Abstract
Androgen receptor (AR) interacts with β-catenin and can suppress its coactivation of T cell factor 4 (Tcf4) in prostate cancer (PCa) cells. Pin1 is a peptidyl-prolyl cis/trans isomerase that stabilizes β-catenin by inhibiting its binding to the adenomatous polyposis coli gene product and subsequent glycogen synthase kinase 3β (GSK-3β)-dependent degradation. Higher Pin1 expression in primary PCa is correlated with disease recurrence, and this study found that Pin1 expression was markedly increased in metastatic PCa. Consistent with this result, increased expression of Pin1 in transfected LNCaP PCa cells strongly accelerated tumor growth in vivo in immunodeficient mice. Pin1 expression in LNCaP cells enhanced β-catenin/Tcf4 transcriptional activity, as assessed using Tcf4-regulated reporter genes, and increased expression of endogenous Tcf4 and c-myc. However, in contrast to results in cells with intact PTEN and active GSK-3β, Pin1 expression in LNCaP PCa cells, which are PTEN deficient, did not increase β-catenin. Instead, Pin1 expression markedly inhibited the β-catenin interaction with AR, and Pin1 abrogated the ability of AR to antagonize β-catenin/Tcf4 binding and transcriptional activity. These findings demonstrate that AR can suppress β-catenin signaling, that the AR-β-catenin interaction can be regulated by Pin1, and that abrogation of this interaction can enhance β-catenin/Tcf4 signaling and contribute to aggressive biological behavior in PCa.
Prostate cancer (PCa) is the most common noncutaneous cancer in men in the United States and the second leading cause of cancer-related deaths in men in industrialized countries, but the molecular mechanisms involved in the development and progression of this disease are poorly understood. Nonetheless, many lines of evidence indicate that the androgen receptor (AR) functions as a positive regulator of cell proliferation in PCa, and androgen deprivation therapy is still the standard treatment for metastatic disease. AR is a member of the steroid hormone receptor subfamily of ligand-regulated nuclear receptors, and its natural ligands are testosterone and 5α-dihydrotestosterone (DHT) (14). As with other steroid receptors, AR is a modular protein that contains an N-terminal transactivation domain, a conserved DNA-binding domain (DBD), and a C-terminal ligand-binding domain (LBD). Ligand binding to the LBD induces conformational changes that generate binding sites for coactivator proteins, which stimulate transcription through chromatin remodeling and recruitment of the transcriptional machinery.
One recently identified protein that can interact with and coactivate the AR is β-catenin, which binds to the DHT-liganded AR LBD via a site that is distinct from the hydrophobic cleft that mediates binding of LXXLL motifs found in many other coactivator proteins (8, 29, 33, 46, 48, 57). However, the biological role of AR interactions with β-catenin has not been established and may be complex given further direct interactions between AR and T-cell factor 4 (Tcf4) as well as between AR and amino-terminal enhancer of split (a Tcf corepressor and member of the Groucho/TLE family) (1, 59). Although β-catenin can function as an AR coactivator and may selectively regulate a subset of AR-responsive genes, another function for the AR-β-catenin interaction in normal prostate epithelium may be to sequester nuclear β-catenin and thereby suppress β-catenin/Tcf4 signaling, consistent with AR functioning in normal prostate epithelium to suppress growth and stimulate terminal differentiation (1, 10, 27, 30, 33, 43, 46). The vitamin D and retinoic acid receptors can similarly bind to β-catenin and interfere with Tcf4 coactivation by β-catenin (13, 32, 43).
The best established functions of β-catenin are in the nucleus as a transcriptional coactivator for the Tcf family of sequence-specific transcription factors and on the plasma membrane as a bridge molecule connecting E-cadherin to the cytoskeleton (17). Coactivator activity is determined by the level of free β-catenin, which is tightly regulated by a β-catenin degradation complex (18, 21, 36, 41). This complex includes glycogen synthase kinase 3β (GSK-3β), the adenomatous polyposis coli gene product (APC), and Axin. APC binds to free β-catenin and recruits it to this complex, where it is phosphorylated at N-terminal sites by GSK-3β and thereby targeted for ubiquitination and proteolysis. Wnt signaling stabilizes β-catenin by inhibiting GSK-3β activity, leading to increased cytoplasmic and nuclear β-catenin levels and activation of Tcf transcription factors. Tcf4 is the predominant Tcf in epithelia, and transcriptional targets of the β-catenin/Tcf4 complex include growth regulatory genes such as c-myc and cyclin D1 (4, 5, 8, 20, 47, 49, 52).
The β-catenin/Tcf signaling pathway plays a critical role in normal development, stem cell renewal, and tumorigenesis. The importance of β-catenin/Tcf signaling in cancer has been most clearly demonstrated in hereditary colorectal cancer, where loss of APC leads to stabilization of β-catenin and increased expression of the β-catenin/Tcf4 target gene c-myc (20, 28, 49). Defects leading to β-catenin stabilization, including loss of APC or Axin function, or mutations in the N terminus of β-catenin that prevent GSK-3β-mediated phosphorylation have been described in sporadic colon cancer and in many other tumor types. β-Catenin mutations have been identified in approximately 5% of prostate cancers, but a role for β-catenin in PCa development or progression has not been established (9, 51). Nonetheless, immunohistochemical studies have shown increased cytoplasmic and nuclear β-catenin expression in 20 to 30% of PCa, with greater expression in more advanced tumors (8, 11).
One mechanism for increased β-catenin expression in PCa may be PTEN loss, which is common in advanced PCa and results in activation of the phosphatidylinositol 3-kinase and downstream Akt signaling pathways (7, 12, 50). Akt can phosphorylate and inactivate GSK-3β, leading to stabilization and increased levels of β-catenin. Indeed, GSK-3β suppression and subsequent β-catenin stabilization have been demonstrated directly in the PTEN-deficient LNCaP PCa cell line (34, 44). However, LNCaP cells do not show substantial nuclear accumulation of β-catenin, and transfection studies with Tcf4 regulated reporter genes have shown minimal β-catenin/Tcf4 transcriptional activity, indicating that additional GSK-3β-independent mechanisms may regulate β-catenin/Tcf4 activity in PCa (10, 11).
An alternative mechanism for β-catenin stabilization is via Pin1-mediated proline isomerization, which can prevent β-catenin binding to APC (42). Pin1 is a peptidyl-prolyl cis/trans isomerase that targets phosphorylated Ser/Thr-Pro (pSer/Thr-Pro) peptide bonds and has been found to regulate the activities of multiple proteins involved in cell cycle progression and other functions (23, 24, 37, 56). The WW domain of Pin1 appears to bind to pSer246-Pro247 in the third Armadillo repeat of β-catenin, with isomerization of this proline disrupting the interaction between β-catenin and APC (42). Overexpression of Pin1 has been implicated in cell transformation and correlated with increased levels of β-catenin, cyclin D1, and c-myc in human breast cancer and other cancers (3, 22, 38, 45, 53-55). Significantly, Pin1 overexpression has also been observed in a subset of primary prostate cancers, and its expression correlates with increased risk of recurrence after radical prostatectomy (2). However, the functional effects of Pin1 overexpression on β-catenin nuclear signaling in PCa cells (and in particular in PTEN-deficient cells), and how it contributes to more aggressive biological behavior have not been determined.
In this study, we have assessed the role of Pin1 in regulating β-catenin activity in PCa. We found initially that Pin1 expression was markedly increased in metastatic versus primary PCa. Consistent with this result, increased expression of Pin1 in transfected LNCaP PCa cells strongly accelerated tumor growth in vivo in immunodeficient mice. Increased Pin1 expression in LNCaP cells enhanced β-catenin/Tcf4 transcriptional activity, as assessed using Tcf4-regulated reporter genes, and increased expression of endogenous Tcf4 and c-myc. However, in contrast to results in cells with intact PTEN and active GSK-3β, Pin1 expression in PTEN-deficient LNCaP PCa cells did not increase the levels of total or free β-catenin. Significantly, while Pin1 expression in cells with intact PTEN could markedly enhance β-catenin coactivation of Tcf4, Pin1 expression markedly inhibited β-catenin coactivation of AR in vivo and AR binding in vitro. Moreover, Pin1 abrogated the ability of AR to antagonize β-catenin/Tcf4 binding and transcriptional activity. These findings demonstrate that Pin1 can regulate the AR-β-catenin interaction in the prostate and contribute to aggressive biological behavior in PCa by abrogating this interaction and enhancing β-catenin/Tcf4 signaling.
MATERIALS AND METHODS
Plasmids and reagents.
Expression vectors and reporter genes have been described previously (42). The AR LBD (amino acids 660 to 919) was cloned into the mammalian Gal4 DBD fusion vector pBIND (Promega), to give pBIND-AR-LBD. The AR DBD-LBD and AR N-DBD vectors were constructed in pcDNA3.1 (Invitrogen) and encode amino acids 501 to 919 and 1 to 500, respectively. GST-AR LBD encodes amino acids 676 to 919 of the AR LBD in the pGEX-2TK vector. Unconjugated anti-β-catenin was from BD Transduction Laboratories (San Jose, CA). Anti-AR (PG21), anti-Pin1 (07-091), and anti-Tcf4 (6H5-3) were from Upstate Biotechnology (Lake Placid, NY). Fetal bovine serum (FBS), charcoal-dextran stripped FBS (CDS-FBS), and tetracycline-free FBS were from HyClone (Logan, UT).
Cell lines, stable transfectants, and xenografting.
To generate Pin1-expressing LNCaP cell lines, pcDNA3.1 (control) or pcDNA-Pin1 plasmid was transfected into LNCaP cells and selected in medium containing 0.9 mg/ml G418. For in vivo growth, 2 million stable Pin1-transfected or control LNCaP cells were injected subcutaneously into the flanks of male ICR-scid mice (6 to 8 weeks; Taconic) in 50% Matrigel. Stable Pin1 and control clones derived from these lines were maintained in RPMI 1640 with 10% FBS and 0.3 mg/ml G418. The CWR22D1 cell line was derived from a CWR22 xenograft that was adapted to grow in vitro in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS and was provided by Xin Yuan (Beth Israel Deaconess Medical Center). Similar to the CWR22 xenograft, the cell line has a mutant AR (H874Y), has intact PTEN, and does not have constitutive activation of the phosphatidylinositol 3-kinase/Akt pathway (data not shown).
Immunostaining, immunoblotting, and real-time RT-PCR.
Immunochemistry was done using tissue microarrays that contained normal prostate, primary PCa, and PCa that was metastatic to multiple sites including lymph nodes and bone. Primary antibodies were used at 1:20 for anti-β-catenin and 1:1,000 for anti-Pin1. Free cytosolic and nuclear proteins were isolated with digitonin lysis buffer (1% digitonin, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, and 10 mM MgCl2) containing protease inhibitors. Immunoblotting was carried out using the indicated primary antibodies, followed by horseradish peroxidase conjugates. Real-time reverse transcription (RT)-PCR was done with TaqMan kits (PE Biosystems) and an ABI Prism 7700 sequence detector (Perkin Elmer). The c-myc forward primer is 5′-TGAGGAGACACCGCCCA-3′, the reverse primer is 5′-AACATCGATTTCTTCCTCA-3′, and probe is 5′-6-carboxyfluorescein-CACCAGCAGCGACTCTGA-3′. 18S rRNA was used as internal control.
GST pull downs and coimmunoprecipitations.
For glutathione S-transferase (GST) pull downs, 293T cells cultured in 10-cm dishes were transfected with Lipofectamine 2000 (Invitrogen) as indicated. After overnight incubation, the cell culture medium was replaced with 10 ml DMEM containing 5% FBS. After another 24 h, the cells were fractionated with the NE-PER kit containing protease and phosphatase inhibitors. The cytoplasmic protein fraction was precleared with glutathione-agarose beads (Amersham) and then equally divided and precipitated for 4 h at 4°C with 20 μl of packed glutathione-agarose beads bound with GST or GST-AR-LBD fusion proteins (5 μg). For coimmunoprecipitations, 293T cells were transfected with 3 μg of each plasmid DNA as above. After overnight incubation, the cell culture medium was replaced with 10 ml DMEM containing 10% CDS-FBS and 10 nM DHT. After another 24 h, the cells were lysed in binding buffer (phosphate-buffered saline, 0.5% Triton X-100, 10% glycerol, and protease and phosphatase inhibitors). The cell lysates were precleared for 20 min with 200 μg of nonimmune mouse serum absorbed on protein G-agarose beads. The supernatant was then split and immunoprecipitated for 1 h with 1 μg of nonimmune mouse serum or anti-Tcf4 mouse monoclonal antibody absorbed onto protein G-agarose beads.
Transient transfections and reporter gene assays.
CV1, 293T, LNCaP, and CWR22D1 cells cultured in 48-well plates were transfected using Lipofectamine 2000, using the indicated amounts of each vector. The experiments shown used empty pcDNA3.1 vector to normalize for total DNA content. However, as the empty pcDNA vectors can suppress transcriptional activity, control experiments without adding empty pcDNA, or using pBluescript to normalize for total DNA, were also carried out as indicated. Finally, further controls using empty pcDNA at the same molar concentration as the insert containing expression vectors, with pBluescript added to normalize for total DNA mass, were also carried out as indicated, with comparable results. For experiments with DHT stimulation, cells were cultured in 5 to 10% CDS-FBS. Firefly and internal control Renilla luciferase activities were determined using a dual luciferase reporter assay kit (Promega, Madison, WI), and Renilla activities were not consistently affected by any of the cotransfected vectors. The firefly luciferase was divided by the control Renilla luciferase and the results, given as relative luciferase units, reflect the means and standard deviations from triplicate samples.
RESULTS
Pin1 expression is increased in metastatic PCa.
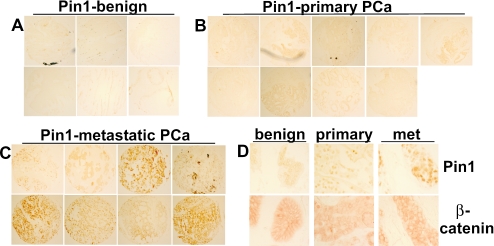
A previous study of Pin1 in PCa radical prostatectomy specimens found a correlation between higher levels of Pin1 expression and increased risk of PCa recurrence (2). Immunostaining on a series of normal prostate, primary PCa, and metastatic PCa samples showed that Pin1 expression was markedly increased in the metastatic PCa relative to the normal prostate (Fig. 1A to C and Table 1). Significantly, Pin1 expression in the metastatic PCa samples was also markedly increased relative to the primary PCa samples, with 24/29 metastatic tumors showing medium to strong Pin1 expression versus only 6/30 primary tumors showing medium staining (and none showing strong staining) (P < 0.0001). Controls for immunostaining included anti-AR, which showed consistent nuclear expression in the epithelium (data not shown). This further increase of Pin1 in the metastatic versus primary tumors was consistent with a role for Pin1 in metastatic behavior.
FIG. 1.
Expression of Pin1 and β-catenin in metastatic PCa. (A to C) Tissue microarrays containing benign prostate, primary PCa, and metastatic PCa samples were immunostained for Pin1. (D) Representative samples of Pin1 and β-catenin immunostaining in adjacent sections of benign prostate, primary PCa, and metastatic (met) PCa.
TABLE 1.
Pin1 expression in benign prostate and primary and metastatic prostate cancera
| Pin1 expression staining result | No. of samples of:
|
||
|---|---|---|---|
| Benign prostate | Primary PCa | Metastatic PCa | |
| Negative | 16 | 10 | 0 |
| Weak | 9 | 14 | 5 |
| Moderate | 0 | 6 | 10 |
| Strong | 0 | 0 | 14 |
| Total no. of samples | 25 | 30 | 29 |
Differences between Pin1 expression in the benign versus primary PCa and in primary versus metastatic PCa were significant (exact two-sided; P = 0.008 and P < 0.0001, respectively) using Kruskall-Wallis tests.
These metastatic tumors also showed increased cytoplasmic and nuclear β-catenin expression relative to the predominant plasma membrane expression in normal prostate epithelium (Fig. 1D). However, this increase was similarly observed in some primary PCa samples with low or intermediate levels of Pin1, implicating additional factors (such as loss of E-cadherin and PTEN that occur frequently in PCa) in the altered β-catenin expression. Indeed, as Pin1-mediated proline isomerization stabilizes β-catenin by preventing its APC binding and subsequent GSK-3β-mediated degradation, it was not clear to what extent increased Pin1 would enhance β-catenin activity or tumor growth in PCa cells with suppressed GSK-3β activity due to PTEN loss and Akt activation.
Pin1 expression enhances tumor growth and β-catenin/Tcf4 activity in PTEN-deficient PCa cells.
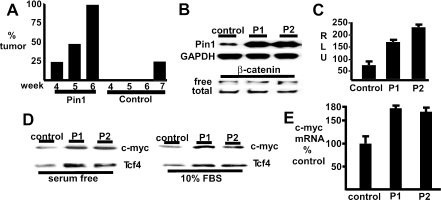
To determine whether increased Pin1 expression could enhance tumor growth and β-catenin activity in PTEN-deficient PCa cells, we examined the PTEN-deficient LNCaP PCa cell line stably transfected with a Pin1 or control expression vector. Immunodeficient male ICR-scid mice were implanted subcutaneously with Pin1-transfected (8 mice) or control transfected (4 mice) LNCaP cell lines in 50% Matrigel. As shown in Fig. 2A, growth of the Pin1-transfected cells was detected as early as 4 weeks after implantation (2 of 8 mice, 25%), with tumors in all 8 (100%) of the mice bearing Pin1-transfected cells by 6 weeks. In contrast, there was no detectable growth of the control transfected LNCaP cells at 6 weeks, with a small tumor detected in only 1 mouse (25%) at week 7. This difference was highly significant (P < 0.01 by Fisher's exact test), indicating that Pin1 expression could enhance in vivo tumor growth.
FIG. 2.
Pin1 expression enhances tumor growth and β-catenin/Tcf4 activity in PTEN-deficient LNCaP PCa cells. (A) Pin1 (8 mice) or vector control (4 mice) stable LNCaP cell lines were implanted subcutaneously in 50% Matrigel into the flanks of male SCID mice, and the percentage of mice with palpable tumors was determined weekly. (B) Pin1-expressing LNCaP clones (P1 and P2) or control clones were lysed in digitonin buffer or in 1% sodium dodecyl sulfate (SDS), and lysates were immunoblotted to identify free and total β-catenin, respectively. (C) Control or Pin1-expressing LNCaP clones were transfected with pTopflash (50 ng) and cytomegalovirus-Renilla (2.5 ng) reporter plasmids. Firefly versus Renilla luciferase activities were determined and expressed as relative light units (RLU). (D) Control and Pin1 clones in 10% FBS or in serum-free medium for 24 h were lysed in 1% SDS and immunoblotted for c-myc and Tcf4. (E) RNA was extracted from control or Pin1 clones, and c-myc gene expression was measured by quantitative real-time RT-PCR.
A series of independent Pin1 or control LNCaP clones expressing varying levels of Pin1 were then generated and examined for β-catenin levels and for β-catenin coactivation of Tcf4 transcriptional activity. Significantly, there was no increase in the levels of total or free (digitonin soluble) β-catenin in a series of clones expressing varying levels of Pin1, including clones expressing high levels of Pin1 relative to control LNCaP cells transfected with the vector alone (Fig. 2B). To assess β-catenin/Tcf4 transcriptional activity, clones were transfected with a Tcf-regulated luciferase reporter plasmid (pTopflash). Consistent with previous reports, pTopflash-specific activity was very low in control LNCaP cells (Fig. 2C). However, this activity was increased in Pin1-expressing clones. Similar results were obtained using a reporter gene derived from the c-myc promoter, which contains two previously characterized Tcf-responsive elements (data not shown).
Expression of endogenous c-myc was next assessed to determine whether increased Pin1 levels enhanced expression of an endogenous β-catenin/Tcf4 target gene in PTEN-deficient PCa cells. The expression of c-myc protein was increased in independent stable Pin1 transfectants compared to the vector-alone LNCaP transfectant (control) (Fig. 2D). Real-time RT-PCR confirmed that the increased c-myc protein reflected increased mRNA levels (Fig. 2E). Significantly, Tcf4 protein levels were also increased in the stable Pin1 transfectants. These findings indicated that Pin1 increased β-catenin coactivation of Tcf4 and suggested that enhanced β-catenin coactivation of Tcf4 resulted in selection for cells with increased Tcf4 levels and expression of β-catenin/Tcf4 regulated genes. Taken together, these studies showed that Pin1 could enhance β-catenin coactivation of Tcf4 in PCa cells by a mechanism that appeared to be distinct from its ability to increase β-catenin protein levels by suppressing β-catenin degradation.
Pin1 suppresses β-catenin coactivation of AR.
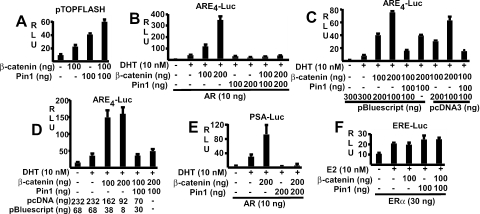
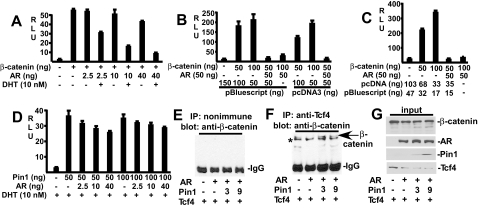
Several groups have shown that β-catenin can also function as an AR coactivator and that there may be cross-competition between AR and Tcf4 for limiting nuclear β-catenin (1, 8, 10, 27, 29, 30, 33, 43, 46, 48, 57). Therefore, a series of transfection studies were next carried out to determine how Pin1-mediated changes in β-catenin affected Tcf4 versus AR activity. Consistent with previous data with HeLa cells (42), Pin1 transfection enhanced the activity of the β-catenin/Tcf-regulated pTopflash reporter gene in CV1 cells and could further enhance the stimulation by cotransfected β-catenin (Fig. 3A). As shown in Fig. 3B, AR transcriptional activity could also be markedly enhanced by β-catenin transfection. However, in marked contrast to the Pin1-mediated enhancement of pTopflash activity, Pin1 suppressed AR activity on an ARE4 reporter in the absence of transfected β-catenin and completely abolished AR coactivation by transfected β-catenin (Fig. 3B). Similar results were obtained in 293T cells using either empty pcDNA3 or pBluescript to control for total transfected DNA (Fig. 3C) or using equal molar amounts of pcDNA3 expression vectors in all samples and pBluescript to control for total DNA (Fig. 3D).
FIG. 3.
Pin1 enhances Tcf4 and suppresses AR coactivation by β-catenin. (A) CV1 cells were transfected with pTopflash (20 ng), Pin1 and β-catenin expression vectors were used as indicated (−, not used), and pRL-CMV (2.5 ng) was used as an internal control. (B) CV1 cells were transfected with ARE4-luciferase (10 ng), AR, Pin1, and β-catenin expression vectors as indicated, and pRL-CMV (2.5 ng) was used as an internal control. (C and D) 293T cells were transfected with AR (10 ng), ARE4-luciferase (10 ng), pRL-CMV (1 ng), empty pcDNA, and pBluescript as indicated. In panel D, the added empty pcDNA3 is calculated to yield equimolar amounts of pcDNA3 vector in all samples. (E and F) CV1 cells were transfected with PSA-luciferase (10 ng) (E) or ERE2-luciferase (10 ng) (F) reporters, together with AR, ERα, Pin1, and β-catenin expression vectors as indicated and pRL-CMV (2.5 ng) as an internal control. DHT or estradiol (E2) was added at a final concentration of 10 nM as indicated. Luciferase activities were determined 24 h after hormone treatment. Results are given in relative light units (RLU).
To determine whether Pin1 inhibition of AR coactivation by β-catenin was dependent on a particular promoter context, we examined a luciferase reporter regulated by the androgen-dependent promoter and enhancer from the prostate-specific antigen (PSA) gene (PSA-Luc). As observed with the ARE4 reporter, Pin1 suppressed AR activity and completely abrogated AR coactivation by transfected β-catenin (Fig. 3E). These opposite effects of Pin1 on AR and Tcf4 coactivation were not due to decreased AR protein expression and were similarly observed in transfected 293T, indicating that they were not cell type specific (data not shown). Finally, to assess whether Pin1 had a generalized inhibitory effect on steroid hormone receptors, we examined its effects on the estrogen receptor α (ERα). Consistent with previous data, β-catenin did not coactivate ERα activity on an ERE2-Luc reporter gene (Fig. 3F). Moreover, Pin1 transfection did not repress ERα transcriptional activity in the absence or presence of β-catenin. Taken together, these results indicated that the isomerization of β-catenin by Pin1 may prevent its interaction with AR.
Pin1 inhibits β-catenin interaction with the AR LBD.
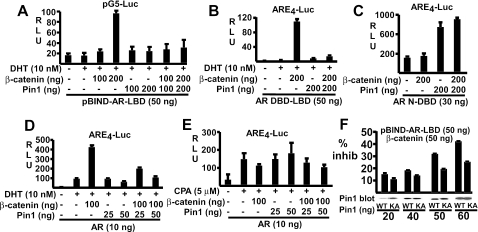
It was shown previously that β-catenin interacts with the AR LBD region, particularly helices 3, 5, 6, and 12 (46, 57). Therefore, to further test the hypothesis that Pin1 antagonizes the β-catenin-AR interaction, we examined the effect of Pin1 on β-catenin coactivation of the isolated AR LBD. The LBD was expressed as a fusion protein with the Gal4 DNA binding domain (pBIND-AR-LBD) and was tested using a Gal4-regulated luciferase reporter (pG5-Luciferase). The pBIND-AR-LBD protein had minimal transcriptional activity, consistent with previous data showing that this domain in the AR lacks a strong transactivation function (Fig. 4A). However, it could be strongly coactivated by transfection with β-catenin. As observed for the full-length AR, Pin1 did not stimulate the LBD and completely antagonized the coactivation by transfected β-catenin.
FIG. 4.
Pin1 represses β-catenin coactivation of the AR LBD but does not repress CPA-liganded AR. (A) CV1 cells were transfected with pBIND-AR-LBD (50 ng), pG5-Luciferase (10 ng), β-catenin, and Pin1 vectors as indicated (−, not used). Luciferase activities were determined 24 h after DHT treatment. (B and C) CV1 cells were transfected with AR DBD-LBD (50 ng) (B) or AR N-DBD (30 ng) (C) vectors, ARE4-luciferase reporter (10 ng), β-catenin, and Pin1 as indicated. (D and E) CV1 cells were transfected with pCIneo-AR (10 ng), ARE4-luciferase reporter (10 ng), and β-catenin and Pin1 expression vectors as indicated. Transfected cells were then treated for 24 h with DHT (D) or CPA (E). pRL-CMV (2.5 ng) was used as an internal control. (F) CV1 cells were transfected as above with pBIND-AR-LBD (50 ng), pG5-Luciferase (10 ng), β-catenin (50 ng), and wild-type (WT) or K63A (KA) mutant Pin1. The percent inhibition (inhib) of control (no Pin1) activity is shown. Pin1 immunoblots were carried out on pooled protein from the triplicate samples. For panels A to E, results are given in relative light units (RLU).
Similar results were obtained when we examined the AR DBD-LBD using an ARE4-luciferase reporter. This construct was strongly coactivated by β-catenin, and Pin1 completely abrogated this activation (Fig. 4B). As a further control, we tested the effect of Pin1 on the transcriptional activity of the AR N terminus, which harbors a strong ligand-independent activation function (termed activation function 1). As shown in Fig. 4C, β-catenin had no effect on AR activation function 1 transactivation, confirming that β-catenin does not interact directly with the AR N terminus. Importantly, AR N terminus activity was not suppressed by Pin1 but was instead enhanced. This enhancement appears to be independent of β-catenin and may reflect Pin1 effects on additional N-terminal coactivators or corepressors (25).
Previous studies have shown that cyproterone acetate (CPA) functions as an AR partial agonist, and that the CPA-liganded AR is not coactivated by β-catenin (1, 27). Therefore, if the inhibitory effect of Pin1 on the DHT-liganded AR is due to blocking β-catenin-AR interaction, then Pin1 should not antagonize the CPA-liganded AR. To test this hypothesis, CV1 cells were transfected with AR, β-catenin, and Pin1 and were then treated with DHT or CPA. Consistent with previous results, β-catenin stimulated AR activity in the presence of DHT but not CPA (Fig. 4D and E). Indeed, β-catenin had a modest inhibitory effect on the CPA-liganded AR, which likely reflected sequestration of other coactivators. Importantly, the CPA-liganded AR was not inhibited by Pin1 in the absence or presence of exogenous β-catenin (Fig. 4E).
Taken together, these data indicated that Pin1 was inhibiting the interaction between the AR LBD and β-catenin. To determine whether this inhibition was dependent on the peptidyl-prolyl isomerase activity of Pin1, we examined a previously described catalytically inactive Pin1 mutant, K63A (42). CV1 cells were transfected with the AR LBD expressed as a fusion protein with the Gal4 DNA binding domain (pBIND-AR-LBD), β-catenin, and varying amounts of wild-type or K63A (KA) mutant Pin1. As shown in Fig. 4F, the wild-type Pin1 was more active than the K63A mutant at inhibiting β-catenin coactivation of the AR LBD, although the mutant also had inhibitory activity. Immunoblotting confirmed that the proteins were expressed at comparable levels. This result supports a role for the isomerase activity, while it is not yet clear whether the inhibitory activity of the K63A mutant reflects residual enzymatic activity or β-catenin blockade by binding to the Pin1 WW domain.
AR LBD is not a direct target of Pin1.
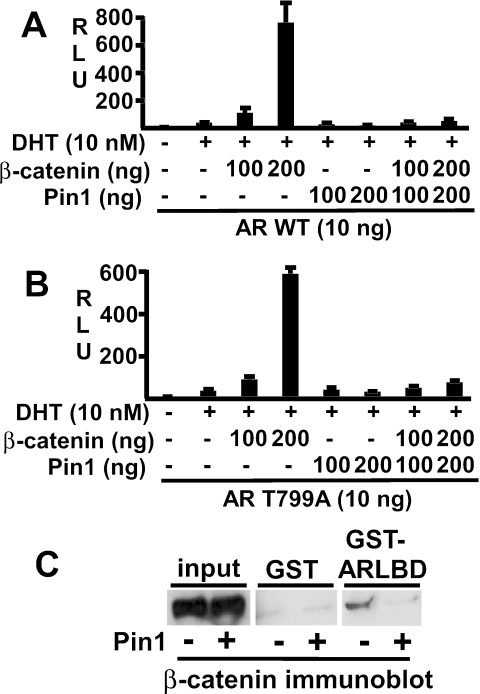
Although β-catenin has been shown to be a direct Pin1 target, it was possible that Pin1 abrogation of the AR-β-catenin interaction was due to a direct effect of Pin1 on the AR LBD. The AR LBD contains a single potential Pin1 target site, Thr799-Pro800, which lies in the kink between helices 7 and 8. The proposed Pin1 target site on β-catenin is similarly located in a kink between two helices in armadillo repeat 3 (42). Therefore, as AR is an extensively phosphorylated protein, phosphoThr799-Pro800 may serve as a Pin1 substrate. To test this hypothesis, we generated a Thr799Ala mutant AR and assessed the effects of Pin1 on this mutant versus the wild-type AR. As shown in Fig. 5A and B, the wild-type and Thr799Ala mutant AR were similarly stimulated by DHT and coactivated by β-catenin. Significantly, Pin1 suppressed the activity of the T799A mutant and abrogated its coactivation by β-catenin (Fig. 5B). These data indicate that Pin1 inhibition of the AR-β-catenin interaction is mediated through β-catenin and not by Pin1 isomerization of the AR LBD.
FIG. 5.
Inhibitory effect of Pin1 is mediated through β-catenin by disruption of its binding to the AR LBD. (A and B) CV1 cells were transfected with pRL-CMV (2.5 ng), ARE4-luciferase (10 ng), and pCIneo-AR (wild-type AR) (A), or pCIneo-AR(Thr799Ala) (10 ng) (B). Additional plasmids were cotransfected as indicated, and cells were treated with vehicle or DHT (10 nM). −, not used. Results are given in relative light units (RLU). (C) 293T cells were transfected with either 10 μg of pcDNA3.1 vector (−) or pcDNA-Pin1 (+), as indicated. Lysates were precipitated with 5 μg of GST or GST-AR LBD fusion proteins bound to glutathione-agarose beads, and bound β-catenin was determined by immunoblotting.
Pin1 inhibits β-catenin binding to the AR LBD.
The most straightforward interpretation of these results was that Pin1 abrogates β-catenin coactivation of AR by acting on β-catenin to prevent its binding to the AR LBD. To test this hypothesis, we directly examined the effects of Pin1 on β-catenin binding to the AR LBD. Cell lysates from control or Pin1-transfected 293T cells (which express substantial levels of β-catenin and can be transfected at a very high efficiency) were incubated with GST or GST-AR-LBD fusion proteins linked to glutathione-agarose beads, and bound β-catenin was detected by immunoblotting. As shown in Fig. 5C, the endogenous β-catenin bound specifically to the GST-AR LBD beads compared to the GST control beads. However, specific binding was markedly diminished when lysates from Pin1-transfected 293T cells were analyzed. These results, in conjunction with the above functional studies, indicated that Pin1 isomerization of β-catenin abrogated its coactivation of AR by inhibiting β-catenin binding to the AR LBD.
Pin1 antagonizes the inhibition of Tcf4 signaling by the DHT-liganded AR.
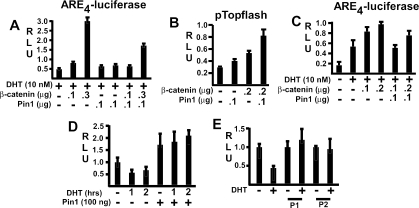
While these data showed that Pin1 could suppress β-catenin coactivation of AR activity, a function of the AR-β-catenin interaction appears to be sequestration of nuclear β-catenin and consequent inhibition of β-catenin/Tcf4 signaling (1, 10, 27, 30, 33, 43, 46). Therefore, further studies were carried out to determine the effect of Pin1 on AR inhibition of β-catenin/Tcf signaling. As has been shown previously, β-catenin strongly stimulates the Tcf-regulated pTopflash reporter, and this activity can be markedly repressed by AR in a dose- and DHT-dependent manner (Fig. 6A). This AR-mediated repression was also observed either using empty pcDNA3 or pBluescript to control for total transfected DNA (Fig. 6B) or using equal molar amounts of pcDNA3 expression vectors and pBluescript to control for total DNA (Fig. 6C). Similar to the effects of β-catenin transfection, the pTopflash reporter was activated by the Pin1-mediated increase in β-catenin (Fig. 6D). However, in this case, the AR inhibition of pTopflash activity was markedly diminished. These results indicated that Pin1 could enhance β-catenin/Tcf signaling in AR-expressing cells by preventing β-catenin sequestration by AR.
FIG. 6.
Pin1 antagonizes the inhibition of Tcf4 signaling by the DHT-liganded AR. (A to D) 293T cells were transfected with pRL-CMV (1 or 2.5 ng), pTopflash (20 ng), pCIneo-AR, and β-catenin expression vectors as indicated (+, used; −, not used). Total DNA was normalized using empty pcDNA3 in panel A, pBluescript or empty pcDNA3 in panel B, and a mixture of empty pcDNA3 and pBluescript in panel C (with the amount of pcDNA3 adjusted to be equimolar in each sample). Results are given in relative light units (RLU). (E to G) 293T cells were transfected with 3 μg of β-catenin and Tcf4 plasmids in every case, 3 μg of AR as indicated, and 3 or 9 μg of Pin1 vector as indicated. pcDNA3.1 vector was used to equalize the total plasmid amount. Cell lysates were precleared and then immunoprecipitated (IP) with control nonimmune mouse serum (E) or mouse anti-Tcf4 antibody (F), followed by immunoblotting for β-catenin. The position of β-catenin is indicated with an arrow, while the lower band (*) is an immunoglobulin (IgG) dimer present in the anti-Tcf4 antibody preparation that is recognized by the secondary anti-mouse antibody alone (not shown). (G) Inputs (1%) for the indicated proteins.
Coimmunoprecipitation experiments were next carried out to directly test the hypothesis that AR can sequester β-catenin from Tcf4 and that this action can be blocked by Pin1. Lysates from transfected 293T cells were immunoprecipitated with anti-Tcf4 or control antibodies and then immunoblotted to detect Tcf4-associated β-catenin. In cells transfected with Tcf4 alone, β-catenin was coimmunoprecipitated by anti-Tcf4 but not the control antibody (Fig. 6E and F, lane 1). In contrast, AR cotransfection caused a marked decrease in the amount of Tcf4-associated β-catenin (Fig. 6F, lane 2). Although there was also a small decrease in the level of total β-catenin and Tcf4 (Fig. 6G), this result provided direct evidence for AR sequestration of β-catenin. Importantly, cotransfection of Pin1 with AR restored the coimmunoprecipitation of β-catenin by anti-Tcf4 (Fig. 6F, lanes 3 and 4). Moreover, this was not due to an increase in Tcf4 or β-catenin or due to a decrease in AR (Fig. 6E). Taken together, these biochemical studies and the above functional data showed that Pin1 can prevent AR-mediated repression of β-catenin/Tcf4 signaling by abrogating AR binding to β-catenin.
Pin1 antagonizes β-catenin coactivation of endogenous AR in PCa cells.
We next determined whether Pin1 would prevent β-catenin coactivation of the endogenous AR in PCa cells. As shown in LNCaP PCa cells, AR transactivation of an ARE reporter could be stimulated by β-catenin, and this stimulation was antagonized by cotransfection with Pin1 (Fig. 7A). Similar results were obtained using another unrelated PCa cell line, CWR22D1, which was derived from the CWR22 xenograft and is not PTEN deficient. Pin1 transfection in the CWR22D1 cells could stimulate the pTopflash reporter and further enhanced the activation of pTopflash by cotransfected β-catenin (Fig. 7B). In contrast, Pin1 transfection antagonized β-catenin coactivation of the endogenous AR in these cells (Fig. 7C). It should be noted that the CWR22 AR, like the LNCaP AR, has a mutation in the LBD (H874Y in CWR22 versus T877A in LNCaP), but these results indicate that the mutants and the wild-type AR interact similarly with β-catenin.
FIG. 7.
Pin1 expression in PCa cells antagonizes β-catenin coactivation of AR and prevents AR-mediated suppression of β-catenin/Tcf4 activity. (A) LNCaP cells were transfected with ARE4-luciferase reporter (100 ng), pRL-CMV control (2.5 ng), β-catenin, and Pin1 expression vectors for 24 h as indicated (+, used; −, not used). Cells were then stimulated with DHT (10 nM) for 24 h, and firefly versus Renilla luciferase activities were determined. (B) CWR22D1 cells in medium with 10% FBS were transfected with pTopflash (20 ng), pRL-CMV (2.5 ng), β-catenin, and Pin1 plasmids as indicated for 24 h and assayed after another 24 h as described for panel A. (C) CWR22D1 cells were transfected and treated as described for panel A. (D) LNCaP cells were transfected with pTopflash reporter (50 ng), pCMV-RL (2.5 ng), and Pin1 (10 ng) expression vectors for 24 h as indicated, followed by another 24 h in steroid hormone-depleted medium. They were then stimulated for 1 or 2 h with 10 nM DHT and assayed for luciferase versus Renilla activity. (E) Control or stable Pin1-expressing LNCaP cells (P1 and P2) were transfected with pTopflash (50 ng) and pCMV-RL (2.5 ng) vectors for 24 h, and luciferase versus Renilla activities were determined after another 24 h. Results are given in relative light units (RLU).
Finally, we examined LNCaP cells to determine whether Pin1 could abrogate β-catenin inhibition by the endogenous AR in PTEN-deficient PCa cells. LNCaP cells were transfected with the pTopflash reporter, minus or plus Pin1, and activity of the pTopflash reporter in response to DHT was assessed. Treatment with DHT caused a rapid decline in pTopflash activity, and this inhibition was completely prevented by Pin1 (Fig. 7D). Similar results were observed in LNCaP cells stably transfected with Pin1. In control vector-transfected cells, pTopflash activity was repressed by DHT. In contrast, there was no inhibition in Pin1-expressing clones (Fig. 7E). These results confirmed β-catenin inhibition by endogenous AR in PCa cells and showed that abrogation of this inhibition is a mechanism by which Pin1 can enhance β-catenin/Tcf4 activity in PCa.
DISCUSSION
The increased expression of β-catenin plays a major role in many cancers, but its contribution to PCa and role of the AR-β-catenin interaction have not been clear. Previous transient-transfection studies using Tcf4-regulated reporter genes have indicated that a function of the AR-β-catenin interaction may be to sequester limited nuclear β-catenin and thereby suppress β-catenin/Tcf4 signaling (10, 30, 46). Pin1 has been shown to stabilize β-catenin in cells with active APC/GSK-3β-mediated β-catenin degradation, and increased Pin1 expression in radical prostatectomy specimens has been correlated with greater risk of PCa recurrence (2, 42). This study found that Pin1 was markedly increased in advanced metastatic PCa and therefore assessed increased Pin1 as a mechanism for enhanced β-catenin expression and function in PCa (and specifically in PTEN-deficient PCa cells). Pin1 expression enhanced β-catenin/Tcf4 signaling in LNCaP cells, and stable expression of Pin1 in LNCaP transfectants markedly enhanced tumor growth in immunodeficient mice. However, consistent with PTEN loss and the constitutive suppression of GSK-3β activity in these cells, increased Pin1 did not increase β-catenin levels (34, 44). Instead, Pin1 abrogated the AR-β-catenin interaction and suppressed the ability of AR to antagonize β-catenin/Tcf4 activity. Taken together, these data indicate that Pin1 can stimulate β-catenin/Tcf4 signaling in PCa, including PTEN-deficient prostate cancers, by abrogating AR-mediated suppression of β-catenin function. These results demonstrate roles for Pin1 and β-catenin in PCa progression and support a physiological role for the AR-β-catenin interaction in suppressing β-catenin/Tcf4 signaling.
The hypothesis that Pin1 augments β-catenin/Tcf4 signaling in PCa was supported by increased expression of c-myc, an endogenous β-catenin/Tcf4 target gene, in LNCaP cell lines stably transfected with Pin1. Significantly, the Pin1 stable LNCaP cell lines also had increased expression of Tcf4. The relatively low levels of endogenous Tcf4 expression in LNCaP cells as well as weak β-catenin/Tcf4 signaling, as assessed by transfection with the Tcf-regulated pTopflash reporter, have been noted previously (10). As Tcf4 functions as a strong transcriptional repressor in the absence of nuclear β-catenin through recruitment of the Grouch/TLE family of corepressor proteins, there is presumably selective pressure to keep its level low in the absence of coactivation by nuclear β-catenin. Conversely, the increased availability of nuclear β-catenin in Pin1-expressing LNCaP cells likely selects for cells with increased Tcf4 levels, which can take advantage of the increased β-catenin to enhance expression of β-catenin/Tcf4-regulated genes such as c-myc.
Transient-transfection assays showed that Pin1 prevented β-catenin coactivation of the isolated AR LBD but did not repress the isolated AR N terminus or the CPA-liganded full-length AR (which does not recruit β-catenin). These results indicated that the Pin1-mediated isomerization of β-catenin, which blocks its interaction with APC, was similarly preventing β-catenin interaction with the AR LBD. This interpretation was supported by decreased inhibitory activity of a catalytically inactive Pin1 mutant and by site-directed mutagenesis to remove the single potential Pin1 recognition site in the AR LBD, as this did not prevent Pin1-mediated abrogation of the β-catenin-AR interaction. Direct binding studies further confirmed that Pin1 could prevent β-catenin binding to the AR LBD. Finally, β-catenin/Tcf4 coimmunoprecipitation experiments showed directly that AR could suppress β-catenin association with Tcf4 and that this suppression could be abrogated by Pin1. Interestingly, the AR may also interact with a number of other Pin1 target proteins (including c-Jun, cyclin D1, and p53), suggesting that Pin1 may further regulate AR function through modulation of interactions with additional proteins.
The WW domain of Pin1 recruits this enzyme to pSer/pThr-Pro motifs, and proline isomerization at these sites can both regulate dephosphorylation and alter interactions with other proteins (60). Pin1 appears to bind to a pSer-Pro site in the third Armadillo repeat of β-catenin, and mutation in this serine (Ser246) can block the ability of Pin1 to prevent β-catenin-APC binding in vitro (42). The site on β-catenin that mediates AR binding is within the first six Armadillo repeats, indicating that Pin1 may abrogate β-catenin binding to APC and AR by altering the same site (57). Efforts have been made to directly test this hypothesis using a previously described β-catenin Ser246Ala mutant, but this mutant is expressed at extremely low levels in transient transfections and does not yield any detectable coactivation of AR or Tcf4 (data not shown) (42). Therefore, it is not yet clear whether Pin1 modulates β-catenin binding to APC and AR via the same or distinct sites or whether different kinases regulate Pin1 recognition of these sites. It should also be noted that further direct or indirect effects of Pin1 on AR are also possible, based on Pin1 suppression of AR activity in the absence of exogenous β-catenin (although this may in part reflect isomerization of endogenous β-catenin) and augmentation of the isolated AR N terminus.
The levels of total and nuclear β-catenin are tightly regulated by binding to APC, which mediates GSK-3β-dependent degradation of β-catenin and can also stimulate its nuclear export (16, 31, 36, 39-41). Therefore, although Pin1-mediated abrogation of β-catenin binding to APC does not increase β-catenin stability in PTEN-deficient PCa cells, it may nonetheless further increase β-catenin/Tcf4 activity by decreasing the nuclear export of β-catenin. Indeed, immunofluorescence studies indicate that Pin1 can cause a relative increase in the levels of nuclear β-catenin in LNCaP cells (data not shown). In support of the hypothesis that APC may continue to mediate nuclear export of β-catenin in advanced PCa, loss of heterozygosity in the APC locus, hypermethylation of the APC promoter, and APC mutations have been reported in PCa and may correlate with more advanced disease (6, 15, 19, 26, 35, 58).
In a previous study we found a correlation between AR ligands that support β-catenin binding and stimulate LNCaP cell growth and suggested that AR recruitment of β-catenin may be necessary to stimulate the expression of one or more growth-promoting genes (27). In contrast, this study shows that the β-catenin-AR interaction can function to suppress β-catenin/Tcf4 signaling and tumorigenesis. Taken together, these findings suggest that the β-catenin-AR interaction may have a dual function. AR coactivation and stimulation of growth-promoting genes may predominate in cells with active β-catenin degradation, while AR sequestration of β-catenin may play an important role in suppressing the tumorigenic activity of excess free nuclear β-catenin in cells with physiological active Wnt signaling or pathological loss of regulated β-catenin degradation. In the latter cases, increased Pin1 expression would abrogate the AR sequestration of β-catenin and contribute to tumor progression.
In summary, these studies indicate that Pin1 contributes to the development of aggressive PCa by abrogating the AR-β-catenin interaction and thereby increasing β-catenin coactivation of Tcf4 and expression of Tcf4-regulated genes. These findings also strongly support a physiological role for AR in the negative regulation of β-catenin/Tcf4 signaling. Importantly, this may provide a rationale for the early use of intermittent androgen ablation therapy to suppress β-catenin function and suggests that this therapy may eventually fail in part due to increased Pin1 expression. Finally, this study indicates that drugs targeting Pin1 or selective AR antagonists that maintain or enhance AR-β-catenin binding may be more effective than conventional androgen ablation therapies in a subset of PCa patients.
Acknowledgments
We thank Lirim Shemshedini for plasmids, Xin Yuan for the CWR22D1 cells, Victoria Petkova for real-time RT-PCR, Meredith Regan for statistical analyses, Nicole McKnight for GST pull downs, Mike Stanbrough for help with figures, Eunis Choi for technical assistance, and Akihide Ryo for helpful discussions.
This work was supported by grants from the NIH (DK61047 to S.P.B., GM58556 to K.P.L., K08-CA093655 to G.W., CA022082 to X.Z.Z.), the Dana Farber/Harvard Cancer Center Prostate SPORE, and the Hershey Family Prostate Cancer Research Fund. S.-Y.C. was supported by a DOD Prostate Cancer Postdoctoral Award (PC040499). K.P.L. is a Pew Scholar and a Leukemia and Lymphoma Society Scholar.
REFERENCES
- 1.Amir, A. L., M. Barua, N. C. McKnight, S. Cheng, X. Yuan, and S. P. Balk. 2003. A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4. J. Biol. Chem. 278:30828-30834. [DOI] [PubMed] [Google Scholar]
- 2.Ayala, G., D. Wang, G. Wulf, A. Frolov, R. Li, J. Sowadski, T. M. Wheeler, K. P. Lu, and L. Bao. 2003. The prolyl isomerase Pin1 is a novel prognostic marker in human prostate cancer. Cancer Res. 63:6244-6251. [PubMed] [Google Scholar]
- 3.Bao, L., A. Kimzey, G. Sauter, J. M. Sowadski, K. P. Lu, and D. G. Wang. 2004. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, N., G. Huls, V. Korinek, and H. Clevers. 1999. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am. J. Pathol. 154:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brantjes, H., J. Roose, M. van De Wetering, and H. Clevers. 2001. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 29:1410-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster, S. F., S. Browne, and K. W. Brown. 1994. Somatic allelic loss at the DCC, APC, nm23-H1 and p53 tumor suppressor gene loci in human prostatic carcinoma. J. Urol. 151:1073-1077. [DOI] [PubMed] [Google Scholar]
- 7.Cantley, L. C., and B. G. Neel. 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96:4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesire, D. R., C. M. Ewing, W. R. Gage, and W. B. Isaacs. 2002. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene 21:2679-2694. [DOI] [PubMed] [Google Scholar]
- 9.Chesire, D. R., C. M. Ewing, J. Sauvageot, G. S. Bova, and W. B. Isaacs. 2000. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate 45:323-334. [DOI] [PubMed] [Google Scholar]
- 10.Chesire, D. R., and W. B. Isaacs. 2002. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene 21:8453-8469. [DOI] [PubMed] [Google Scholar]
- 11.De la Taile, A., M. A. Rubin, M. W. Chen, F. Vacherot, S. G. De Medina, M. Burchardt, R. Buttyan, and D. Chopin. 2003. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin. Cancer Res. 9:1801-1807. [PubMed] [Google Scholar]
- 12.Deocampo, N. D., H. Huang, and D. J. Tindall. 2003. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 28:145-153. [PubMed] [Google Scholar]
- 13.Easwaran, V., M. Pishvaian, Salimuddin, and S. Byers. 1999. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr. Biol. 9:1415-1418. [DOI] [PubMed] [Google Scholar]
- 14.Gelmann, E. P. 2002. Molecular biology of the androgen receptor. J. Clin. Oncol. 20:3001-3015. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein, A. V., T. A. Almeida, G. Zhao, E. Chess, I. Shih, K. Buhler, K. Pienta, M. A. Rubin, R. Vessella, and N. Papadopoulos. 2002. APC/CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer 34:9-16. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, B. R. 2000. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat. Cell Biol. 2:653-660. [DOI] [PubMed] [Google Scholar]
- 17.Hurlstone, A., and H. Clevers. 2002. T-cell factors: turn-ons and turn-offs. EMBO J. 21:2303-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, D. E., S. Soriano, X. Xia, C. G. Eberhart, B. De Strooper, H. Zheng, and E. H. Koo. 2002. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell 110:751-762. [DOI] [PubMed] [Google Scholar]
- 19.Kang, G. H., S. Lee, H. J. Lee, and K. S. Hwang. 2004. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J. Pathol. 202:233-240. [DOI] [PubMed] [Google Scholar]
- 20.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 21.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 22.Lu, K. P. 2003. Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 4:175-180. [DOI] [PubMed] [Google Scholar]
- 23.Lu, K. P., S. D. Hanes, and T. Hunter. 1996. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380:544-547. [DOI] [PubMed] [Google Scholar]
- 24.Lu, P. J., X. Z. Zhou, M. Shen, and K. P. Lu. 1999. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283:1325-1328. [DOI] [PubMed] [Google Scholar]
- 25.Markus, S. M., S. S. Taneja, S. K. Logan, W. Li, S. Ha, A. B. Hittelman, I. Rogatsky, and M. J. Garabedian. 2002. Identification and characterization of ART-27, a novel coactivator for the androgen receptor N terminus. Mol. Biol. Cell 13:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruyama, R., S. Toyooka, K. O. Toyooka, A. K. Virmani, S. Zochbauer-Muller, A. J. Farinas, J. D. Minna, J. McConnell, E. P. Frenkel, and A. F. Gazdar. 2002. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 8:514-519. [PubMed] [Google Scholar]
- 27.Masiello, D., S. Y. Chen, Y. Xu, M. C. Verhoeven, E. Choi, A. N. Hollenberg, and S. P. Balk. 2004. Recruitment of beta-catenin by wild type or mutant androgen receptors correlates with ligand stimulated growth of prostate cancer cells. Mol. Endocrinol. 18:2388-2401. [DOI] [PubMed] [Google Scholar]
- 28.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 29.Mulholland, D. J., H. Cheng, K. Reid, P. S. Rennie, and C. C. Nelson. 2002. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J. Biol. Chem. 277:17933-17943. [DOI] [PubMed] [Google Scholar]
- 30.Mulholland, D. J., J. T. Read, P. S. Rennie, M. E. Cox, and C. C. Nelson. 2003. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis. Oncogene 22:5602-5613. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld, K. L., F. Zhang, B. R. Cullen, and R. L. White. 2000. APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 1:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer, H. G., J. M. Gonzalez-Sancho, J. Espada, M. T. Berciano, I. Puig, J. Baulida, M. Quintanilla, A. Cano, A. G. de Herreros, M. Lafarga, and A. Munoz. 2001. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 154:369-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlowski, J. E., J. R. Ertel, M. P. Allen, M. Xu, C. Butler, E. M. Wilson, and M. E. Wierman. 2002. Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 277:20702-20710. [DOI] [PubMed] [Google Scholar]
- 34.Persad, S., A. A. Troussard, T. R. McPhee, D. J. Mulholland, and S. Dedhar. 2001. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol. 153:1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips, S. M., D. G. Morton, S. J. Lee, D. M. Wallace, and J. P. Neoptolemos. 1994. Loss of heterozygosity of the retinoblastoma and adenomatous polyposis susceptibility gene loci and in chromosomes 10p, 10q and 16q in human prostate cancer. Br. J. Urol. 73:390-395. [DOI] [PubMed] [Google Scholar]
- 36.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 37.Ranganathan, R., K. P. Lu, T. Hunter, and J. P. Noel. 1997. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89:875-886. [DOI] [PubMed] [Google Scholar]
- 38.Rippmann, J. F., S. Hobbie, C. Daiber, B. Guilliard, M. Bauer, J. Birk, H. Nar, P. Garin-Chesa, W. J. Rettig, and A. Schnapp. 2000. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell Growth Differ. 11:409-416. [PubMed] [Google Scholar]
- 39.Rosin-Arbesfeld, R., A. Cliffe, T. Brabletz, and M. Bienz. 2003. Nuclear export of the APC tumour suppressor controls beta-catenin function in transcription. EMBO J. 22:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosin-Arbesfeld, R., F. Townsley, and M. Bienz. 2000. The APC tumour suppressor has a nuclear export function. Nature 406:1009-1012. [DOI] [PubMed] [Google Scholar]
- 41.Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272:1023-1026. [DOI] [PubMed] [Google Scholar]
- 42.Ryo, A., M. Nakamura, G. Wulf, Y. C. Liou, and K. P. Lu. 2001. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 3:793-801. [DOI] [PubMed] [Google Scholar]
- 43.Shah, S., A. Hecht, R. Pestell, and S. W. Byers. 2003. Trans-repression of beta-catenin activity by nuclear receptors. J. Biol. Chem. 278:48137-48145. [DOI] [PubMed] [Google Scholar]
- 44.Sharma, M., W. W. Chuang, and Z. Sun. 2002. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 277:30935-30941. [DOI] [PubMed] [Google Scholar]
- 45.Shen, M., P. T. Stukenberg, M. W. Kirschner, and K. P. Lu. 1998. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 12:706-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song, L. N., R. Herrell, S. Byers, S. Shah, E. M. Wilson, and E. P. Gelmann. 2003. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol. Cell. Biol. 23:1674-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 48.Truica, C. I., S. Byers, and E. P. Gelmann. 2000. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 60:4709-4713. [PubMed] [Google Scholar]
- 49.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 50.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 51.Voeller, H. J., C. I. Truica, and E. P. Gelmann. 1998. Beta-catenin mutations in human prostate cancer. Cancer Res. 58:2520-2523. [PubMed] [Google Scholar]
- 52.Wong, N. A., and M. Pignatelli. 2002. Beta-catenin-a linchpin in colorectal carcinogenesis? Am. J. Pathol. 160:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wulf, G., A. Ryo, Y. C. Liou, and K. P. Lu. 2003. The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res. 5:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wulf, G. M., Y. C. Liou, A. Ryo, S. W. Lee, and K. P. Lu. 2002. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J. Biol. Chem. 277:47976-47979. [DOI] [PubMed] [Google Scholar]
- 55.Wulf, G. M., A. Ryo, G. G. Wulf, S. W. Lee, T. Niu, V. Petkova, and K. P. Lu. 2001. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 20:3459-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaffe, M. B., M. Schutkowski, M. Shen, X. Z. Zhou, P. T. Stukenberg, J. U. Rahfeld, J. Xu, J. Kuang, M. W. Kirschner, G. Fischer, L. C. Cantley, and K. P. Lu. 1997. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278:1957-1960. [DOI] [PubMed] [Google Scholar]
- 57.Yang, F., X. Li, M. Sharma, C. Y. Sasaki, D. L. Longo, B. Lim, and Z. Sun. 2002. Linking beta-catenin to androgen-signaling pathway. J. Biol. Chem. 277:11336-11344. [DOI] [PubMed] [Google Scholar]
- 58.Yegnasubramanian, S., J. Kowalski, M. L. Gonzalgo, M. Zahurak, S. Piantadosi, P. C. Walsh, G. S. Bova, A. M. De Marzo, W. B. Isaacs, and W. G. Nelson. 2004. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 64:1975-1986. [DOI] [PubMed] [Google Scholar]
- 59.Yu, X., P. Li, R. G. Roeder, and Z. Wang. 2001. Inhibition of androgen receptor-mediated transcription by aminoterminal enhancer of split. Mol. Cell. Biol. 21:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, X. Z., O. Kops, A. Werner, P. J. Lu, M. Shen, G. Stoller, G. Kullertz, M. Stark, G. Fischer, and K. P. Lu. 2000. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell 6:873-883. [DOI] [PubMed] [Google Scholar]