Abstract
Activation of Akt, or protein kinase B, is frequently observed in human cancers. Here we report that Akt activation via overexpression of a constitutively active form or via the loss of PTEN can overcome a G2/M cell cycle checkpoint that is induced by DNA damage. Activated Akt also alleviates the reduction in CDC2 activity and mitotic index upon exposure to DNA damage. In addition, we found that PTEN null embryonic stem (ES) cells transit faster from the G2/M to the G1 phase of the cell cycle when compared to wild-type ES cells and that inhibition of phosphoinositol-3-kinase (PI3K) in HEK293 cells elicits G2 arrest that is alleviated by activated Akt. Furthermore, the transition from the G2/M to the G1 phase of the cell cycle in Akt1 null mouse embryo fibroblasts (MEFs) is attenuated when compared to that of wild-type MEFs. These results indicate that the PI3K/PTEN/Akt pathway plays a role in the regulation of G2/M transition. Thus, cells expressing activated Akt continue to divide, without being eliminated by apoptosis, in the presence of continuous exposure to mutagen and accumulate mutations, as measured by inactivation of an exogenously expressed herpes simplex virus thymidine kinase (HSV-tk) gene. This phenotype is independent of p53 status and cannot be reproduced by overexpression of Bcl-2 or Myc and Bcl-2 but seems to counteract a cell cycle checkpoint mediated by DNA mismatch repair (MMR). Accordingly, restoration of the G2/M cell cycle checkpoint and apoptosis in MMR-deficient cells, through reintroduction of the missing component of MMR, is alleviated by activated Akt. We suggest that this new activity of Akt in conjunction with its antiapoptotic activity may contribute to genetic instability and could explain its frequent activation in human cancers.
Akt, or protein kinase B (PKB), is a serine/threonine kinase that has been implicated in the control of major cellular functions such as transcription, protein synthesis, and carbohydrate and lipid metabolism, and it is a downstream effector of growth factor-mediated cell survival. Normally, Akt is activated by growth factors that activate phosphoinositol-3-kinase (PI3K). Upon activation, PI3K phosphorylates the inositol ring at the D3 position, which in turn serves to anchor Akt to the plasma membrane, where it is phosphorylated and fully activated by the 3-phosphoinositide-dependent kinases PDK1 and PDK2. Phospholipid phosphatases such as PTEN and SHIP decrease the pool of available phospholipids and therefore are negative regulators of Akt. Activated Ras, at least in certain circumstances, can up-regulate PI3K and therefore is a potential activator of Akt as well (13, 22). Overall, positive regulators of Akt are commonly up-regulated in human cancers, while PTEN is frequently lost or inactivated by mutations (7, 36). Furthermore, heterozygous deletion of PTEN in mice elicits a wide range of spontaneous tumors; this has been attributed mainly to activation of Akt (12, 34, 38). Finally, activated forms of Akt induce cellular transformation (4). Taken together, these observations suggest that Akt activation plays a potent role in the genesis of cancer which is unlikely to be explained solely by the antiapoptotic properties of this kinase. We therefore decided to investigate further whether Akt activation also affects cellular responses to DNA damage and cell cycle checkpoints induced by DNA damage.
MATERIALS AND METHODS
Cell culture and retrovirus infection.
Rat1a cells are immortal nontransformed rat embryo fibroblasts that are susceptible to oncogenic transformation and exhibit serum- and Akt-dependent susceptibility to a variety of apoptotic stimuli. HCT116 cells are human colon carcinoma cells that are deficient in mismatch repair (MMR) function. The HCT116-ch3 cell line was derived from HCT116 by the introduction of normal human chromosome 3, which restores MMR in these cells. HCT116 and HCT116-ch3 were a gift of C. R. Boland. Wild-type and PTEN-deficient embryonic stem (ES) cells were cultured on a monolayer of lethally irradiated mouse embryo fibroblasts (MEFs). The generation of Akt1 null MEFs has been previously described (9). Simian virus 40 (SV40)-immortalized MEFs were generated by SV40 infection of wild-type and Akt1 null MEFs prepared from 13.5-day embryos of littermates. All cell lines were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin, at 37°C in 5% CO2.
Retroviral transduction was performed using Phoenix packaging cell lines, as described earlier (23). Routinely, 104 to 105 infected clones were obtained upon appropriate selection. The cells were pooled, expanded, and maintained as polyclonal populations.
Rat1a derivatives with ectopic expression of c-Myc and Bcl-2, Bcl-2, Bcl-XL, and MyrAkt or tetracycline-regulated expression of MyrAkt were described earlier (16, 23). Constitutive expression of MyrAkt was achieved via infection with SRα-MyrAkt, pBabePuroMyrAkt, and pBabeEGFPMyrAkt retroviruses, followed by selection for neomycin resistance, puromycin resistance, and green fluorescence, respectively. Qualitatively similar results were obtained with all three vector systems. GSE56 in pLXSN was a gift of Andrei Gudkov. The generation of polyclonal HEK293 cells expressing MyrAkt has been previously described (15).
To generate polyclonal HCT116 and HCT116-ch3 cell lines expressing MyrAkt, the cells were infected with pBabePuroMyrAkt amphotropic retrovirus and pBabePuro amphotropic retrovirus as a control. Following infection, the cells were subjected to puromycin selection and several hundred puromycin- resistant clones were collected to generate the polyclonal cell lines. Expression of Akt was assessed by Western blot analysis using anti-Akt antibodies (Abs) (Cell Signaling Technology).
For the studies of DNA damage responses in Rat1a and HCT116 cell lines, the cells were seeded a day before treatment at 5 to 10% confluence with an equal number of cells for all the treated populations. 6-Thioguanine (6-TG) was prepared as a 2 mM stock in 0.1 M NaOH and added to the growth medium as indicated. Gamma irradiation was performed using a J. L. Shepherd and Associates irradiator, according to the manufacturer's recommendations. Medium was changed following irradiation, and cells were allowed to recover as indicated.
For colony formation assays, cells were washed with phosphate-buffered saline (PBS), fixed with methanol, and stained with crystal violet or methylene blue (2% solution of either dye in 50% methanol).
Cell cycle analysis.
Combined detection of bromodeoxyuridine (BrdU) incorporation and DNA content using propidium iodide (PI) was performed, using CellQuest software on a FACSort flow cytometer (Becton Dickinson) and a BrdU flow kit (Pharmingen) according to the manufacturers' recommendations. For irradiation experiments, exponentially grown ES cells were pulse labeled with BrdU for 45 min, trypsinized, and gamma irradiated with 10 Gy prior to plating onto a fresh monolayer of lethally irradiated MEFs. Cells were harvested for the analysis at indicated time points. For the study of cell cycle progression in untreated cells, wild-type and PTEN−/− ES cell cultures were pulse labeled with BrdU and collected for flow cytometry as indicated. BrdU labeling allowed us to exclude MEFs from the analysis and to examine synphasic populations of wild-type and PTEN−/− ES cells without performing physical synchronization. For an S-phase block of HEK293 cells, 2.5 × 105 cells/6-cm-diameter dish were plated and allowed to attach overnight. The cells were then cultured in 0.1% FBS for 48 h, and the medium was replaced with Dulbecco's modified Eagle's medium containing 10% FBS and 5 μg of aphidicoline/ml. After 24 h, the cells were washed twice with PBS and were released in 10% FBS.
For cell cycle analysis of BrdU-labeled SV40-immortalized MEFs, about 2 × 106 proliferating cells were pulse labeled with BrdU for 45 min. Following the pulse labeling, cells were harvested at different time points for analysis of BrdU incorporation and DNA content.
For routine cell cycle analysis, the suspension in 0.3 ml of PBS was fixed by drop-wise addition to ice-cold 70% ethanol and was stored at 4°C. Subsequently, cells were pelleted by centrifugation and resuspended in staining solution containing 40 μg of PI/ml and 100 μg of RNase A/ml. After 60 min of incubation at 37°C, the cell suspension was passed through a 35-μm-pore-size cell strainer (Becton Dickinson) and flow cytometry was performed using a Becton Dickinson FACSort flow cytometer. The data was analyzed using CellQuest and ModFit software (Becton Dickinson). For the purpose of analysis, acquired events were gated to eliminate cell aggregates and debris.
Mitotic indices.
For the measurement of mitotic indices, 6-TG-treated, gamma-irradiated, and untreated Rat1a cells with or without MyrAkt were incubated in the presence of 250 nM nocodazole, fixed at indicated times in paraformaldehyde (4% final concentration), and stained with phospho-histone H3 Ab (UBI) and tetramethyl rhodamine isothiocyanate-labeled anti-rabbit secondary Ab (Pharmingen). The percentage of mitotic cells was quantified by scoring at least 300 cells from four random fields.
CDC2 activity.
For the determination of CDC2 kinase activity, the enzyme was immunoprecipitated from 500 μg of total protein, using the anti-CDC2 Ab C-19 (Santa Cruz). Immunoprecipitated protein was used for an in vitro kinase assay, with histone H1 as a substrate. Kinase activity was normalized for the amount of CDC2 in the immunoprecipitates, as detected by Western blotting with C-19.
Mutation analysis.
Mutations in the herpes simplex virus thymidine kinase 1 (HSV-tk) gene were estimated from the frequency of ganciclovir (GCV)-resistant colonies among cells transduced with the HSV-tk gene. The HSV-tk gene was introduced into pLXSE retroviral vectors that coexpress enhanced green fluorescent protein, and single-cell clones were established by flow sorting, as described earlier (21). The number of GCV-resistant clones was determined by plating 5 × 106 cells in 10 μg of the drug/ml and scoring colonies 2 to 3 weeks later.
RESULTS AND DISCUSSION
Activated Akt overrides a G2/M cell cycle checkpoint induced by 6-TG and gamma irradiation.
Polyclonal Rat1a cells that express an activated Akt (MyrAkt) were compared to cells of the same origin transduced with an empty control vector. Both isogenic polyclonal cell lines were subjected to treatment with 6-TG. The guanine analog 6-TG is efficiently incorporated into DNA. Subsequently, it may base pair with either cytidine or thymidine and thus elicits lesions that are recognized by the DNA MMR machinery (18). Incorporation of 6-TG is known to trigger growth arrest in the S and G2 phases of the cell cycle, as well as the onset of cell death, which is mediated by the MMR (5, 11, 19, 39).
Exposure of Rat1a cells for 3 or more days to 6-TG in concentrations as low as 1 μM caused significant G2 arrest. However, this G2 arrest was diminished in cells expressing activated Akt, which showed a significant decrease of accumulation in G2 (P = 0.02 for G2/G1 ratios, using the paired t test) (Fig. 1A to C).
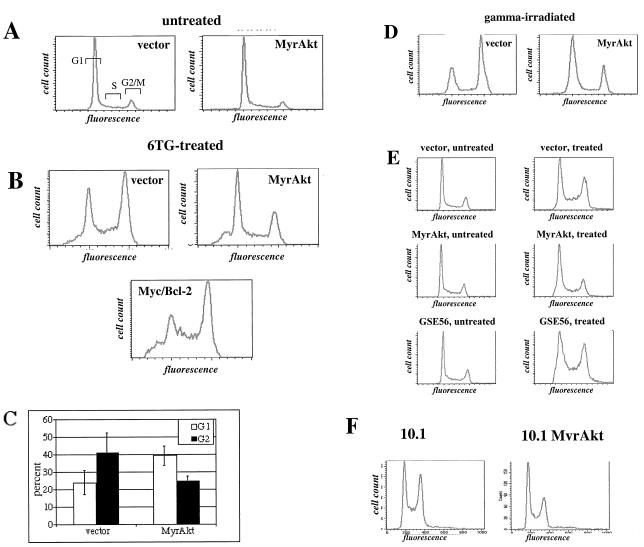
FIG. 1.
Activated Akt attenuates G2 arrest in Rat1a cells following DNA damage. (A to D) Cell cycle evaluation of Akt effects. Cell cycle profiles of untreated Rat1a cells transduced with activated Akt (MyrAkt) or an empty control vector (A) and Rat1a cells treated for 4 days with 2 μM 6-TG and transduced with activated Akt (MyrAkt) or Myc plus Bcl-2 (Myc/Bcl-2) or an empty control vector (B). (C) Summary of three independent experiments performed as described for panel B. The percentages of cells in G1 and G2 are shown. (D) Twelve hours after 7-Gy gamma irradiation. Cells were PI stained and analyzed by flow cytometry. (E) The effect of activated Akt is not reestablished by the presence of dominant-negative p53. Rat1a cells transduced with an empty vector (upper panels) or activated Akt (middle panels) or dominant-negative p53 fragment (GSE56) (bottom panels) were either left untreated or treated with 2 μM 6-TG and analyzed by flow cytometry upon PI staining. (F) Activated Akt attenuates accumulation of 10.1 3T3 cells in the G2 phase of the cell cycle. 10.1 3T3 cells and 10.1 3T3 cells expressing activated Akt were exposed to 2 μM 6-TG as described for panels A to E and analyzed by flow cytometry upon PI staining.
We also subjected the MyrAkt-expressing Rat1a cells and control Rat1a cells to gamma irradiation. Rat1 cells are deficient in the G1 checkpoint, due to the hypermethylation of the cell cycle kinase inhibitor p21cip1/waf1 promoter (2), and thus, these cells respond to gamma irradiation predominantly by a transient G2/M arrest. As shown in Fig. 1D, accumulation of cells in G2/M following gamma irradiation is significantly reduced by expression of activated Akt. Apparently, like 6-TG, gamma irradiation can also induce a transient G2 arrest that can be mediated by MMR, as MMR deficiency is capable of alleviating postirradiation G2 arrest (11, 19). Similarly, we observed abolition of G2/M arrest by activated Akt following exposure to the methylating agent nitroso-methyl urea (E. S. Kandel and N. Hay, unpublished results). Importantly, addition of the mitotic inhibitor nocodazole after gamma irradiation results in identical levels of complete G2 arrest in MyrAkt- and vector-transduced cells, indicating that the observed phenomena cannot be attributed to enhancement of G1 arrest by activated Akt.
The ability of activated Akt to override G2/M arrest is independent of p53 status and cannot be reestablished by coexpression of Myc and Bcl-2.
The ability of activated Akt to override G2/M arrest as induced in Rat1a cells that have little or no expression of p21cip1/waf1 suggests that this activity of Akt is independent of p53. Indeed, as shown in Fig. 1E, Rat1a cells expressing p53 dominant-negative fragments (GSE56) (33) respond to 6-TG exposure in a manner similar to that of control cells. Furthermore, activated Akt attenuates G2 accumulation of p53 null 10.1 3T3 fibroblasts (41) upon exposure to gamma irradiation (Fig. 1F). Finally, overexpression of Myc that overcomes p53-dependent cell cycle checkpoints (41, 42) cannot overcome the arrest induced by 6-TG (Fig. 1B). This suggests that Akt acts in G2 essentially in a p53-independent manner and that growth-enhancing (Myc) and antiapoptotic (Bcl-2) oncogenes together cannot reproduce this effect. Interestingly, exposure of cells to PALA (N-phosphonoacetyl-l-aspartate), which depletes the pool of pyrimidines and attenuates cell cycle progression in a p53-dependent manner (10), can be abolished in GSE56- or Myc/Bcl-2-expressing cells but not in cells expressing activated Akt (data not shown). Thus, Akt activation is not equivalent to functional loss of p53-dependent cell cycle control.
Activation of Akt maintains high mitotic index, CDC2 activity, and long-term survival upon exposure to DNA damage.
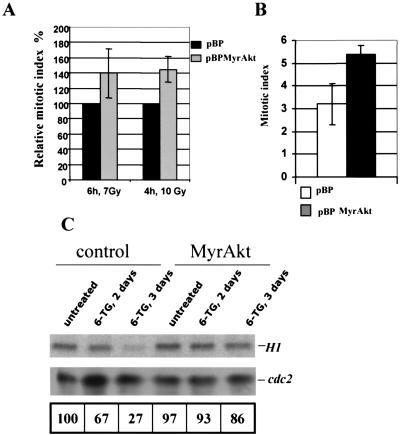
In agreement with the cell cycle data, nocodazole block revealed accumulation of mitotic cells in gamma-irradiated or 6-TG-treated cells in an Akt-dependent manner (Fig. 2A and B). As shown in Fig. 2, reduced G2/M arrest correlates with a higher fraction of mitotic cells in MyrAkt-expressing populations, and the reduction in CDC2 activity was not observed in cells expressing activated Akt. These results imply that the arrest is indeed in the G2 phase of the cell cycle and that activation of Akt can override this arrest.
FIG. 2.
Activated Akt maintains higher mitotic index and CDC2 activity upon exposure to DNA damage. (A) Cells with activated Akt maintain a higher mitotic index following gamma irradiation. Vector (pBP)- and MyrAkt (pBPMyrAkt)-transduced Rat1a cells were treated with the indicated doses of gamma irradiation and analyzed 4 and 6 h posttreatment. The mitotic indices were determined as described in Materials and Methods and are normalized to that of an untreated control for each cell line. The relative mitotic index of vector-transduced cells is shown as 100%. The difference between vector and MyrAkt cell lines is significant, with P = 0.05 (two-tailed t test). (B) Cells with activated Akt maintain a higher mitotic index following 6-TG treatment. The mitotic indices of vector (pBP)- and MyrAkt (pBPMyrAkt)-transduced Rat1a cells were measured following 3 days of 6-TG treatment as described in Materials and Methods. (C) Activated Akt reduces the decline in CDC2 activity following 6-TG treatment. CDC2 was immunoprecipitated from treated (MyrAkt) and untreated (control) cells as indicated, and its activity was measured and normalized for the total amount of this protein as described in Materials and Methods. Normalized activity is shown as a percentage of that in an untreated vector-transduced control.
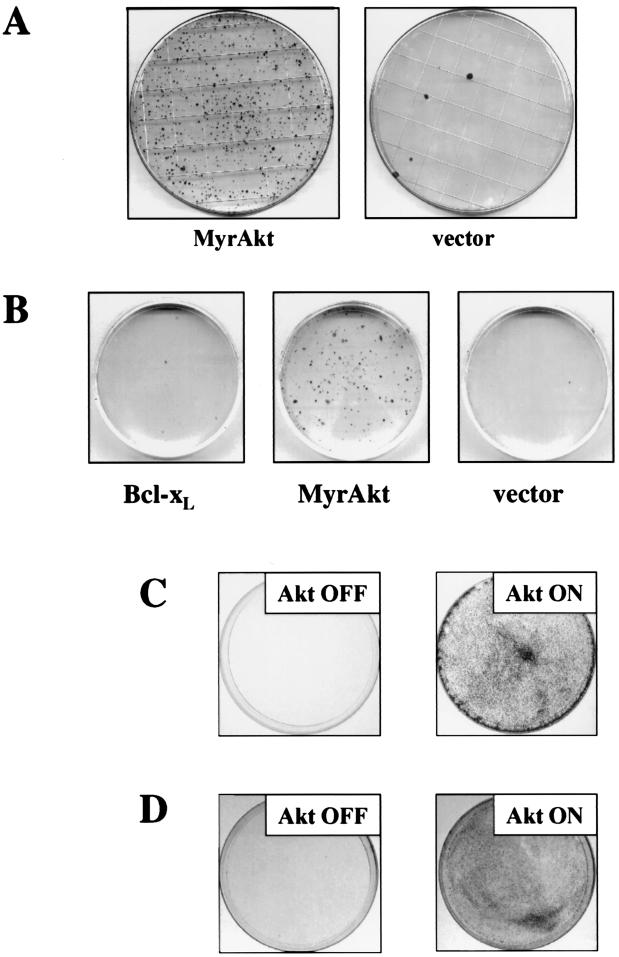
We next wanted to determine whether the bypass of the G2/M checkpoint by activated Akt, in conjunction with its antiapoptotic function, enables cells to go through mitosis and continue dividing without maintaining checkpoint control and genome integrity. The frequency by which cells become resistant to long-term exposure of 6-TG is about 1 per 106 cells. This acquired resistance of cells to 6-TG is predominantly due to mutations in the HGPRT gene. However, a deficiency in MMR dramatically increases the tolerance to low doses of 6-TG in an HGPRT-independent manner. The explanation for the higher tolerance of MMR-deficient cells for low doses of 6-TG is that both cell cycle arrest and apoptosis, as mediated by 6-TG, are alleviated in these cells (11, 19). We therefore set forth to investigate whether activation of Akt elicits a similar phenotype and assessed the effect of long-term and continuous exposure to 6-TG on cells with activated Akt. Essentially complete elimination of Rat1a cells was observed at 6-TG concentrations as low as 1 μM, while substantial survival of Rat1a cells expressing activated Akt was observed at concentrations as high as 4 μM. For concentrations of 6-TG in the range of 1 to 2 μM, activated Akt increased the colony yield by approximately 2 orders of magnitude (Fig. 3A). This effect has been observed in eight independent experiments (P < 0.005 [by Sign's criterion]). This cannot be explained solely by the antiapoptotic function of Akt, as Bcl-XL- and Bcl-2-overexpressing cells cannot form colonies after long-term exposure to 6-TG, although they exhibit increased resistance after short-term exposure to the mutagen (Fig. 3B and data not shown).
FIG. 3.
(A) Akt enhances long-term survival upon 6-TG treatment. Rat1a cells transduced with MyrAkt or an empty control vector were treated with 2 μM 6-TG, as described in Materials and Methods, followed by methylene blue staining. (B) Expression of Bcl-XL does not reestablish the long-term effect of Akt. Rat1a cells transduced with MyrAkt or Bcl-XL or an empty vector were treated and visualized as described for panel A. The experiment shown is representative of eight independent experiments. (C) 6-TG resistance is coregulated with Akt expression. Rat1a pBPSTR-1 MyrAkt cells were treated as described for panel A in the presence (Akt OFF) or absence (Akt ON) of tetracycline. (D) Continuous Akt function is required for long-term 6-TG resistance. Cells were passed through 6-TG treatment in the Akt ON state as described for panel C and were retested for 6-TG resistance in the presence or absence of tetracycline. The Tet-inducible MyrAkt cell line has been described previously (16).
Although 6-TG resistance can result from a failure to incorporate the drug into DNA, as happens in HGPRT-deficient cells, several lines of evidence ruled this out as an explanation for the resistance of MyrAkt cells. First, acquired genetic resistance to 6-TG is usually selected at high doses of 6-TG (14). Second, the high frequency of resistant clones is not likely to be due to mutations in HGPRT. Third, Rat1a MyrAkt cells pooled after 2 weeks of growth in the presence of 6-TG retain high clonogenicity in HAT medium, in which HGPRT-deficient cells are unable to grow and therefore die (14; Kandel and Hay, unpublished). Finally, to further confirm that continuous Akt activity is required for survival per se rather than for acquisition of secondary resistance mutations, we employed a Rat1a cell line with regulated expression of MyrAkt (16). The cells were routinely cultured in the absence of activated Akt (Akt OFF), yet activation of Akt just prior to 6-TG treatment was sufficient to induce a resistance phenotype (Fig. 3C). Thus, 6-TG tolerance cannot be explained by acquisition of additional mutations during prolonged Akt activation. Moreover, cells surviving 6-TG treatment in an Akt ON state remained sensitive to subsequent exposures to the drug when Akt was turned off (Fig. 3D). Thus, Akt activation is indeed the primary determinant of the resistance phenotype in these cells and provides protection both from apoptosis and from G2 arrest mediated by MMR.
We have also assessed the effect of proliferation in the presence of 6-TG on the accumulation of mutations in cells expressing activated Akt by measuring HSV-tk gene inactivation. Expression of the HSV-tk gene renders the cells sensitive to GCV; therefore, the frequency with which the HSV-tk gene is mutated and inactivated is commonly measured by the frequency of resistance to GCV (1). Thus, Rat1a cells with tetracycline-dependent expression of MyrAkt were infected with a retrovirus carrying the HSV-tk gene and single-cell clones were isolated (see Materials and Methods). The isolated clones were expanded in an Akt OFF state to generate clonal cell lines. Two clonal cell lines were chosen for subsequent experiments. The cells were propagated in the presence of 6-TG in an Akt ON state, as shown in Fig. 3C, for about a month. The 6-TG-resistant cells were then examined for mutations in the HSV-tk gene, as reflected by the number of colonies resistant to GCV. The 6-TG-resistant cells were examined for resistance to GCV in an Akt ON or Akt OFF state. The values were compared to those obtained in the Akt ON state for the same clones without prior 6-TG exposure. Two important observations were made (see Table 1). First, a significantly higher number of GCV-resistant colonies were observed after 6-TG treatment; second, there was no significant difference in the number of GCV-resistant colonies when Akt expression was turned off. Thus, the inactivation of the HSV-tk gene had occurred in these clones during the period that activated Akt was turned on, and once it had occurred it was no longer dependent on Akt activation. On the basis of these observations, we concluded that Akt, in fact, permits survival and proliferation of cells by decreasing sensitivity to mismatched nucleotides rather than through prevention of uptake or incorporation of 6-TG. Consequently, the cells with transiently activated Akt accumulate mutations at secondary loci and exhibit novel phenotypes that are no longer affected by Akt status. These observations suggest that even transient up-regulation of Akt activity in conditions of DNA damage may result in a permanent higher mutation load in the surviving cell population.
TABLE 1.
Mutation analysis of HSV-tk gene as measured by GCV resistancea
| Cell line | No preselectionb | No. of GCV-resistant clones with:
|
|
|---|---|---|---|
| With Akt inductionc | Without Akt inductiond | ||
| 1 (expt 1) | 0 | 120 | 80 |
| 2 (expt 2) | 3 | 137 | 240 |
| 2 | 50 | >1,500 | >1,500 |
Clonal cell lines of Ratla pBPSTR-1 MyrAkt expressing HSV-tk were examined for the number of GCV-resistant clones out of 5 × 106 cells. Two independent clonal cell lines were examined. Results of two independent experiments with cell line 1 and one experiment with cell line 2 are shown.
Cells with no prior 6-TG treatment. GCV selection done in an Akt ON state.
Cells passed through 6-TG selection and GCV selection, both with induction.
Cells passed through 6-TG selection in an Akt ON state but selected on GCV without Akt induction.
PTEN−/− ES cells are deficient in G2/M checkpoints induced by gamma irradiation.
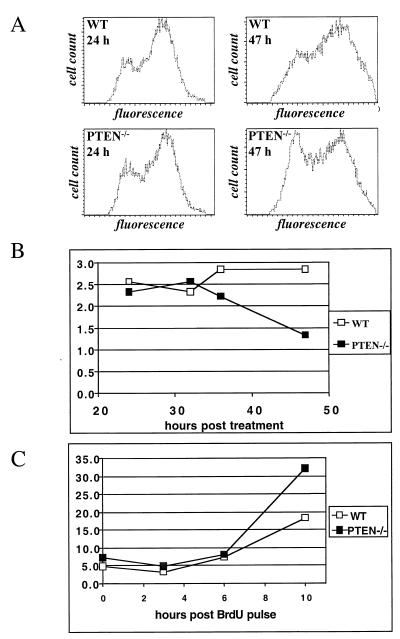
The predominant mode of Akt activation in human malignancies is probably via inactivation of PTEN. Therefore, we set out to investigate whether PTEN deficiency may exhibit a phenotype similar to that which we observed in MyrAkt-expressing cells. After pulsing with BrdU for 45 min, wild-type or PTEN-deficient ES cells (12) were irradiated, plated on a fresh layer of lethally irradiated embryonic fibroblasts, and collected at various time points. In two independent experiments, analysis of a BrdU-positive population revealed that PTEN-deficient cells were released from G2 arrest and entered G1 significantly earlier than their wild-type counterparts (Fig. 4A and B).
FIG. 4.
PTEN deficiency affects exit from G2 phase. (A and B) PTEN-deficient ES cells recover faster from gamma irradiation-induced G2 arrest. Wild-type and PTEN−/− ES cells were pulse labeled with BrdU and irradiated as described in Materials and Methods. Cell cycle distribution of BrdU-positive cells was monitored by flow cytometry at the indicated times posttreatment. Representative histogram plots (A) and changes over time in the G2-to-G1 ratio (B) are shown. (C) PTEN deficiency facilitates G2/M-to-G1 transition in untreated ES cells. Wild-type (WT) and PTEN−/− ES cells were labeled with BrdU, and the fraction of BrdU-positive cells in G1 was measured by flow cytometry at the indicated times. The results are representative of two independent experiments.
Finally, we investigated whether the PTEN/PI3K/Akt pathway affects cell cycle progression in untreated cells. PTEN deficiency had been reported to cause a small increase in the growth rate (37). While increased survival or faster transition through the G1 checkpoint has not been ruled out, we set out to examine whether other phases of the cell cycle might be affected in these cells. Wild-type or PTEN-deficient ES cells were pulsed with BrdU for 45 min and periodically sampled for the next 10 h. By the end of the experiment, the fraction of BrdU-positive cells in the G1 phase was substantially higher in the PTEN-deficient population (Fig. 4C). Thus, PTEN deficiency mimics ectopic expression of activated Akt in its ability to overcome the G2/M checkpoint.
The PI3K/Akt pathway is required for G2/M transition.
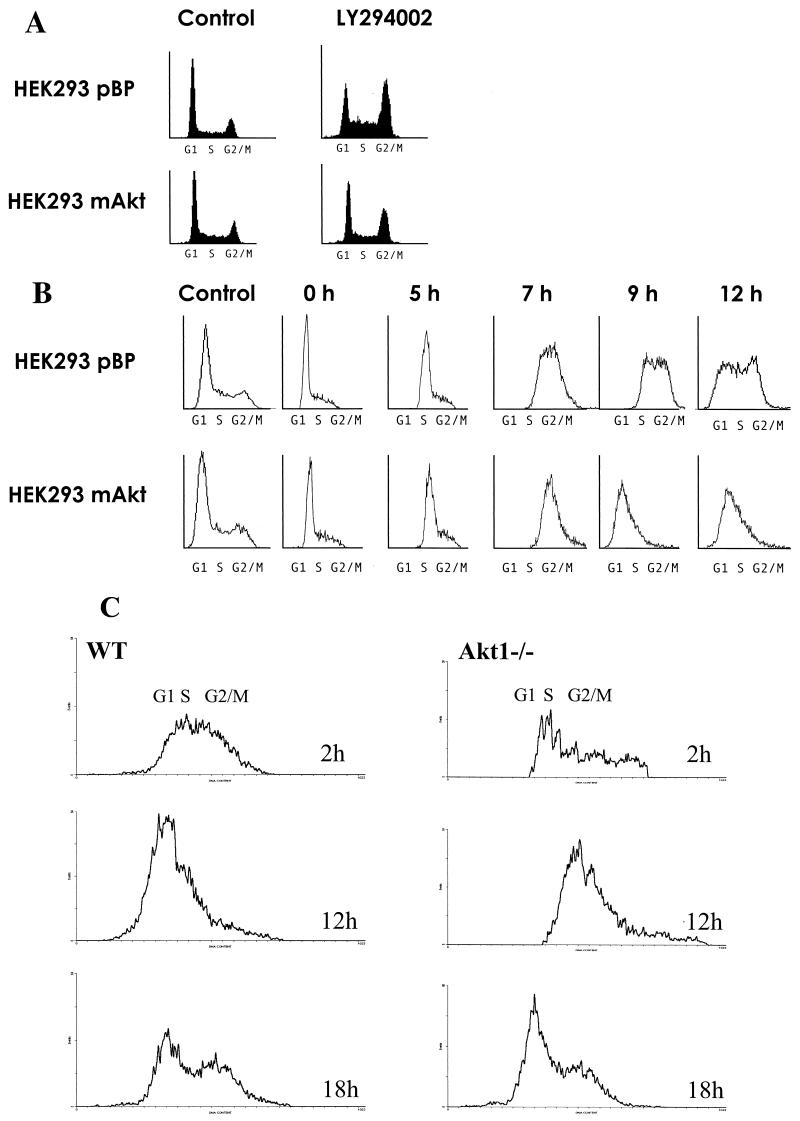
The results shown in Fig. 4C suggest that the PI3K/Akt signaling pathway can regulate G2/M transition. To further explore this observation, we subjected cells to the PI3K inhibitor LY294002 (LY). In many cell lines, inhibition of PI3K leads to G1 arrest (8, 26). However, some cell lines respond to LY by a G2 cell cycle arrest. For example, HEK293 cells respond to LY mainly by G2 cell cycle arrest, which is abolished in HEK293 cells expressing activated Akt (HEK293mAkt) (Fig. 5A ). This can be observed in other cell lines when they are blocked in the G1/S boundary and released to progress through the cell cycle in the presence of LY (40; data not shown). We also found that, following the release of S-phase block, HEK293mAkt cells showed accelerated progression through G2/M and a significant diminution of G2 arrest in the presence of LY when compared with control cells (Fig. 5B and data not shown). As shown in Fig. 5B, at 7 h after release, both HEK293pBP and HEK293mAkt cells enter the G2/M phase of the cell cycle. However, at 9 h after release, the HEK293mAkt cells exit the G2/M and enter the G1 phase, whereas the HEK293pBP cells are still in the G2/M phase; at 12 h after release, the HEK293mAkt cells enter the second S phase, whereas only about 50% of HEK293pBP cells exit the G2/M and enter the G1 phase of the cell cycle at this time point.
FIG. 5.
(A) Activation of Akt overcomes G2 cell cycle arrest induced by LY. Asynchronously growing HEK293 cells infected with pBabePuro (HEK293pBP) or pBabePuroMyrAkt (HEK293pBPmAkt) were either left untreated or treated with LY (20 μm) for 12 h and then subjected to flow cytometry analysis. (B) Akt accelerates the transition from G2/M to G1. HEK293pBP and HEK293mAkt cells were subjected to aphidicoline-mediated S-phase block and were released from the block as described in Materials and Methods. Samples were taken for flow cytometry analysis at the indicated time points. The profiles of asynchronously growing cells (control) are also shown. (C) Akt1−/− MEFs are attenuated in the transition from G2/M to G1. Asynchronously growing SV40-immortalized wild-type (left panels) and Akt1−/− (right panels) MEFs were pulse labeled with BrdU for 45 min and subjected to flow cytometry analysis at the indicated time points as described in Materials and Methods. x axis, DNA content; y axis, number of events.
Finally, we have analyzed the G2/M-to-G1 transition of Akt1 null MEFs. Proliferating SV40-immortalized wild-type and Akt1−/− MEFs were pulse labeled with BrdU and analyzed by flow cytometry, as described in Materials and Methods. As expected, 2 h after the pulse, most of the BrdU-labeled wild-type and Akt1−/− cells were in the S phase (Fig. 5C). However, 12 h after the pulse, the wild-type-labeled cells appeared mostly in the G1 phase, whereas the Akt1−/− cells were still in the G2/M phase at this time point (Fig. 5C). Although we cannot completely exclude the possibility that the Akt1−/− cells are also impaired in the transition from S phase to G2 phase, these results, together with the results described above, strongly suggest that Akt is required for the G2/M transition.
Activation of Akt resembles DNA MMR deficiency in ability to overcome the G2/M cell cycle checkpoint and apoptosis induced by DNA damage.
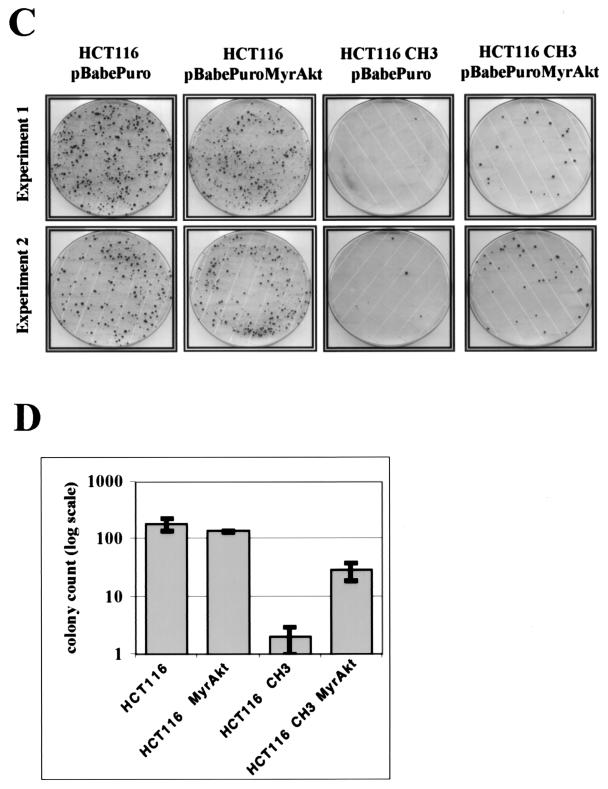
Attenuation of cell cycle arrest in a p53-independent manner and increased survival of certain types of DNA damage upon Akt activation resembled the phenotype of MMR-deficient cells (11, 19; for a review, see reference 6). To further investigate this issue, we employed the colon carcinoma cell line HCT116. These cells, although they express wild-type p53, are not susceptible to pronounced G2 arrest upon exposure to gamma irradiation or 6-TG, because they are MMR deficient (11, 24) (Fig. 6B ). This phenotype of HCT116 cells is rescued by introducing human chromosome 3, which encodes the missing MMR component hMLH1, into the cells (HCT116-ch3 cells) but is not affected by the loss of p53 (11, 24). We used retrovirus infection in HCT116 and HCT116-ch3 cells to generate polyclonal cell lines expressing MyrAkt (see Materials and Methods). Expression levels of Akt in control and MyrAkt-expressing cells are shown in Fig. 6A. As shown in Fig. 6B, we found that expression of activated Akt in HCT116-ch3 cells reestablishes the phenotype of the parental HCT116 cells. The differences between vector- and MyrAkt-transduced HCT116-ch3 cells are significant, with P = 0.014 and P = 0.013 for the 19 -h and 24 -h time points, respectively (paired t test). We also observed that long-term 6-TG tolerance of HCT116-ch3 cells increases when MyrAkt is expressed, which resembles the phenotype of parental MMR-deficient HCT116 cells (Fig. 6C and D). Incomplete restoration of long-term 6-TG resistance may be due in part to frequent inactivation of long-terminal-repeat-driven transgenes in both HCT116 and HCT116-ch3 cell lines, as was previously shown (27).
FIG. 6.
Activated Akt attenuates G2 arrest following gamma irradiation in HCT116-ch3 cells and mimics MMR deficiency in HCT116 cells. (A) Immunoblotting analysis of expression of Akt in HCT116 and HCT116-ch3 transduced with empty vector (lanes 1 and 3) or activated Akt (lanes 2 and 4). β-Actin is shown as a loading control. (B) The isogenic cell lines HCT116 and HCT116-ch3, after being transduced with activated Akt or an empty control vector, were gamma irradiated (7 Gy), and their cell cycle profiles were determined by PI staining and flow cytometry. Ratios of G2-to-G1 fractions are shown. Cumulative data from two independent experiments are presented. (C) Akt activation increases long-term survival upon 6-TG treatment. A total of 1.5 × 106 HCT116 and HCT116-ch3 cells transduced with activated Akt or an empty control vector were continuously treated with 2 μM 6-TG. Colonies were visualized by crystal violet staining. Results of two independent experiments are shown. (D) Quantification of the results shown in panel B. Colony counts (presented as averages with standard deviations) are plotted on a logarithmic scale.
The phenotype of cells with Akt activation resembles that of MMR-deficient cells. The results in Fig. 4C, showing that the transition from G2/M to G1 is facilitated in PTEN null cells, suggest that activation of Akt is more likely to be affecting the cell cycle machinery than is the activity of MMR proteins. Furthermore, the results in Fig. 5, showing that inhibition of PI3K induces G2 cell cycle arrest that can be alleviated by activated Akt and that Akt1 null MEFs display a delayed transition from G2/M to G1, strongly suggest that the PI3K/Akt signaling pathway is required for normal G2/M transition. However, the putative common target for MMR proteins and Akt that mediates the G2/M cell cycle checkpoint is yet unknown. This elusive target either can participate in the signaling to the cell cycle machinery or is an integral part of the cell cycle machinery. One potential candidate that may link MMR, Akt, and the cell cycle is BRCA1, as it was shown that BRCA1 interacts with MMR proteins (43-45) and is also a target for Akt phosphorylation (3). In addition, it was shown that BRCA1 exon 11 isoform null cells are defective in the G2/M cell cycle checkpoint and do not arrest in G2 following exposure to gamma irradiation (46). It was also shown that overexpression of BRCA1 induces mainly G2/M cell cycle arrest (30). Because Chk1 can execute a G2/M cell cycle checkpoint that is p53 independent and the deletion of Chk1 elicits apoptosis in a p53-independent and MMR-dependent manner (20, 25, 28, 29, 31), it is also possible that Akt exerts its effects through direct or indirect modulation of Chk1. Interestingly, it was recently shown that BRCA1 mediates its effect through Chk1 (47). While this paper was under review, it was shown that inhibition of PI3K in MDCK cells elicits G2 arrest that is abolished by activated Akt. Because the inhibition of PI3K activates Chk1 and activated Akt alleviates this activation, it was suggested that the PI3K/Akt signaling pathway may regulate G2/M transition, at least in part through modulating Chk1 activity (35). Clearly, more studies are required to delineate the exact mechanism by which Akt exerts its effect on the G2/M transition. Another avenue through which Akt may exert its effect is through the inhibition of the FOXO transcription factors that are downstream phosphorylation targets of Akt. It was recently shown that FOXO3a modulates the expression of several genes that regulate response to stress at the G2/M cell cycle checkpoint (40).
Several lines of evidence indicate that G2 arrest, which is alleviated by activated Akt, is p53 independent: (i) Rat1a cells in which the p21cip1/waf1 promoter is inactive and which are thus deficient in p53-dependent cell cycle checkpoints still respond to gamma irradiation and 6-TG by transient G2 arrest, which is alleviated by activated Akt (Fig. 1A, B, and D). (ii) Rat1a cells expressing dominant-negative p53 still arrest in G2 upon exposure to 6-TG (Fig. 1E). (iii) Overexpression of Myc that overcomes p53-dependent cell cycle checkpoints (41, 42) cannot overcome the arrest induced by 6-TG (Fig. 1B). (iv) 10.1 3T3 cells that are null for p53 accumulate in G2 upon exposure to 6-TG, and activated Akt alleviates this accumulation (Fig. 1F). (v) Inhibition of PI3K in HEK293 cells elicits G2 arrest that is alleviated by activated Akt (Fig. 5A). In HEK293 cells, the transcription activation function of p53 is diminished due to expression of the adenovirus E1B 55K protein, which binds to the transactivation domain of p53 and inhibits its activity (17, 48). (vi) SV40-immortalized Akt1−/− MEFs in which p53 is not functional are attenuated in the transition from G2/M to G1 phase (Fig. 5C). (vii) The MMR-proficient cells, HCT116-ch3, arrest in G2 upon exposure to gamma irradiation, and activated Akt diminishes this arrest (Fig. 6). It was previously shown that MMR-dependent G2 arrest in HCT116-ch3 cells cannot be abolished by ectopic expression of the human papillomavirus E6 protein, which degrades p53 (11).
In summary, our findings document a novel role for Akt in the control of G2/M cell cycle progression and show that activation of Akt can overcome both the p53-independent G2/M cell cycle checkpoint and apoptosis induced by DNA damage. In addition, it was recently shown that activation of Akt also has the potential of alleviating the p53-mediated cell cycle checkpoints through phosphorylation and sequestration of p21cip1/waf1 and through enhanced degradation of p53 (32, 49, 50). Therefore, Akt activation is similar to inactivation of p53, which abolishes the cell cycle checkpoints and at the same time inhibits apoptosis. The significance of this observation is underscored by the high incidence of tumor-associated changes that lead to Akt activation. The results also suggest that activation of Akt may influence tumor response to therapy, in particular to antipurine treatment, and that the status of Akt as a prognostic marker should be evaluated in efforts to improve the outcome of the associated disease.
Acknowledgments
We thank Andrei Gudkov for GSE56 expression construct and C. R. Boland for HCT116-ch3 and HCT116 cell lines as well as Joel Jacobs for his help in organizing the manuscript.
This work was supported by NIH grants AG16927 and CA90764 to N.H.
REFERENCES
- 1.Abuin, A., H. Zhang, and A. Bradley. 2000. Genetic analysis of mouse embryonic stem cells bearing Msh3 and Msh2 single and compound mutations. Mol. Cell. Biol. 20:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, L. A., T. Duhig, M. Read, and M. Fried. 2000. The p21WAF1/CIP1 promoter is methylated in Rat-1 cells: stable restoration of p53-dependent p21WAF1/CIP1 expression after transfection of a genomic clone containing the p21WAF1/CIP1 gene. Mol. Cell. Biol. 20:1291-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altiok, S., D. Batt, N. Altiok, A. Papautsky, J. Downward, T. M. Roberts, and H. Avraham. 1999. Heregulin induces phosphorylation of BRCA1 through phosphatidylinositol 3-kinase/AKT in breast cancer cells. J. Biol. Chem. 274:32274-32278. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., O. Batista, A. Bellacosa, P. Tsichlis, and P. K. Vogt. 1998. The akt kinase: molecular determinants of oncogenicity. Proc. Natl. Acad. Sci. USA 95:14950-14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aquilina, G., M. Crescenzi, and M. Bignami. 1999. Mismatch repair, G2/M cell cycle arrest and lethality after DNA damage. Carcinogenesis 20:2317-2326. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A. 2001. Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ. 8:1076-1092. [DOI] [PubMed] [Google Scholar]
- 7.Cantley, L. C., and B. G. Neel. 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96: 4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casagrande, F., D. Bacqueville, M. J. Pillaire, F. Malecaze, S. Manenti, M. Breton-Douillon, and J. M. Darbon. 1998. G1 phase arrest by the phosphatidylinositol 3-kinase inhibitor LY 294002 is correlated to up-regulation of p27Kip1 and inhibition of G1 CDKs in choroidal melanoma cells. FEBS Lett. 422:385-390. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernova, O. B., M. V. Chernov, Y. Ishizaka, M. L. Agarwal, and G. R. Stark. 1998. MYC abrogates p53-mediated cell cycle arrest in N-(phosphonacetyl)-l-aspartate-treated cells, permitting CAD gene amplification. Mol. Cell. Biol. 18:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, T. W., C. Wilson-Van Patten, M. Meyers, K. A. Kunugi, S. Cuthill, C. Reznikoff, C. Garces, C. R. Boland, T. J. Kinsella, R. Fishel, and D. A. Boothman. 1998. Defective expression of the DNA mismatch repair protein, MLH1, alters G2-M cell cycle checkpoint arrest following ionizing radiation. Cancer Res. 58:767-778. [PubMed] [Google Scholar]
- 12.Di Cristofano, A., B. Pesce, C. Cordon-Cardo, and P. P. Pandolfi. 1998. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19:348-355. [DOI] [PubMed] [Google Scholar]
- 13.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 14.Fenwick, R. G., Jr., and C. T. Caskey. 1975. Mutant Chinese hamster cells with a thermosensitive hypoxanthine-guanine phosphoribosyltransferase. Cell 5:115-122. [DOI] [PubMed] [Google Scholar]
- 15.Gingras, A. C., S. G. Kennedy, M. A. O'Leary, N. Sonenberg, and N. Hay. 1998. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12:502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlob, K., N. Majewski, S. Kennedy, E. S. Kandel, R. B. Robey, and N. Hay. 2001. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial metabolism. Genes Dev. 15:1406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grand, R. J., P. S. Lecane, D. Owen, M. L. Grant, S. Roberts, A. J. Levine, and P. H. Gallimore. 1995. The high levels of p53 present in adenovirus early region 1-transformed human cells do not cause up-regulation of MDM2 expression. Virology 210:323-334. [DOI] [PubMed] [Google Scholar]
- 18.Griffin, S., P. Branch, Y. Z. Xu, and P. Karran. 1994. DNA mismatch binding and incision at modified guanine bases by extracts of mammalian cells: implications for tolerance to DNA methylation damage. Biochemistry 33:4787-4793. [DOI] [PubMed] [Google Scholar]
- 19.Hawn, M. T., A. Umar, J. M. Carethers, G. Marra, T. A. Kunkel, C. R. Boland, and M. Koi. 1995. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 55:3721-3725. [PubMed] [Google Scholar]
- 20.Hirose, Y., M. S. Berger, and R. O. Pieper. 2001. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 61:5843-5849. [PubMed] [Google Scholar]
- 21.Kandel, E. S., B. D. Chang, B. Schott, A. A. Shtil, A. V. Gudkov, and I. B. Roninson. 1997. Applications of green fluorescent protein as a marker of retroviral vectors. Somat. Cell Mol. Genet. 23:325-340. [DOI] [PubMed] [Google Scholar]
- 22.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701-713. [DOI] [PubMed] [Google Scholar]
- 24.Koi, M., A. Umar, D. P. Chauhan, S. P. Cherian, J. M. Carethers, T. A. Kunkel, and C. R. Boland. 1994. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-n′-nitro-n-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 54:4308-4312. (Erratum, 55:201, 1995.) [PubMed] [Google Scholar]
- 25.Koniaras, K., A. R. Cuddihy, H. Christopoulos, A. Hogg, and M. J. O'Connell. 2001. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene 20:7453-7463. [DOI] [PubMed] [Google Scholar]
- 26.Le, X. F., R. Vadlamudi, A. McWatters, D. S. Bae, G. B. Mills, R. Kumar, and R. C. Bast, Jr. 2000. Differential signaling by an anti-p185(HER2) antibody and heregulin. Cancer Res. 60:3522-3531. [PubMed] [Google Scholar]
- 27.Lengauer, C., K. W. Kinzler, and B. Vogelstein. 1997. DNA methylation and genetic instability in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 94:2545-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 29.Luo, Y., S. K. Rockow-Magnone, M. K. Joseph, J. Bradner, C. C. Butler, S. K. Tahir, E. K. Han, S. C. Ng, J. M. Severin, E. J. Gubbins, R. M. Reilly, A. Rueter, R. L. Simmer, T. F. Holzman, and V. L. Giranda. 2001. Abrogation of G2 checkpoint specifically sensitizes p53 defective cells to cancer chemotherapeutic agents. Anticancer Res. 21:23-28. [PubMed] [Google Scholar]
- 30.MacLachlan, T. K., K. Somasundaram, M. Sgagias, Y. Shifman, R. J. Muschel, K. H. Cowan, and W. S. El-Deiry. 2000. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J. Biol. Chem. 275:2777-2785. [DOI] [PubMed] [Google Scholar]
- 31.Mailand, N., J. Falck, C. Lukas, R. G. Syljuasen, M. Welcker, J. Bartek, and J. Lukas. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science 288:1425-1429. [DOI] [PubMed] [Google Scholar]
- 32.Mayo, L. D., and D. B. Donner. 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98:11598-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossovskaya, V. S., I. A. Mazo, M. V. Chernov, O. B. Chernova, Z. Strezoska, R. Kondratov, G. R. Stark, P. M. Chumakov, and A. V. Gudkov. 1996. Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc. Natl. Acad. Sci. USA 93:10309-10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podsypanina, K., L. H. Ellenson, A. Nemes, J. Gu, M. Tamura, K. M. Yamada, C. Cordon-Cardo, G. Catoretti, P. E. Fisher, and R. Parsons. 1999. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 96:1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shtivelman, E., J. Sussman, and D. Stokoe. 2002. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr. Biol. 12:919-924. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, L., and R. Parsons. 2001. Pten: life as a tumor suppressor. Exp. Cell Res. 264:29-41. [DOI] [PubMed] [Google Scholar]
- 37.Sun, H., R. Lesche, D. M. Li, J. Liliental, H. Zhang, J. Gao, N. Gavrilova, B. Mueller, X. Liu, and H. Wu. 1999. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 96:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki, A., J. L. de la Pompa, V. Stambolic, A. J. Elia, T. Sasaki, I. del Barco Barrantes, A. Ho, A. Wakeham, A. Itie, W. Khoo, M. Fukumoto, and T. W. Mak. 1998. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8:1169-1178. [DOI] [PubMed] [Google Scholar]
- 39.Swann, P. F., T. R. Waters, D. C. Moulton, Y. Z. Xu, Q. Zheng, M. Edwards, and R. Mace. 1996. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 273:1109-1111. [DOI] [PubMed] [Google Scholar]
- 40.Tran, H., A. Brunet, J. M. Grenier, S. R. Datta, A. J. Fornace, Jr., P. S. DiStefano, L. W. Chiang, and M. E. Greenberg. 2002. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296:530-534. [DOI] [PubMed] [Google Scholar]
- 41.Wagner, A. J., J. M. Kokontis, and N. Hay. 1994. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 8:2817-2830. [DOI] [PubMed] [Google Scholar]
- 42.Wagner, A. J., M. B. Small, and N. Hay. 1993. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol. Cell. Biol. 13:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Q., H. Zhang, R. Fishel, and M. I. Greene. 2000. BRCA1 and cell signaling. Oncogene 19:6152-6158. [DOI] [PubMed] [Google Scholar]
- 44.Wang, Q., H. Zhang, S. Guerrette, J. Chen, A. Mazurek, T. Wilson, A. Slupianek, T. Skorski, R. Fishel, and M. I. Greene. 2001. Adenosine nucleotide modulates the physical interaction between hMSH2 and BRCA1. Oncogene 20:4640-4649. [DOI] [PubMed] [Google Scholar]
- 45.Wang, Y., D. Cortez, P. Yazdi, N. Neff, S. J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927-939. [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, X., Z. Weaver, S. P. Linke, C. Li, J. Gotay, X. W. Wang, C. C. Harris, T. Ried, and C. X. Deng. 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3:389-395. [DOI] [PubMed] [Google Scholar]
- 47.Yarden, R. I., S. Pardo-Reoyo, M. Sgagias, K. H. Cowan, and L. C. Brody. 2002. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 30:285-289. [DOI] [PubMed] [Google Scholar]
- 48.Yew, P. R., and A. J. Berk. 1992. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 357:82-85. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, B. P., Y. Liao, W. Xia, Y. Zou, B. Spohn, and M. C. Hung. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973-982. [DOI] [PubMed] [Google Scholar]