EcoRV-digested and EcoRI-digested DNA. Of 138 offspring from crosses between F2 heterozygous mice, 35 were +/+, 70 were +/−, and 33 were −/−. (C) Northern blot analysis of total RNA from wild-type (lanes 1 to 3) and SH2-B−/− (lanes 4 to 6) mice showed no SH2-B mRNA expression in SH2-B−/− mice. Lanes 1 and 4, cerebrum; lanes 2 and 5, cerebellum; lanes 3 and 6, testis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels are shown as an internal control. (D) Growth of SH2-B KO mice. The body weights of 4- and 6-week-old male (M) and female (F) mice were measured. The number of analyzed mice are as follows: 4-week-old male mice (+/+, 11; +/−, 15; −/−, 12); 4-week-old female mice (+/+, 7; +/−, 15; −/−, 9); 6-week-old male mice (+/+, 13; +/−, 15; −/−, 9); and 6-week-old female mice (+/+, 8; +/−, 14; −/−, 8). Results are shown as mean ± standard error of the mean. (E) Tissue distribution of SH2-B mRNA. Ten micrograms of total RNA was loaded in each lane. Lanes: 1, cerebrum; 2, cerebellum; 3, thymus; 4, skeletal muscle; 5, stomach; 6, small intestine; 7, colon; 8, lymph node; 9, spleen; 10, kidney; 11, liver; 12, ovary; 13, testis.
Flow cytometric analysis.
Single-cell suspensions of lymphocytes from bone marrow, thymus, spleen, or lymph nodes were prepared. Red blood cells were lysed and removed using 0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA (pH 7.2) prior to staining. Predetermined optimal concentrations of the respective antibodies were used to stain 0.5 × 106 to 1.0 × 106 cells at 4°C for 20 min. Cells were washed and analyzed on a FACScan instrument (Becton Dickinson, Mountain View, Calif.) using CellQuest software (Becton Dickinson). The following monoclonal antibodies (MAbs) were used: fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (53-6.7), phycoerythrin (PE)-conjugated anti-CD4 (RM4-5), biotin-conjugated anti-CD3ɛ (145-2C11), PE- or biotin-conjugated anti-CD43 (S7), PE-conjugated anti-Gr-1 (RB6-8C5), and FITC-conjugated anti-Mac-1 (M1/70) (all purchased from Pharmingen, San Diego, Calif.); FITC- or PE-conjugated anti-B220 (RA3-6B2) (Caltag Laboratories, Burlingame, Calif.); biotin-conjugated anti-IgD (CS/15; gift from K. Miyake, Tokyo, Japan). FITC- or PE-conjugated F(ab′)2 fragments of polyclonal anti-IgM were purchased from Caltag Laboratories, and PE- or TRI-COLOR-conjugated streptavidin (Caltag Laboratories) was used to reveal biotin-coupled antibody staining.
Lymphocyte proliferation and cytokine production.
Splenic B cells were purified using a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) after incubation with biotin-conjugated anti-CD43 and streptavidin-coupled microbeads (Miltenyi Biotec). B cells (105) were cultivated in 0.2 ml of RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a 96-well plate. Cells were stimulated with anti-mouse IgM F(ab′)2 (Organon Teknika, Durham, N.C.), interleukin-4 (IL-4), 10 μg of lipopolysaccharide (Difco Laboratories, Detroit, Mich.) per ml, or 3 μg of anti-CD40 (HM40-3; Pharmingen) per ml. Cells were pulse-labeled with [3H]thymidine (0.2 μCi per well) during the last 16 h of a 66-h culture period, and the incorporated [3H]thymidine was measured using a Matrix 96 Direct Beta Counter (Packard, Meriden, Conn.).
For T-cell proliferation assays, splenocytes (106) were cultured in 0.2 ml of medium in a 96-well plate in the presence of various concentrations of anti-CD3ɛ (145-2C11). Cells were pulse-labeled with [3H]thymidine (0.2 μCi per well) during the last 8 h of a 48-h culture period, and the incorporated [3H]thymidine was measured.
For the assessment of cytokine production, splenocytes (106) were cultured in 0.2 ml of medium in the presence of 10 μg of anti-CD3ɛ/ml for 48 h. Culture supernatants were collected, and the levels of IL-2 and IL-4 in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (41). All of the MAbs used for the capture and detection of cytokines were purchased from Pharmingen.
Serology.
Concentrations of each immunoglobulin isotype in serum were determined in 6-week-old mice by isotype-specific ELISA (41). To measure production of the antibodies against thymus-independent antigens, mice were intraperitoneally injected with 10 μg of trinitrophenol (TNP)-Ficoll in saline and were bled on day 10 after injection. Serial dilutions of serum were analyzed for TNP-specific immunoglobulin isotypes by ELISA using dinitrophenol (DNP)-coupled bovine serum albumin (BSA), which cross-reacts with anti-TNP antibodies) as a capture reagent. To examine the response against thymus-dependent antigens, mice were immunized intraperitoneally with 100 μg of BSA in a 1:1 homogenate of incomplete Freund's adjuvant (Nacalai Tesque, Kyoto, Japan) and saline. A booster dose of 100 μg of BSA in saline was given on day 20. Mice were bled on day 30 and the presence of anti-BSA antibodies in each immunoglobulin subclass was determined using a BSA-specific ELISA.
Mast cell cultures and functional assays.
Bone marrow-derived mast cells (BMMCs) were obtained from a culture of bone marrow cells in RPMI 1640 supplemented with 5 ng of murine IL-3/ml (Pepro Tech EC), 8% FCS, nonessential amino acids (Gibco BRL), 100 IU of penicillin/ml, 100 μg of streptomycin/ml, and 10 μM 2-mercaptoethanol. We used BMMCs cultured for 4 to 10 weeks for all studies. BMMCs were cultured in 96-well plates (5 × 104 cells; 0.2 ml/well) for 48 h in RPMI 1640 supplemented with 8% FCS and various concentrations of stem cell factor (SCF) or IL-3 as indicated. [3H]thymidine (0.2 μCi per well) was added 12 h before cells were harvested, and the incorporated [3H]thymidine was measured. BMMCs were sensitized with anti-DNP IgE by incubation in cultured supernatants of Igel α2(15.3) hybridoma cells at 37°C for 1 h, after which they were washed, resuspended in Tyrode's buffer (10 mM HEPES [pH 7.4], 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA), and left unstimulated or stimulated with DNP-BSA for 1 h at 37°C. The degranulation was evaluated by measuring the amount of granular enzyme β-hexosaminidase released from cells. The enzymatic activities of β-hexosaminidase in supernatants and cell pellets solubilized with 0.5% Triton X-100 and Tyrode's buffer were measured with p-nitrophenyl N-acetyl-β-d-glucosaminide (Sigma Chemical Co.) in 0.1 M sodium citrate (pH 4.5) for 90 min at 37°C. The reaction was stopped by adding 0.2 M glycine (pH 10.7). The product 4-p-nitrophenol was detected by absorbance at 405 nm. Percentages of degranulation were calculated by dividing absorbance in the supernatants by total absorbance in the supernatants and cell pellets.
Sperm assay.
For sperm analysis, we collected sperm from the epididymides of SH2-B-null and wild-type mice as described previously (19). The sperm was incubated in R18S3 medium (180 mg of Raffinose/ml and 30 mg of skim milk/ml) for 1 h in a 5% CO2 atmosphere equilibrated with 5% CO2. The sperm were diluted 1:100 with isotonic saline, and a phase-contrast microscope was used to count the sperm and to evaluate the morphology. We followed the procedure of the World Health Organization laboratory manual for the examination of human semen and semen-cervical mucus interaction to measure sperm motility (43a).
IVF.
For in vitro fertilization (IVF), we collected sperm from the epididymides of SH2-B-null and wild-type males as described previously (19). Then, we incubated the sperm in HTF medium supplemented with 0.75 μg of penicillin G/ml and 0.5 μg of streptomycin/ml in a 5% CO2 atmosphere, equilibrated with 5% CO2, for at least 1 h for capacitation. Females were treated to induce superovulation, and oocytes were collected from the ampullae of their oviducts. Only fully matured oocytes, as confirmed by the first polar body release, were used for IVF experiments. We excluded oocytes with no first polar body. Oocytes were treated with 0.1% hyaluronidase to remove surrounding granulosa and cumulus cells. Thereafter, 10 μl of the sperm suspension solution was transferred into the oocytes in saline followed by incubation for 24 h under 5% CO2 at 37°C. After 24 h, these inseminated eggs were beginning cleavage, and the number of two-cell-stage eggs was counted. These two-cell-stage embryos were transplanted into ICR mice (purchased from Japan SLC Inc.) as described previously (19).
COC expansion assay.
Briefly, 48 h after administration of pregnant mare serum gonadotropin (PMSG), cumulus cell-oocyte complexes (COCs) were collected from the oviduct ampullae of wild-type and SH2-B−/− mice sacrificed by cervical dislocation. The large Graafian follicles were punctured with 27-gauge needles, and the COCs were extruded into Waymouth's medium MB752/1 (Gibco BRL). Because exposure to IGF-I and FSH leads to drastic changes in cumulus cells due to the hyaluronidase hypersensitivity of the cumulus oophori, we cultured COCs in Waymouth's medium MB752/1 containing 3 mg of BSA (Gibco BRL) per ml rather than FCS (control), in order to reduce hyaluronic acid synthesis and to avoid this effect of IGF-1 in the serum (13). Cultures were maintained in a humidified atmosphere of 5% O2-5% CO2-90% N2 at 37°C for 24 h. Other COCs were cultured in Waymouth's medium containing 3 mg of BSA/ml and 1 μg of highly purified rat FSH (Biogenesis Ltd.) per ml, or 3 mg of BSA/ml plus 10 ng of IGF-I (Pepro Tech EC, Ltd) per ml, into which 1 μg of highly purified mouse FSH/ml was added after 4 h. For immunoblotting with anti-phosphotyrosine and anti-phosphorylated mitogen-activated protein (MAP) kinase (ERK2) antibodies, COCs were incubated with 100 ng of IGF-1/ml for 20 min and then cell extracts were prepared.
Northern hybridization and RT-PCR.
Total RNA was extracted from tissues and cells using Trizol reagent (Gibco BRL). Total RNA (10 μg) was separated on 1.0% agarose gels containing 2.4% formaldehyde and then transferred to positively charged nylon membranes. After fixation under calibrated UV irradiation, the membranes were hybridized with [32P]CTP-labeled cDNA probes as described previously (44). SH2-B probe (334 bp) was obtained by PCR from a mouse testis cDNA library and primer sets (5′-GAGGAAGTCGCTTGGAGTTCTTTGTAC-3′ and 5′-TCCTGGCTAGGCAGACTCTCTGAATGA-3′). FSH-R cDNA (about 2 kb) was obtained by PCR using ovary cDNA and primer sets (5′-ATGGCCTGGCTCCTGGTCTCCTTGCT-3′ and 5′-GAGGGACAAGCACGTAACTATTGGTGACT). To detect SH2-B, APS, and Lnk mRNA in mast cells and splenocytes, standard reverse transcription-PCR (RT-PCR) was performed using a Standard GeneAmp RNA PCR kit (PE Biosystems) according to the manufacturer's instructions. The following primer sets were used: SH2-B (about 150 bp), 5′-TCTACTATTACTGATGTCCGCACAGCC-3′ and 5′-TGTACTCTGAAGGGCCTTCTACCTTAA-3′; Lnk (930 bp), 5′-ATGCCTGACAACCTCTACAC-3′ and 5′-ATTCACACGTCTGCCTCTCT-3′; APS (530 bp), 5′-GAAAGGGATTCTGGCTGCGTAACA and 5′-ATCCACACAGCCCTGGATGTCAGC. PCR primers for SH2-B splice variants have been described by Yousaf et al. (46).
Immunoblotting and immunostaining.
For immunoblotting, total cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were detected by immunoblotting as described previously (44). Anti-phospho-STAT5 and phospho-ERK2-specific antibodies were purchased from Cell Signaling and Upstate Biotechnology.
An anti-SH2-B antibody (G-17) which reacts with the N-terminal region of the SH2-B protein was purchased from Santa Cruz Biotechnology, Inc., and an anti-murine APS antibody was affinity purified as described previously (21). For immunofluorescence microscopy, tissues were fixed overnight in 4% paraformaldehyde and embedded in optimal cutting temperature compound. Tissues were cryostat sectioned to a 10-μm thickness and incubated with a 1:100 dilution of anti-SH2-B antibody, followed by staining with a FITC-conjugated anti-goat IgG antibody. Then, the samples were examined with fluorescent microscopy. For immunohistochemistry, tissues were fixed with 10% buffered formalin, dehydrated through a graded series of alcohol, cleared in xylene, and embedded in paraffin. Paraffin blocks were sectioned at 3-μm thickness. Slides were incubated with a 1:100 dilution of anti-APS antibody and stained with a peroxidase-conjugated anti-rabbit IgG using an Envision+ kit (Dako, Carpinteria, Calif.). The samples were then stained with Mayer's hematoxylin.
RESULTS
Generation of SH2-B knockout (KO) mice.
To clarify the physiological role of SH2-B, we developed mice with a targeted disruption in the SH2-B gene locus. Since the PH and SH2 domains are predicted to be essential for SH2-B to function as an adaptor protein, the exons encoding these domains (second to eighth exons) were deleted (Fig. 1A). For genotyping of the F2 offspring generated by intercrossing the F1 heterozygotes, Southern blot analysis was carried out. This revealed a ratio of offspring within the Mendelian expectation for transmission (1 (+/+):2 (+/−):1 (−/−) (Fig. 1B). The disruption of SH2-B gene expression was confirmed by Northern blot analysis (Fig. 1C). SH2-B homozygotes exhibited no significantly abnormal appearance at birth.
At age 2 to 6 weeks, the body weight of the SH2-B−/− mice was 60 to 80% that of wild-type littermates (Fig. 1D). In this period, SH2-B-null mice showed postnatal growth retardation and proportionate dwarfism. However, the size and weight of SH2B−/− mice became similar to that of wild-type mice after 6 to 10 weeks. These phenotypes may be related to a reduced response to GH or IGF-I. Occasionally, some of the SH2-B-null mice died shortly after birth (8 out of 78 SH2-B−/− pups), although the cause of death was not clear.
Normal development and function of T, B, and mast cells in SH2-B KO mice.
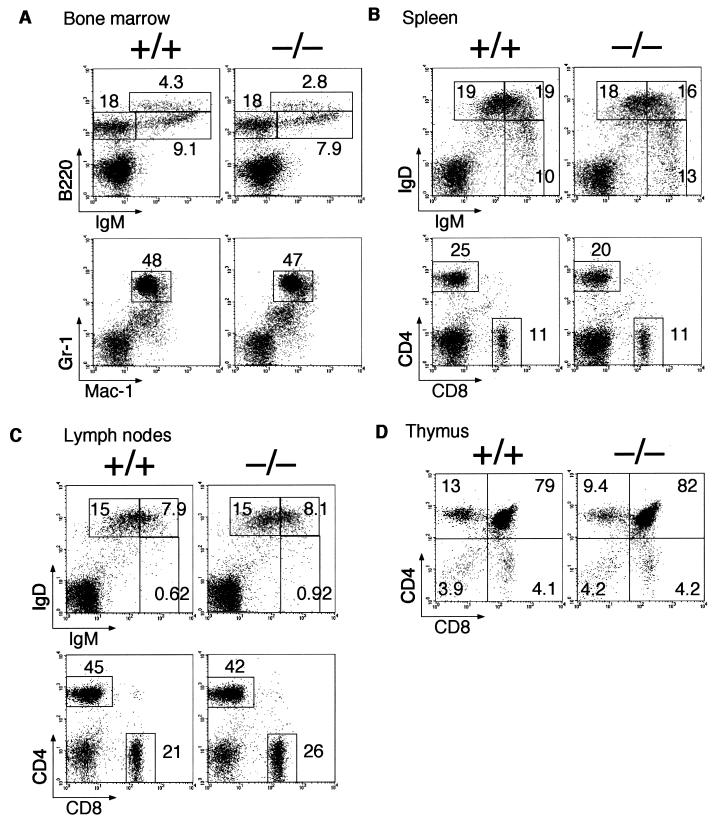
We initially investigated SH2-B mRNA distribution in wild-type mice by using Northern blot analysis (Fig. 1E). SH2-B mRNA is widely expressed in most organs, but the strongest expression was observed in the thymus (lane 3), lymph node (lane 8), and spleen (lane 9), in addition to the brain (lanes 1 and 2), ovary (lane 12), and testis (lane 13). SH2-B has been shown to be an activator of JAK2 (34), which is essential for cytokine signal transduction and hematopoiesis (30). We therefore analyzed the development and function of lymphocytes and myeloid cells in SH2B−/− mice. The cellularity in the bone marrow, thymus, spleen, and lymph nodes was grossly normal in SH2-B−/− mice (data not shown), and B- and T-cell development was also normal as assessed by flow cytometry (Fig. 2). The cellular distribution in each lymphoid organ was comparable to that of normal mice, and expression levels of various surface proteins on lymphocytes or myeloid cells were also comparable.
FIG. 2.
Normal lymphocyte development in SH2-B−/− mice. Representative two-color fluorescence plots showing expression of B220 and IgM (measuring B-cell development) or Gr-1and Mac-1 (granulocytes and macrophages) on bone marrow cells (A), IgD and IgM (B-cell development), or CD4 and CD8 (T-cell development) on splenocytes and lymph node cells (B, C), and CD4 and CD8 on thymocytes (D). Percentages represent the fractions of the total gated live cells that fall into the indicated boxes. Cellular distribution in each lymphoid organ and expression levels of various surface proteins on lymphocytes or myeloid cells were comparable between SH2-B−/− mice and normal littermates.
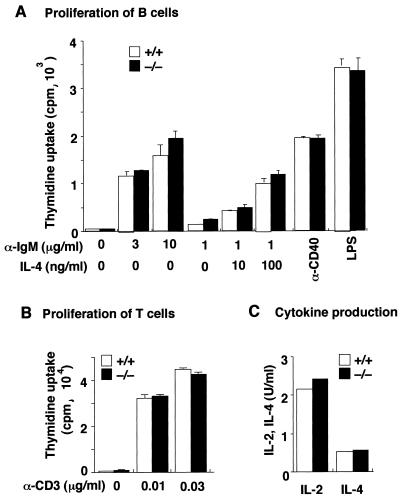
We then examined SH2-B−/− lymphocyte functions in vitro and in vivo. The proliferative responses of B cells induced by various stimuli were normal (Fig. 3A). SH2-B−/− T cells proliferated normally upon stimulation with anti-CD3 and produced normal levels of IL-2 and IL-4 (Fig. 3B and C). SH2-B−/− mice had normal antibody responses against immunization with both a thymus-independent antigen, TNP-Ficoll, and a thymus-dependent antigen, BSA (Table 1). Thus, SH2-B seems dispensable for lymphocyte development and steady-state hematopoiesis and for functions of lymphocytes and antigen-presenting cells.
FIG. 3.
Normal proliferative response and cytokine production by SH2-B−/− lymphocytes. (A) Proliferation of splenic B cells induced by anti-IgM, anti-CD40, or lipopolysaccharide (LPS). Splenic B cells from SH2-B−/− mice or wild-type littermates were stimulated with indicated stimuli, and proliferation was measured on day 3 by [3H]thymidine incorporation. The values are the mean counts per minute × 10−3 (± standard deviation [SD]) of triplicate determinations. Representative results of three independent experiments are shown. (B) Splenocytes from SH2-B−/− mice or wild-type littermates were stimulated with the indicated concentrations of anti-CD3ɛ, and proliferation was measured on day 2. The values are the mean counts per minute × 10−3 (± SD) of triplicate determinations. Representative results of two independent experiments are shown. (C) Splenocytes from SH2-B−/− mice or wild-type littermates were stimulated with 10 μg of anti-CD3ɛ/ml and cultured for 48 h. Supernatants were collected, and IL-2 or IL-4 in the supernatants was measured by ELISA. The results are presented as the average of duplicate assays.
TABLE 1.
Antibody production in SH2-B−/− mice
| Expt and genotype (n) | Antibody response
|
|||||
|---|---|---|---|---|---|---|
| IgM | IgG3 | IgG1 | IgG2a | IgG2b | IgA | |
| Serum immunoglobulina | ||||||
| +/+ (7) | 140 ± 36 | 130 ± 50 | 480 ± 130 | 620 ± 380 | 360 ± 110 | 120 ± 20 |
| −/− (7) | 150 ± 39 | 86 ± 18 | 280 ± 64 | 210 ± 61 | 260 ± 80 | 69 ± 6.0 |
| Thymus-independent antigenbd | ||||||
| +/+ (7) | 14 ± 3.6 | |||||
| −/− (5) | 13 ± 3.3 | |||||
| Thymus-dependent antigencd | ||||||
| +/+ (5) | 12 ± 2.9 | 2.8 ± 0.49 | 17 ± 7.0 | 4.9 ± 0.96 | 10 ± 4.9 | 2.0 ± 0.51 |
| −/− (4) | 8.4 ± 2.3 | 3.1 ± 1.8 | 17 ± 6.3 | 9.2 ± 4.1 | 16 ± 11 | 2.2 ± 0.81 |
Concentrations (mg/ml) of immunoglobulin subclasses in serum were determined by isotype-specific ELISA.
Antibody response to thymus-independent antigen, TNP-Ficoll. Mice were injected with TNP-Ficoll and the amounts of hapten-specific IgM antibodies were measured by ELISA.
Response to thymus-dependent antigen, BSA. Mice were immunized with BSA and BSA-specific immunoglobulin subclasses were measured. Data shown are the mean ± standard error of the mean for the indicated groups of mice.
Results are expressed as relative titer (10−2).
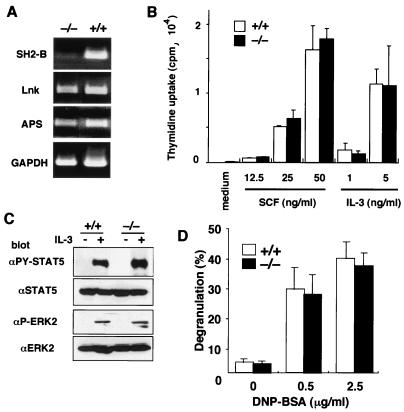
APS and Lnk, members of the adaptor protein family to which SH2-B belongs, have been implicated in functioning in the c-Kit signaling pathway (39, 40, 44). SH2-B was originally reported to be a possible adaptor protein that binds to the immunoreceptor tyrosine-based activation motif of Fcɛ-RI in mast cells (29). We therefore asked if loss of SH2-B would affect the mast cell function largely regulated by c-Kit and Fcɛ-RI signaling. IL-3-dependent BMMCs were established from SH2-B−/− mice and their normal littermates. As shown in Fig. 4A, SH2-B was expressed in wild-type BMMCs, and Lnk and APS was expressed at comparable levels in both wild-type and SH2-B-null BMMCs. BMMCs of both origins expressed c-Kit in comparable amounts (data not shown), and they proliferated equally in response to IL-3 or SCF (Fig. 4B). Activation (phosphorylation) of STAT5 (a substrate of JAK2), and ERK2 MAP kinase (downstream of JAK2) was similarly induced in response to IL-3 in wild-type and SH2-B−/− mast cells (Fig. 4C). Expression levels of high-affinity FcɛR assessed by the binding of IgE MAb were also comparable between SH2-B−/− and normal BMMCs (data not shown), and degranulation was similarly induced by cross-linking of Fcɛ-RI in BMMCs from both sources (Fig. 4D). These results indicate that SH2-B deficiency does not compromise mast cell function.
FIG. 4.
Normal mast cell function in the absence of SH2-B. (A) RT-PCR analysis for the expression of SH2-B, Lnk, APS, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA from 106 BMMCs was used as a template. (B) Proliferation of mast cells induced by IL-3 or SCF. BMMCs obtained from SH2-B−/− mice or wild-type littermates were stimulated with the indicated concentrations of IL-3 or SCF, and proliferation was measured on day 3 by [3H]thymidine incorporation. The values are the mean counts per minute × 10−3 (± standard deviation) of triplicate determinations. Representative results of two independent experiments are shown. (C) STAT5 and ERK2 activation in response to IL-3. BMMCs (106) were stimulated with 5 ng of IL-3/ml for 20 min, and then cell extracts were immunoblotted with the indicated antibodies. (D) Degranulation of mast cells induced by Fcɛ-RI cross-linking. BMMCs were sensitized with anti-DNP IgE and then stimulated with the indicated amount of DNP-BSA. The release of β-hexosaminidase was measured as described in Materials and Methods. Percentages of degranulation were calculated by dividing the released β-hexosaminidase by the total β-hexosaminidase stored in cells. Representative results of three independent experiments are shown.
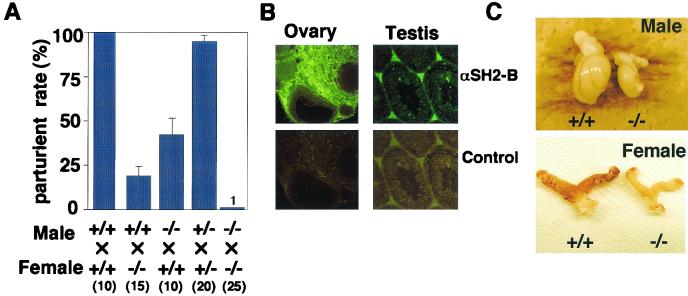
Impaired fertility in SH2-B KO mice.
Although offspring were born normally from intercrosses of heterozygotes, few offspring were born following incrosses of homozygotes (Fig. 5A). Only 1 of 25 breeding pairs of SH2-B−/− male and SH2-B−/− female mice produced offspring. Both SH2-B−/− male and female mice also showed reduced fertility when mated with wild-type mice (Fig. 5A). We monitored SH2-B−/− incrossed females daily for the presence of vaginal plugs, an indication that copulation has occurred. It took a much longer time after mating for vaginal plugs to be observed in the SH2-B−/− mice than it did following wild-type matings (5.3 ± 1.3 days for wild-type female mice [n = 13], and 31.8 ± 5.8 days or no plugs for female SH2-B−/− mice [n = 22]).
FIG. 5.
Reduced fertility and hypoplasia of gonadal tissues in SH2-B−/− mice. (A) Parturition rate of wild-type, SH2-B+/−, and SH2-B−/− females and males was examined by caging one male with one or two females with various SH2-B genotypes. The average parturition rate (± standard error of the mean) is shown. The numbers in parentheses indicate the numbers of mating pairs. (B) Localization of SH2-B in wild-type testis and ovary. Immunofluorescence staining was carried out on 5-μm sections with anti-mouse SH2-B polyclonal antibody. Magnification, ×40. (C) Hypoplasia of SH2-B−/− female and male gonads in contrast to those from wild-type mice. Testes were from the wild-type and SH2-B−/− littermates at 73 days after birth. Ovaries were from wild-type and SH2-B−/− littermates at 83 days after birth.
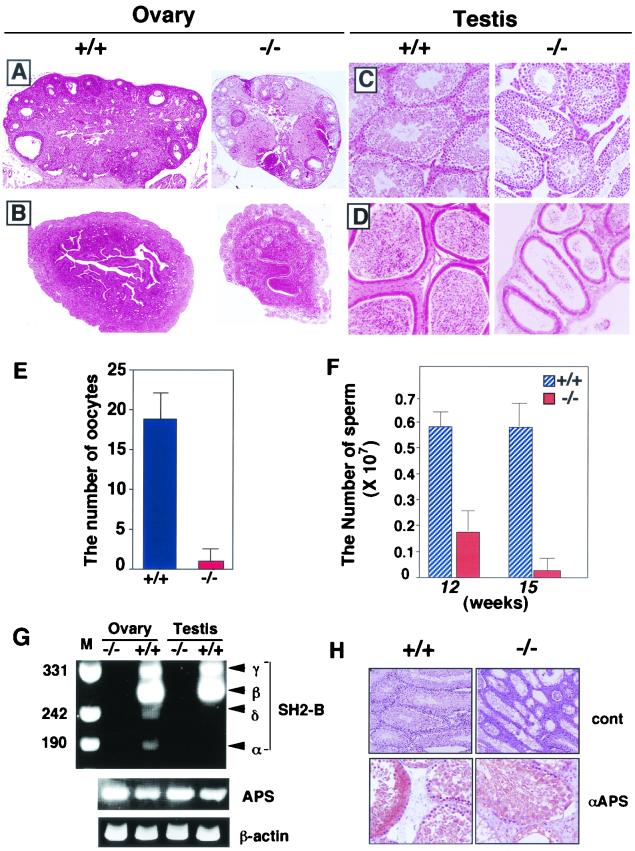
To investigate the cause of this phenotype, we immunohistochemically stained gonadal tissues (testis and ovary) from wild-type mice (Fig. 5B). SH2-B was localized in Leydig cells of the testis and stromal cells of the ovary. Therefore, reduced fertility of SH2-B-null mice might be related to the immaturity or deficiency of the reproductive systems. Dissection of SH2-B−/− mice revealed hypoplasia of the testis and ovary (Fig. 5C), even though their body weights were almost the same as those in controls at 10 weeks of age. Although the seminal vesicle and prostate gland (so-called accessory reproductive organs) were normal (data not shown), the testes and ovaries from SH2-B−/− mice showed size and weight reductions of approximately 50% compared to those from the wild-type and heterozygous controls.
Ovarian sections from SH2-B−/− mice revealed evidence of anovulation, based on the lack of antral follicles and the presence of many preantral follicles (Fig. 6A). The uterine tubules in SH2-B−/− mice were also immature compared to those in wild-type mice (Fig. 6B). We also observed a significant decrease in the number of ovulated oocytes under natural conditions in the mutant mice (Fig. 6E). These observations suggest a dysregulation of the estrous cycle. In the testis, the number of Leydig cells was also reduced in SH2-B−/− male mice compared with wild-type controls (Fig. 6C). Epididymides were also immature, and the number of sperm collected from the epididymides of SH2-B−/− mice was significantly reduced (Fig. 6D and F). The sperm from SH2-B-null mice had an abnormal morphological appearance and much-reduced motility compared to sperm from wild-type mice (data not shown).
FIG. 6.
Dysfunction of gonads of SH2-B-deficient mice. Histological analysis of ovary (A), uterus (B), testis (C), and epididymis (D) from wild-type (+/+) and SH2-B KO (−/−) mice. All sections were fixed overnight in 10% buffered formalin, dehydrated with ethanol, and then embedded in paraffin. Five-micrometer sections stained with hematoxylin and eosin are shown. Magnification, ×50. (E) Number of naturally ovulated oocytes from wild-type and SH2-B-deficient mice. The values are the mean ± standard deviation of triplicate determinations. Mice in estrous were used. (F) Analysis of sperm number from SH2-B-deficient and wild-type male mice. Sperm were collected from the epididymis, suspended in R18S3 medium, and then diluted with PBS. The sperm number is shown as the mean ± standard error of the mean (n = 4). (G) Expression of SH2-B splice variants and APS. After RT of mRNA from ovary and testis of wild-type (+/+) and SH2-B−/− mice, PCR was carried out with specific diagnostic primers (46) to amplify variant-specific fragments of carboxyl-terminal coding regions (indicated on the right) and in parallel to amplify APS and a control β-actin fragment. Products were compared on an ethidium bromide-stained agarose gel with specific size markers (in base pairs) shown on the left. (H) APS expression detected by immunohistochemistry. Testes of wild-type (+/+) and SH2-B KO (−/−) mice were stained with preimmune rabbit IgG (control) or affinity-purified anti-murine APS antibody. Magnification, ×40 (control) and ×100 (αAPS).
To determine which splice variant of SH2-B is expressed in gonadal tissues, we performed RT-PCR with primers which distinguish between the four variants to amplify short PCR fragments, as described by Yousaf et al. (46) (Fig. 6G). Consistent with their data, β and γ isoforms were predominately expressed in the ovary and testis, while levels of α and δ isoforms were low. To determine if APS is expressed in compensation in the ovary and testis, we examined APS expression using RT-PCR (Fig. 6G) as well as immunohistochemistry (Fig. 6H; data on the ovary are not shown). We found no overexpression of APS in SH2-B−/− mice. Immunohistochemistry revealed that APS protein is expressed in seminiferous tubules containing spermatogonia in the testis (Fig. 6H), while SH2-B is expressed in Leydig cells (Fig. 5B). SH2-B and APS are both expressed in stromal cells in the ovary (data not shown).
IVF experiments show reduced sperm and egg activity.
To investigate possible defects in the germ cells of SH2-B-null mice, we conducted IVF experiments. Oocytes collected from SH2-B−/− and wild-type females were inseminated with sperm from wild-type males. As shown in Table 2, 84% of wild-type oocytes had developed to the two-cell stage just 24 h after insemination. When sperm collected from SH2-B−/− mice was used to inseminate wild-type oocytes, a much lower percentage of zygotes developed (15% of that with +/+ × +/+). SH2-B-null oocytes were also significantly impaired in development: only 13 or 10% of SH2-B−/− oocytes developed to the two-cell stage when SH2-B−/− oocytes were inseminated with wild-type or SH2-B−/− sperm, respectively. These observations suggest that both male and female germ cells were defective in fertilization.
TABLE 2.
Results of IVF and embryonic transfera
| Genotypes | Total no. of oocytes | No. (%) of two-cell-stage embryos | No. of transplanted embryos | No. of offspring | |
|---|---|---|---|---|---|
| Sperm | Oocyte | ||||
| +/+ | +/+ | 83 | 70 (81.4) | 65 | 28 |
| −/− | +/+ | 66 | 10 (15.2) | 10 | 3 |
| +/+ | −/− | 71 | 9 (12.6) | 9 | 2 |
| −/− | −/− | 77 | 8 (10.3) | 8 | 1 |
Sperm from the epididymides were incubated with oocytes treated with hyaluronidase. After 24 h, these inseminated eggs were beginning cleavage, and the number of two-cell eggs was counted. Then, these two-cell-stage embryos were transplanted into ICR mice and the number of offspring was scored.
Next, to examine sperm-egg fusion, we performed a partial zona dissection on SH2-B−/− eggs followed by insemination with wild-type sperm, or a partial zona dissection on wild-type eggs followed by insemination with SH2B−/− sperm. These procedures did not increase the efficiency of fertilization (data not shown), thereby suggesting that the lower fertilization efficiency of SH2-B−/− egg and sperm is not due to a defect of egg-sperm fusion but rather to the lower activity of the sperm and to immaturity of the eggs.
To determine whether the impaired fertility was due to a defect in germ cells, we did a transplantation experiment. Two-cell-stage zygotes developed by IVF were transplanted into the ampullae of oviducts of female ICR mice, and the number of offspring was counted. Although offspring were obtained with all combinations (Table 2), offspring from the mutant egg-mutant sperm combination were much fewer than from the wild-type-wild-type combination. These results suggest that impaired fertility and the reduced number of offspring of SH2-B-null mice may be caused by a defect in the initial development of fertilized eggs.
Oocytes from SH2-B-null mice have a reduced response to FSH and IGF-1.
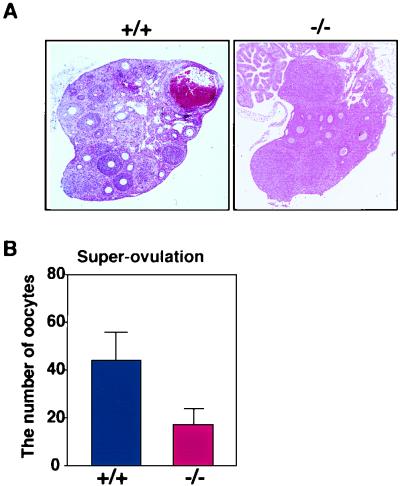
We examined the response of SH2-B-null female mice to reproductive hormones. Serum hormone levels did not differ between wild-type and SH2-B KO mice (Table 3). Gonadotropin (FSH plus luteinizing hormone [LH]) treatment of wild-type and heterozygous mice resulted in the development of many preantral follicles into antral follicles, while in SH2-B−/− female mice this development was impaired (Fig. 7A). There was a significant decrease in the number of ovulated oocytes in mutant mice, even following treatment to induce superovulation (Fig. 7B). These data indicate that the SH2-B-deficient ovary reduced the response to exogenous gonadotropin stimulation.
TABLE 3.
Serum hormone levelsa
| Genotype | Testosterone | Estradiol | FSH | LH | IGF-I |
|---|---|---|---|---|---|
| +/+ | 1.4 ± 0.7 | 10.6 ± 2.7 | 4.0 ± 3.4 | 1.6 ± 1.1 | 234 ± 16 |
| −/− | 2.6 ± 3.0 | 11.5 ± 3.2 | 6.1 ± 3.6 | 1.7 ± 0.9 | 235 ± 10 |
Serum hormone levels (ng/ml) were measured using radioimmunoassay. Four to six mice were used for each assay.
FIG. 7.
Comparison of the responsiveness of wild-type and SH2-B-deficient female mice to gonadotropin. (A) Ovaries were treated with gonadotropin to induce superovulation in wild-type and SH2-B-deficient females, fixed with 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Magnification, ×50. (B) Number of superovulated oocytes. After treatment with PMSG and human chorionic gonadotropin, oocytes were collected from the ampullae of the oviducts of wild-type and SH2-B-deficient mice (n = 5) and the ovulated oocytes were counted.
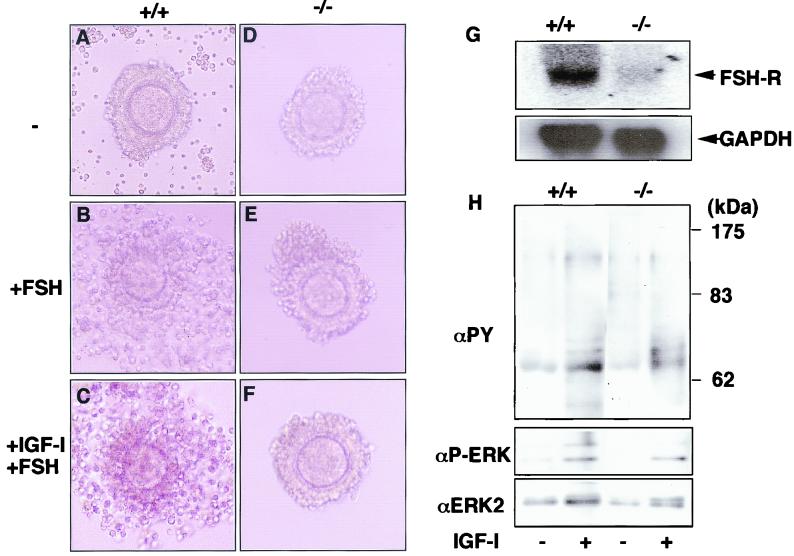
To examine function in the microenvironment of the ovary, we did in vitro experiments on COC expansion. Exposure to FSH leads to drastic changes in cumulus cells because of the hyaluronidase sensitivity of the cumulus oophori (Fig. 8A and B). These experiments showed that in wild-type COCs, FSH caused ovarian follicles to expand. In contrast, SH2-B−/− COCs did not expand in 1 μg of FSH/ml (Fig. 8E). We also observed reduced FSH-R mRNA levels in SH2B−/− COC cells (Fig. 8G). Since FSH-R levels have been shown to be regulated by IGF-I, we examined the effect of IGF-I on COC expansion. IGF-I pretreatment did not increase FSH sensitivity of SH2-B−/− COCs (Fig. 8F), which suggested that COCs from SH2-B−/− mice could not respond to IGF-I. FSH stimulates steroid synthesis in granulosa cells, thereby promoting maturation of oocytes in a paracrine fashion. Since SH2-B is highly expressed in supporting cells (Fig. 5B), these results suggest that dysfunction of supporting cells, probably an IGF-I insensitivity, contributes to the reduced maturation of SH2-B−/− oocytes.
FIG. 8.
SH2-B is essential for developing cumulus oophori. A COC expansion assay was performed with wild-type (A, B, and C) and SH2-B-deficient (D, E, and F) COCs. Forty-eight hours after administration of 5 IU of PMSG, Graafian follicles were punctured with needles and the COCs were removed into control Waymouth's medium. COCs were cultured in Waymouth's medium containing 3 mg of BSA/ml without FCS overnight at 37°C (A and D). Wild-type COCs were expanded with 1 μg of FSH/ml (B and E) in Waymouth's medium. COCs were also cultured in Waymouth's medium containing 10 ng of IGF-I/ml for 4 h, followed by 1 μg of FSH/ml (C and F). Similar results were obtained with up to 100 ng of IGF-I/ml. Magnification, ×100. Representative results of three independent experiments are shown. (G) Expression of FSH-R. Total RNA was extracted from ovaries of wild-type (+/+) and SH2-B-null (−/−) mice and hybridized with FSH-R (upper panel) and control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (lower panel). (H) Tyrosine phosphorylation of cellular proteins and ERK2 phosphorylation in COCs in response to IGF-I. COCs were collected from two female mice and stimulated with 100 ng of IGF-I/ml for 20 min. Cell extracts were immunoblotted with antiphosphotyrosine (αPY), anti-phosphorylated ERK (αP-ERK), or anti-ERK2 antibodies.
To examine the effect of SH2-B in IGF-I signal transduction, tyrosine phosphorylation of cellular proteins as well as ERK2 MAP activation in COCs were examined in response to IGF-I stimulation (Fig. 8H). A similar phosphorylation of cellular proteins and ERK2 was observed in wild-type and SH2-B−/− mice COCs. This suggests that SH2-B is involved in a signal transduction pathway which is different from the classical MAP kinase pathway.
DISCUSSION
SH2-B function in gonad development.
In the present work, we demonstrated that SH2-B is essential for the normal development of gonadal tissues in both male and female mice. IGF-I and FSH signaling has been shown to be essential for spermatogenesis and maintenance of normal sperm production in males (6, 24), and in females it is thought to tightly regulate follicle and oocyte maturation (2, 3, 15, 47). As shown in Fig. 8G, FSH-R levels decreased in SH2-B−/− ovaries and isolated COCs did not respond to IGF-I. The phenotype of SH2-B-null mice partially resembles that of IGF-I-, FSH-, and FSH-R-null mice. Although female FSH-null mice are infertile, males are fertile despite a significant reduction in testis size; the same is true of FSH-R-null mice (1, 11, 24). Based on in vitro analyses, SH2-B was proposed to be a signal-transducing adaptor molecule located downstream of GH, insulin, IGF-I, hepatocyte growth factor, PDGF, and fibroblast growth factor. Of these hormones, IGF-I is thought to be tightly involved in maintaining gonadal function (2, 3, 6). Therefore, the primary defect of the SH2-B−/− mice is probably impaired signaling of IGF-I in gonadal tissues.
In FSH-R-null males, no differences in the levels of FSH and LH were found in the pituitary, even though levels of these hormones in serum were elevated. Therefore, it has been suggested that FSH-R-null males are fertile. However, we observed no differences in levels of these hormones in serum in SH2-B-null male and female mice (Table 3). Furthermore, weight and gross morphology of the pituitary gland in SH2-B-null mice were similar to those of wild-type littermates (data not shown), which suggests that dysfunction of the gonads in SH2-B-deficient mice is not due to abnormal function of the pituitary gland. However, one could argue that reduced FSH-R in the ovary should result in an increase in both FSH and LH through negative feedback mechanisms. Similar levels of FSH and LH in SH2-B KO mice to those in wild-type mice could be explained by a small reduction in sex steroid hormone levels in serum. We could not exclude the possibility of dysfunction of the hypothalamus and pituitary, since SH2-B was highly expressed in the brain. Interestingly, insulin receptor substrate 2 (IRS-2) KO mice exhibit similar defects in folliculogenesis, yet LH levels are reduced (7). Therefore, a negative feedback system may be defective in the SH2-B as well as in the IRS-2 KO mice.
IGF-I is produced by various types of cells, and this growth factor has been implicated in a variety of reproductive processes. Leydig and Sertoli cells in males and granulosa and cumulus cells in females carry the IGF-I receptor (15-17, 28). Ovarian granulosa cells contain IGF-I mRNA, secrete IGF-I in vitro and in vivo, and express the IGF-I receptor. IGF-I stimulates both proliferation and differentiation of granulosa and thecal cells in vitro (6, 9, 17). Moreover, IGF-I has been shown to potentiate FSH-stimulated cyclic AMP production, aromatase activity, and LH receptor expression by granulosa cells. IGF-I-deficient mice show partially similar, but more severe, defects than do SH2-B−/− mice. IGF-I-deficient mice show growth retardation, delayed puberty, and ovarian dysfunction (6, 26). Therefore, SH2-B is likely to be an important, although probably not the only, signaling molecule of the IGF-I receptor. A similar pattern of female infertility, including small anovulatory ovaries with reduced numbers of follicles, was observed in female mice lacking the IRS-2 gene, a component of the insulin and IGF-I signaling cascade (7, 22). Thus, SH2-B and IRS-I could be independently necessary for IGF-I signaling pathways in the ovary.
Although APS is expressed in ovary and testis, there was no strong overexpression of APS in gonadal tissues in SH2-B−/− mice (Fig. 6G and H). Furthermore, we found no defect in reproduction in APS−/− and Lnk−/− mice 39; M. Iseki, S. Takaki, and K. Takatsu, unpublished data). Phenotypes of Lnk KO mice (39, 40) as well as our biochemical analyses (42, 44) suggest that Lnk and APS negatively regulate tyrosine kinase signal transduction, while our present study suggests that SH2-B transmits unknown positive signals from the IGF-I receptor. The reason for such differences in functions of SH2-B and APS or Lnk are not clear at present.
Yousaf et al. (46) reported that all SH2-B isoforms augmented IGF-I- and PDGF-induced mitogenesis, although the most pronounced effect was observed with the γ variant. A high level of expression of SH2-Bγ and -β in gonadal tissues (Fig. 6G) may be responsible for the phenotype of SH2-B KO mice. As shown in Fig. 8H, MAP kinase activation in response to IGF-I in COCs was not affected by SH2-B deficiency, suggesting that other pathways are mediated by SH2-B in these cells. Recently, Diakonova et al. reported that SH2-B directly interacts with Rac (10). SH2-B KO mice will provide a useful tool to define the signaling pathway in which SH2-B mediates the response to IGF-I.
SH2-B is not necessary for other tyrosine kinase signaling.
It has been reported that SH2-B binds to the cytoplasmic phosphotyrosine residue of the insulin receptor (4, 27, 43). SH2-B interacts with insulin receptor activation loop phosphorylation sites and undergoes insulin-stimulated tyrosine phosphorylation in vitro (5, 23). Obesity is associated with infertile conditions such as polycystic ovary syndrome (14, 25). We measured blood glucose concentrations after intraperitoneal injection of 2 g of d-(+)-glucose/kg of body weight, but we observed no differences in blood glucose concentrations compared to that in wild-type control mice. These results suggest that SH2-B may not be involved in blood glucose homeostasis in vivo (data not shown). However, IRS-2-null male mice had increased blood glucose concentrations compared to wild-type controls (7). Moreover, in IRS-2 KO mice the plasma concentrations of LH, prolactin, and sex steroids were low, but this was not the case in SH2-B KO mice. This is probably because the expression of SH2-B is restricted to tissues surrounding germ cells in gonadal organs and SH2-B is not directly involved in the function of the germ cells and pituitary gland.
The anterior pituitary gland plays an important role in regulating the normal reproductive status in mammals. However, the size, gross anatomy, and histology of the pituitary gland of SH2-B-null mice is normal (data not shown). These observations support the idea that the cause of the impaired fertility in SH2-B−/− mice is in the cells surrounding the germ cells, rather than in the germ cells themselves.
Both APS and SH2-B have been shown to be involved in the NGF-induced Ras-MAP kinase signaling cascade. A dominant negative mutant of SH2-Bβ when overexpressed in PC12 cells acts to block NGF-induced neurite outgrowth (12). Thus, SH2-B may be downstream of the Trk tyrosine kinase. Consistent with this finding, SH2-B is highly expressed in the brain. However, when we conducted an in vitro NGF-induced neurite outgrowth assay with dorsal root ganglion cells from SH2-B-null mice, we observed no differences in NGF sensitivity of the dorsal root ganglion cells (data not shown). Thus, either SH2-B is not a major player in NGF signaling or APS can compensate for SH2-B function in SH2-B-null mice.
SH2-Bβ has been reported to be involved in the JAK2 signaling activated by GH (8, 18, 35). Importantly, SH2-Bβ potentiates JAK2 kinase activity (31, 32). Partial growth retardation of SH2-B−/− mice suggests some impairment of GH signaling. However, we obtained no evidence that SH2-B is a JAK2 activator. For example, when stimulated by IL-3, the response of mast cells from SH2-B−/− mice was similar to that of wild-type mice (Fig. 4). We also observed that GH-induced JAK2/STAT5 activation as well as IGF-I induction occurred normally in SH2-B-null mouse liver (data not shown). Thus, SH2-B may be dispensable or unnecessary for JAK2 activity. Further study is necessary to define the role of SH2-B in the GH/JAK2 pathway.
Acknowledgments
We thank H. Ohgusu and Y. Kawabata for technical assistance, M. Kojima, M. Kamei, Y. Komatsu, and M. Okabe for critical discussion and comments, and M. Ohara for language assistance.
This work was supported by Grants-in-Aid (for Y.A.) and Special Coordination Funds (for T.K.) from the Ministry of Education, Science, Technology, Sports and Culture of Japan, the Japan Health Science Foundation, the Human Frontier Science Program, Mochida Memorial Foundation, and the Japan Research Foundation for Clinical Pharmacology. S.O. is supported by a fellowship from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Abel, M. H., A. N. Wootton, V. Wilkins, I. Huhtaniemi, P. G. Knight, and H. M. Charlton. 2000. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795-1803. [DOI] [PubMed] [Google Scholar]
- 2.Adashi, E. Y., C. E. Resnick, and R. G. Rosenfeld. 1990. Insulin-like growth factor-I (IGF-I) and IGF-II hormonal action in cultured rat granulosa cells: mediation via type I but not type II IGF receptors. Endocrinology 126:216-222. [DOI] [PubMed] [Google Scholar]
- 3.Adashi, E. Y., C. E. Resnick, A. J. D'Ercole, M. E. Svoboda, and J. J. Van Wyk. 1985. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 6:400-420. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, Z., and T. S. Pillay. 2001. Functional effects of APS and SH2-B on insulin receptor signalling. Biochem. Soc. Trans. 29:529-534. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed, Z., B. J. Smith, K. Kotani, P. Wilden, and T. S. Pillay. 1999. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem. J. 341:665-668. [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, J., M. P. Hardy, J. Zhou, C. Bondy, F. Lupu, A. R. Bellve, and A. Efstratiadis. 1996. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 10:903-918. [DOI] [PubMed] [Google Scholar]
- 7.Burks, D. J., J. F. de Mora, M. Schubert, D. J. Withers, M. G. Myers, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White. 2000. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377-382. [DOI] [PubMed] [Google Scholar]
- 8.Carter-Su, C., L. Rui, and M. R. Stofega. 2000. SH2-B and SIRP: JAK2 binding proteins that modulate the actions of growth hormone. Recent Prog. Horm. Res. 55:293-311. [PubMed] [Google Scholar]
- 9.Davoren, J. B., B. G. Kasson, C. H. Li, and A. J. Hsueh. 1986. Specific insulin-like growth factor (IGF) I- and II-binding sites on rat granulosa cells: relation to IGF action. Endocrinology 119:2155-2162. [DOI] [PubMed] [Google Scholar]
- 10.Diakonova, M., D. R. Gunter, J. Herrington, and C. Carter-Su. SH2-Bβ is a Rac binding protein that regulates cell motility. J. Biol. Chem., in press. [DOI] [PubMed]
- 11.Dierich, A., M. R. Sairam, L. Monaco, G. M. Fimia, A. Gansmuller, M. LeMeur, and P. Sassone-Corsi. 1998. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 95:13612-13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggert, A., N. Ikegaki, X. Liu, T. T. Chou, V. M. Lee, J. Q. Trojanowski, and G. M. Brodeur. 2000. Molecular dissection of TrkA signal transduction pathways mediating differentiation in human neuroblastoma cells. Oncogene 19:2043-2051. [DOI] [PubMed] [Google Scholar]
- 13.Eppig, J. J. 1979. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro. J. Exp. Zool. 208:111-120. [DOI] [PubMed] [Google Scholar]
- 14.Franks, S., C. Gilling-Smith, H. Watson, and D. Willis. 1999. Insulin action in the normal and polycystic ovary. Endocrinol. Metab. Clin. North Am. 28:361-378. [DOI] [PubMed] [Google Scholar]
- 15.Hammond, J. M., J. L. Baranao, D. Skaleris, A. B. Knight, J. A. Romanus, and M. M. Rechler. 1985. Production of insulin-like growth factors by ovarian granulosa cells. Endocrinology 117:2553-2555. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez, E. R., A. Hurwitz, A. Vera, A. Pellicer, E. Y. Adashi, D. LeRoith, Jr., and C. T. Roberts. 1992. Expression of the genes encoding the insulin-like growth factors and their receptors in the human ovary. J. Clin. Endocrinol. Metab. 74:419-425. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez, E. R., Jr., C. T. Roberts, D. LeRoith, and E. Y. Adashi. 1989. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology 125:572-574. [DOI] [PubMed] [Google Scholar]
- 18.Herrington, J., M. Diakonova, L. Rui, D. R. Gunter, and C. Carter-Su. 2000. SH2-B is required for growth hormone-induced actin reorganization. J. Biol. Chem. 275:13126-13133. [DOI] [PubMed] [Google Scholar]
- 19.Hogan, B., R. Beddington, F. Constantini, and E. Lucy. 1994. Manipulating the mouse embryo, a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 20.Huang, X., Y. Li, K. Tanaka, K. G. Moore, and J. I. Hayashi. 1995. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 92:11618-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iseki, M., S. Takaki, and K. Takatsu. 2000. Molecular cloning of the mouse APS as a member of the Lnk family adaptor proteins. Biochem. Biophys. Res. Commun. 272:45-54. [DOI] [PubMed] [Google Scholar]
- 22.Joshi, R. L., B. Lamothe, N. Cordonnier, K. Mesbah, E. Monthioux, J. Jami, and D. Bucchini. 1996. Targeted disruption of the insulin receptor gene in the mouse results in neonatal lethality. EMBO J. 15:1542-1547. [PMC free article] [PubMed] [Google Scholar]
- 23.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 335:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, T. R., Y. Wang, N. Lu, and M. M. Matzuk. 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 15:201-204. [DOI] [PubMed] [Google Scholar]
- 25.Legro, R. S., R. Spielman, M. Urbanek, D. Driscoll III, J. F. Strauss, and A. Dunaif. 1998. Phenotype and genotype in polycystic ovary syndrome. Recent Prog. Horm. Res. 53:217-256. [PubMed] [Google Scholar]
- 26.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59-72. [PubMed] [Google Scholar]
- 27.Nelms, K., T. J. O'Neill, S. Li, S. R. Hubbard, T. A. Gustafson, and W. E. Paul. 1999. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm. Genome 10:1160-1167. [DOI] [PubMed] [Google Scholar]
- 28.Oliver, J. E., T. J. Aitman, J. F. Powell, C. A. Wilson, and R. N. Clayton. 1989. Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology 124:2671-2679. [DOI] [PubMed] [Google Scholar]
- 29.Osborne, M. A., S. Dalton, and J. P. Kochan. 1995. The yeast trihybrid system--genetic detection of trans-phosphorylated ITAM-SH2-interactions. Bio/Technology 13:1474-1478. [DOI] [PubMed] [Google Scholar]
- 30.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385-395. [DOI] [PubMed] [Google Scholar]
- 31.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 32.Riedel, H., J. Wang, H. Hansen, and N. Yousaf. 1997. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J. Biochem. (Tokyo) 122:1105-1113. [DOI] [PubMed] [Google Scholar]
- 33.Rui, L., and C. Carter-Su. 1998. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bβ with PDGF receptor and phosphorylation of SH2-Bβ. J. Biol. Chem. 273:21239-21245. [DOI] [PubMed] [Google Scholar]
- 34.Rui, L., and C. Carter-Su. 1999. Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rui, L., D. R. Gunter, J. Herrington, and C. Carter-Su. 2000. Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol. Cell. Biol. 20:3168-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rui, L., J. Herrington, and C. Carter-Su. 1999. SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 274:26485-26492. [DOI] [PubMed] [Google Scholar]
- 37.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takaki, S., J. D. Watts, K. A. Forbush, N. T. Nguyen, J. Hayashi, J. Alberola-Ila, R. Aebersold, and R. M. Perlmutter. 1997. Characterization of Lnk, an adaptor protein expressed in lymphocytes. J. Biol. Chem. 272:14562-14570. [DOI] [PubMed] [Google Scholar]
- 39.Takaki, S., K. Sauer, B. M. Iritani, S. Chien, Y. Ebihara, K. Tsuji, K. Takatsu, and R. M. Perlmutter. 2000. Control of B cell production by the adaptor protein Lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaki, S., H. Morita, Y. Tezuka, and K. Takatsu. 2002. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J. Exp. Med. 195:151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehara, S., Y. Hitoshi, F. Numata, M. Makino, M. Howard, T. Mizuochi, and K. Takatsu. 1994. An IFN-gamma-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int. Immunol. 6:1937-1947. [DOI] [PubMed] [Google Scholar]
- 42.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J., and H. Riedel. 1998. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 273:3136-3139. [DOI] [PubMed] [Google Scholar]
- 43a.World Health Organization. 1992. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge, England.
- 44.Yokouchi, M., R. Suzuki, M. Masuhara, S. Komiya, A. Inoue, and A. Yoshimura. 1997. Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene 15:7-15. [DOI] [PubMed] [Google Scholar]
- 45.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Yasukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl, and inhibits PDGF-induced mitogenesis. Oncogene 18:759-768. [DOI] [PubMed] [Google Scholar]
- 46.Yousaf, N., Y. Deng, Y. Kang, and H. Riedel. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 276:40940-40948. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, J., T. R. Kumar, M. M. Matzuk, and C. Bondy. 1997. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 11:1924-1933. [DOI] [PubMed] [Google Scholar]