Abstract
Runx2 (Cbfa1, AML-3) is multifunctional transcription factor that is essential for osteoblast development. Runx2 binds specific DNA sequences and interacts with transcriptional coactivators and corepressors to either activate or repress transcription of tissue-specific genes. In this study, the p21CIP/WAF1 promoter was identified as a repressible target of Runx2. A carboxy-terminal repression domain distinct from the well-characterized TLE/Groucho-binding domain contributed to Runx2-mediated p21 repression. This carboxy-terminal domain was sufficient to repress a heterologous GAL reporter. The repressive activity of this domain was sensitive to the histone deacetylase inhibitor trichostatin A but not to trapoxin B. HDAC6, which is insensitive to trapoxin B, specifically interacted with the carboxy terminus of Runx2. The HDAC6 interaction domain of Runx2 was mapped to a region overlapping the nuclear matrix-targeting signal. The Runx2 carboxy terminus was necessary for recruitment of HDAC6 from the cytoplasm to chromatin. HDAC6 also colocalized and coimmunoprecipitated with the nuclear matrix-associated protein Runx2 in osteoblasts. Finally, we show that HDAC6 is expressed in differentiating osteoblasts and that the Runx2 carboxy terminus is necessary for maximal repression of the p21 promoter in preosteoblasts. These data identify Runx2 as the first transcription factor to interact with HDAC6 and suggest that HDAC6 may bind to Runx2 in differentiating osteoblasts to regulate tissue-specific gene expression.
Runx2 (Cbfa1, AML-3, PEBP2αA) is one of three mammalian homologues of the Drosophila transcription factors Runt and Lozenge (8; A. Daga, J. E. Tighe, and F. Calabi, Letter, Nature 356:484, 1992). Mammalian Runt-related genes are essential for blood, skeletal, and gastric development and are commonly mutated in acute leukemias and gastric cancers (31). Runx2 is a crucial regulator of both intramembranous and endochondral bone formation, as it is required for osteoblast development and differentiation as well as chondrocyte differentiation (10, 12, 28, 42). Mutations altering Runx2 expression or protein structure cause the rare skeletal disorder cleidocranial dysplasia (39, 41). Runx2 is also expressed in T lymphocytes and cooperates with oncogenes c-myc, p53, and Pim1 to accelerate T-cell lymphoma development in mice (5, 48).
Runx factors are DNA binding proteins that can facilitate tissue-specific gene activation or repression (32). Although much has been learned about the mechanisms of Runx2-mediated transcriptional activation, less is understood about how Runx2 represses transcription. Runt/Runx factor-mediated repression, however, clearly involves multiple mechanisms (2). Runx2 interacts with the transcriptional corepressor proteins mSin3A and TLE/Groucho (25, 33, 51). The conserved amino acids, VWRPY, at the extreme carboxy termini of Runx proteins are required for the binding of TLE/Groucho proteins (23, 25, 30, 51). The TLE binding domains of Runt, Runx1, and Runx2, however, are not required for repression of the engrailed, p21, and bone sialoprotein promoters, respectively (2, 24, 33), suggesting that other repression mechanisms exist for Runx proteins. A second Runx2 repression domain was functionally identified and broadly defined to a 150-amino-acid stretch between the Runx2 activation domain and the TLE binding domain (51). The mechanism by which these amino acids repress transcription has not yet been defined. The carboxy-terminal region is crucial for Runx2 function, however, because mice expressing a truncated Runx2 protein lacking both repression domains have a skeletal phenotype similar to that of Runx2-deficient mice (7).
Many transcription factors repress transcription by recruiting histone deacetylases (HDACs) to chromatin and specific nucleosomes. Eleven of a possible 17 HDACs have been cloned from the human genome (18). HDACs are classified into two groups based on structural and functional similarities. Class I HDACs (HDACs 1, 2, 3, 8, and 11) are similar to Rpd3 of Saccharomyces cerevisiae and are expressed in the nuclei of cells in most tissues (17, 18). Class II HDACs (HDACs 4, 5, 6, 7, 9, and 10) are related to the yeast Hda1/2 (4, 15). Class II HDAC expression is more tissue-restricted than that of class I HDACs and is correlated with differentiation status in some cell lineages (4, 14, 15, 19, 38, 53). Class II HDACs appear to be additionally regulated by cellular localization, as they are predominantly cytoplasmic and are shuttled to the nucleus (20, 34, 52). HDAC6 (19, 53) and the recently cloned HDAC10 (13, 21, 26) are unique among HDACs in that they contain two catalytic domains whereas other HDACs contain just one catalytic domain. HDAC6 and HDAC10 are also unique in that they are resistant to the HDAC inhibitor trapoxin B (TPX-B) (16, 21). The function(s) of these HDACs in regulating tissue-specific gene expression is unknown, as they have not yet been found to be associated with any DNA binding transcription factors. However, HDAC6 may provide a link between acetylation and ubiquitin pathways (45).
The goal of this study was to identify the molecular mechanism by which the second repression domain in the Runx2 carboxy terminus inhibits transcription. The p21CIP/WAF1 promoter is inhibited by Runx1, the Runt-related factor required for hematopoiesis (33). Here, we demonstrate that Runx2 also represses the p21CIP/WAF1 promoter in fibroblasts and osteoblast lineage cells. The second repression domain in the Runx2 carboxy terminus is required for maximal p21CIP/WAF1 promoter repression in both cell types. HDAC6 specifically interacts with this Runx2 domain and is recruited to chromatin and specific nuclear structures by Runx2. These data demonstrate a novel mechanism of Runx2-mediated repression and identify Runx2 as the first transcription factor to interact with HDAC6.
MATERIALS AND METHODS
Cell culture.
C2C12, COS, and HeLa cells were cultured in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (FBS), 200 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml (P/S). NIH-3T3 cells were maintained in Dulbecco's modified Eagle medium containing 10% bovine calf serum, 200 mM l-glutamine, and 1% P/S. Cells from the osteoblast lineage lines MC3T3-E1, ROS17/2.8, and UMR 106 were grown in minimal essential medium (Gibco BRL) supplemented with 10% FBS, 1% nonessential amino acids, 200 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. To induce differentiation, confluent MC3T3-E1 cells were cultured in minimal essential medium α (Gibco BRL) containing 10% FBS, 1% P/S, 50 μg of ascorbic acid/ml, and 10 mM β-glycerolphosphate. The differentiation medium was replaced every 3 days.
Plasmids.
Runx2 deletion mutants were made by PCR using gene-specific oligonucleotides (sequences available from the authors upon request) to amplify the indicated coding regions from the pCMV-PEPB2αA1 template (40). The Runx2 sequences were subcloned into the pENTR-1A vector (Invitrogen) and then transferred into a pCMV5-M2 destination vector using the LR recombinase (Invitrogen) to generate GAL-Runx2 fusion proteins. The pCMV5-Runx1 (AML-1B) plasmid has been described previously (36). The p21WAF1/CIP1-luciferase (Luc) plasmids were kindly provided by Bert Vogelstein and Scott Hiebert (11, 33).
Transcription assays.
NIH-3T3, ROS17/2.8, and MC-3T3 cells were transfected with 700 ng of GAL-thymidine kinase (TK)-Luc or p21WAF1/CIP1-Luc, 200 ng of pCMV-secreted alkaline phosphatase (SEAP), or 100 ng of pRL-TK and, unless otherwise noted, 700 ng of the indicated Runx2 expression plasmid by using Superfect (Qiagen) (NIH-3T3 cells) or Lipofectamine (Invitrogen) (ROS17/2.8 and MC-3T3-E1 cells). Luciferase activity was measured 40 h posttransfection with luciferase assay systems (Promega). SEAP activity was measured as previously described (3, 54). Firefly luciferase activity was corrected with SEAP or renilla luciferase values to control for transfection efficiency. Changes in repression or activation (n-fold) were calculated relative to that in samples transfected with pCMV5 or pCMV-GAL. Trichostatin A (TSA; Sigma) and TPX-B were first added to cultures after removal of the transfection reagent at the indicated concentrations. Because of their instability in serum, additional doses of TSA and TPX-B were added at intervals of 24 h and 8 to 12 h, respectively.
Immunoprecipitations and immunoblots.
COS cells were transfected with the indicated pCMV-Runx2 and pCMV-FLAG-HDAC expression vectors using DEAE-dextran. At 40 h posttransfection, the cells were washed with phosphate-buffered saline (PBS) and then lysed for 5 min on ice with PBS containing 0.5% Triton X-100, 0.5% sodium dodecyl sulfate (SDS), 0.25% sodium deoxycholate, 1 mM EDTA, 5 μg of aprotinin/ml, 5 μg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were precleared with Pansorbin cells (Calbiochem). Anti-FLAG-M2-agarose beads (Sigma) were added to lysates to immunoprecipitate FLAG-HDACs and associated proteins. Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to an Immobilon P membrane (Millipore), and detected by immunoblotting with GAL-DNA binding domain (Upstate Biotechnology, Inc.) or FLAG (M2; Sigma) antibodies and enhanced chemiluminescence (Pierce).
To detect endogenous Runx2 and HDAC6 proteins in differentiating osteoblasts, MC3T3 cells were lysed with microextraction buffer (20 mM HEPES [pH 7.4], 450 mM NaCl, 0.2 mM EDTA, 0.5 mM dithiothreitol, 25% glycerol, 5 μg of aprotinin/ml, 5 μg of leupeptin/ml, 5 μg of pepstatin A/ml, and 1 mM PMSF). Lysates were sonicated and clarified by centrifugation. A fraction of each lysate (100 μg) was resolved by SDS-PAGE and transferred to Immobilon P (Millipore). Proteins were detected by immunoblotting with antibodies specific for Runx2 (37), HDAC6 (kindly provided by Scott Hiebert and Bart Lutterbach, Vanderbilt University, or purchased from Cell Signaling Technology), and β-actin (Sigma).
For coimmunoprecipitation of endogenous proteins, ROS17/2.8 cells were washed twice with ice-cold 1× PBS and then lysed in lysis buffer (150 mM NaCl, 20 mM Tris-HCl [pH 8.0], 1% NP-40, 25 μM MG132, 1× protease inhibitor cocktail [Roche]). Lysates were sonicated and precleared with Pansorbin cells for 30 min. The precleared lysates (2 mg per reaction) were incubated with 3 μg of PEBP2α1A (S-19; Santa Cruz) or HDAC6 (Cell Signaling Technology) antibodies for 16 h. Immune complexes were collected with 30 μl of protein A Sepharose (preblocked with BSA and salmon sperm DNA) for 30 min. The beads were washed three times with washing buffer (0.5% Na-deoxycholate, 0.5% NP-40, 50 mM NaCl, 2 mM EDTA, and protease inhibitors). Proteins were resolved by SDS-8% PAGE, transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore), and detected by immunoblotting with PEBP2α1A (S-19; 1:1,000 dilution) or HDAC6 (1:1,000 dilution) antibodies and enhanced chemiluminescence.
Cell fractionation assays.
COS cells were transfected with the indicated pCMV-Runx2 and pCMV-FLAG-HDAC expression vectors using DEAE-dextran. At 40 h posttransfection, the cells were washed with PBS and lysed with cytoplasmic extraction buffer (10 mM Tris-HCl [pH 7.8], 140 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40, 5 μg of aprotinin/ml, 5 μg of leupeptin/ml, and 1 mM PMSF) for 5 min on ice. Extracts were separated by centrifugation at 5,000 rpm (Eppendorf centrifuge) for 5 min at 4°C. Cytoplasmic proteins were collected from the supernatant. Nuclear pellets were washed one time with cold PBS and lysed with nuclear extraction buffer (microextraction buffer containing 1 M NaCl) for 5 min on ice. Following centrifugation at 12,000 rpm for 20 min at 4°C, soluble nuclear proteins were collected from the supernatant. The remaining chromatin-containing pellet was resuspended in 2× SDS sample buffer and sonicated. Proteins were resolved by SDS-PAGE on an 8 to 15% gradient gel, transferred to Immobilon P (Millipore), and immunoblotted with Runx2-specific (37), FLAG-specific (M2; Sigma), mSin3A-specific (Santa Cruz), and Rac1-specific (Upstate Biotechnology, Inc.) antibodies. Proteins were detected by enhanced chemiluminescence.
In situ immunofluorescence microscopy.
HeLa cells grown on gelatin-coated coverslips were cotransfected with 0.5 μg each of FLAG-tagged HDAC6 and hemagglutinin (HA)-tagged Runx2. At 24 h posttransfection, the cells were washed twice with ice-cold 1× PBS, fixed in whole-cell fixative (4% paraformaldehyde in 1× PBS) for 10 min on ice, and then permeabilized with 0.25% Triton X-100 for 20 min on ice. Cells were incubated with mouse monoclonal anti-FLAG antibody (1:2,000 dilution; Sigma) and rabbit polyclonal anti-HA antibody (1:3,000 dilution; Santa Cruz) for 1 h at 37°C. After being washed four times in ice-cold PBSA (0.5% BSA in 1× PBS), the cells were incubated with Alexa 488 goat anti-rabbit and Alexa 568 goat anti-mouse (1:800 dilution; Molecular Probes, Eugene, Oreg.) antibodies for 1 h and washed again. To visualize nuclei, cells were stained with DAPI (4′,6′-diamidino-2-phenylindole; 5 μg/ml) for 5 min and washed once with PBSAT (0.1% Triton X-100 in PBSA) and twice with PBS. The immunostaining of cells was recorded using an epifluorescence microscope attached to a charge-coupled device camera, and the digital images were analyzed with the Metamorph software program. For leptomycin B (LMB; Sigma) treatment, cells were incubated with 100 nM LMB for 5 h before being processed for in situ immunofluorescence.
Rat osteosarcoma ROS17/2.8 cells grown on gelatin-coated coverslips were fixed and lysed as described above. Cells were incubated with mouse monoclonal anti-Runx2 antibody (1:1,000 dilution; a generous gift from Y. Ito, Kyoto University, Japan) and goat polyclonal anti-HDAC6 antibody (1:500 dilution; Santa Cruz) for 1 h at 37°C. After being washed four times in ice-cold PBSA (0.5% BSA in 1× PBS), the cells were incubated with Alexa 488 donkey anti-goat and Alexa 568 goat anti-mouse (1:800 dilution; Molecular Probes) antibodies for 1 h and washed four times with PBSA and counterstained with DAPI as described above. Digital images were analyzed and deconvolved with the Metamorph software program.
RESULTS
Runx2 represses the p21CIP1/WAF1 promoter.
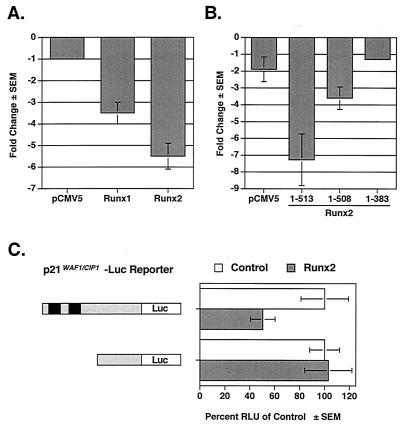
The p21CIP1/WAF1(p21) promoter was recently identified as a target of Runx1, the hematopoietic and leukemia-associated Runx protein (33). Ectopically expressed Runx2 also repressed the p21 promoter by five- to sixfold in nontransformed NIH-3T3 cells, which do not express Runx2 (Fig. 1A), and by twofold in the Runx2-positive osteosarcoma cell line, ROS17/2.8 (Fig. 1C). To determine the region(s) of Runx2 required for repression, we tested a series of Runx2 truncation mutants. A Runx2 protein lacking the last five amino acids and the TLE binding domain repressed the p21 promoter but was approximately 50% less effective than the full-length protein [Runx2 (1-513)] (Fig. 1B). A shorter truncation mutant, Runx2 (1-383), which lacks all residues carboxy terminal to the activation domain (Fig. 2B), did not repress the p21 promoter. Using a previously described p21 promoter mutant (33), we found that the Runx binding sites in the p21 promoter were required for Runx2-mediated repression (Fig. 1C). These data suggest that the carboxy terminus of Runx2 is required for the repression of the p21 promoter and that multiple regions of Runx2 contribute to repression.
FIG. 1.
Runx2 represses the p21 promoter. (A) Runx1 and Runx2 repress the p21 promoter. NIH-3T3 cells were cotransfected with p21-Luc, pCMV5-SEAP, and either pCMV5, pCMV5-Runx1, or pCMV5-Runx2 (MRIPV isoform). (B) Two regions in the Runx2 carboxy terminus are necessary for complete repression of the p21 promoter. NIH-3T3 cells were cotransfected with p21-Luc, pCMV5-SEAP, and either pCMV5-M2 or the indicated pCMV5-M2-Runx2 expression construct. (C) Runx binding sites in the p21 promoter are required for repression by Runx2. ROS17/2.8 cells were transfected with the indicated reporter plasmids and either pCMV5 (Control) or pCMV5-Runx2 (MRIPV). Runx binding sites are indicated by black boxes. The basal activities of the reporters differed significantly; thus the relative light units (RLU) in the Runx2-expressing lysates are shown as percentages of that in the control. Values represent the mean of triplicate samples; error bars indicate standard errors of the means (SEM).
FIG. 2.
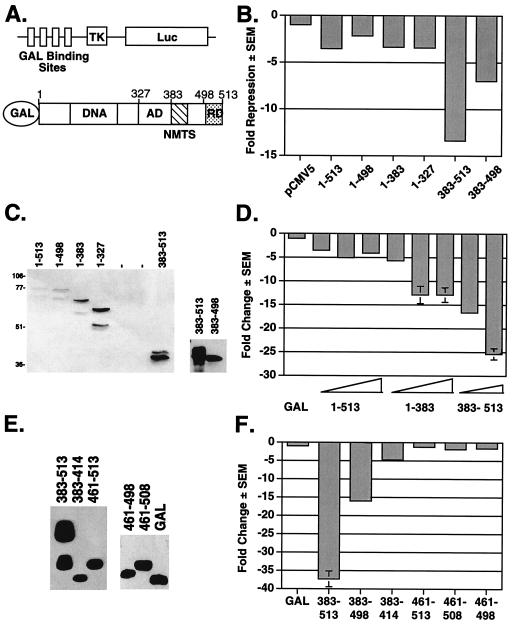
The Runx2 carboxy terminus contains several portable repression domains. (A) Schematics of the heterologous GAL-Luc reporter and GAL-Runx2 (1-513) fusion proteins used in these experiments. The reporter contains a minimal herpes virus TK promoter between four GAL4 binding sites and the Luc reporter gene. Amino acid residue numbers are above the Runx2 diagram. The relative locations of the activation domain (AD), nuclear matrix targeting signal (NMTS), and the TLE binding repression domain (RD) are depicted. (B) The TLE binding domain is not required for GAL-Runx2-mediated repression of a heterologous reporter. NIH-3T3 cells were cotransfected with GAL-TK-Luc, pRL-TK, and 700 ng of the indicated pCMV5-M2 or pCMV5-M2-Runx2 expression constructs. Firefly luciferase activities were corrected for transfection efficiency with renilla luciferase values. The change in repression (n-fold) was calculated relative to that in cells transfected with pCMV5. (C) The relative expression levels of GAL-Runx2 fusion proteins were determined by immunoblot analysis with anti-GAL-DNA binding domain monoclonal antibody. (D) Runx2 contains multiple regions flanking the activation domain that contribute to repression. In these experiments, pCMV5-M2-Runx2 plasmids were added in various quantities (100, 300, or 700 ng). Values were corrected to SEAP activity, and the change in repression (n-fold) was calculated relative to that in cells transfected with pCMV5-GAL. (E) The relative expression levels of GAL-Runx2-C-terminal fusion proteins were determined by immunoblot analysis as described for panel C. (F) The nuclear matrix targeting signal is also a portable transcriptional repressor. These experiments were performed as described above for panel D.
Runx2 contains multiple regions that contribute to transcriptional repression.
To identify the non-TLE repression mechanism(s) of Runx2, a panel of Runx2 truncation mutants was generated. Runx2 residues were fused to the GAL4 DNA binding domain (GAL; amino acids 1 to 147) to ensure nuclear localization and allow each region of Runx2 to be tested on a heterologous reporter containing four GAL4 binding sites and a TK minimal promoter (Fig. 2A). A full-length Runx2 fusion protein [GAL-Runx2 (1-513)] repressed transcription by approximately fivefold in NIH-3T3 cells (Fig. 2B and D). A truncated Runx2 protein, Runx2 (1-498), lacking the last 15 amino acids and the TLE binding domain repressed transcription by approximately twofold (50%) (Fig. 2B). Shorter carboxy-terminal truncation mutants, GAL-Runx2 (1-383) and (1-327), also repressed the GAL reporter (Fig. 2B). These results are in contrast to the effects of Runx2 (1-383) on the p21 promoter but indicate for the first time that residues within the first 327 residues of Runx2 contain a repression domain. We also tested the Runx2 carboxy terminus (amino acids 383 to 513) in this assay. This domain was a very potent repressor (Fig. 2B, D, and F). As it did within the context of the full-length protein, deletion of the TLE binding domain from the GAL-Runx2 carboxy terminus protein reduced repressive activity. This truncation mutant, (383-498), still repressed transcription by 7- to 15-fold (Fig. 2B and F) even though the truncated fusion protein, GAL-Runx2 (383-498), did not appear to be as stable as GAL-Runx2 (383-513) and was consistently found to be expressed at lower levels (Fig. 2C; see also Fig. 4B and C). These data indicate that the region encompassing residues 383 to 498 can repress transcription. These results are consistent with those of Thirunavukkarasu et al., who showed that deletion of this region increased the transcriptional activity of Runx2 (51). Analysis of additional GAL-Runx2 fusion proteins indicated that amino acids 383 to 414 of Runx2 are sufficient to repress transcription by fivefold (80% repression) (Fig. 2F). Runx2 residues 461 to 513, however, did not repress in this assay. These data indicate that a second Runx2 carboxy-terminal repression domain lies within amino acids 383 to 460.
FIG. 4.
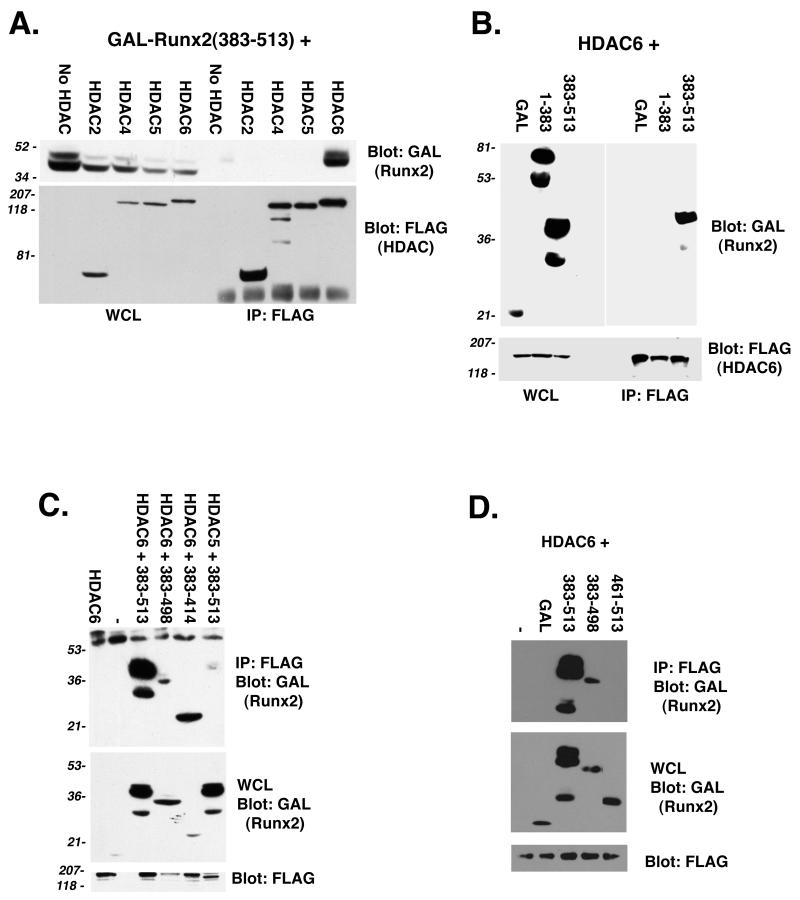
HDAC6 interacts with the Runx2 carboxy terminus. (A) The Runx2 carboxy terminus interacts with HDAC6 but not with HDAC2, HDAC4, or HDAC5. COS7 cells were cotransfected with pCMV5-M2-Runx2 (383-513) in the presence or absence of the indicated pCMV5-FLAG-HDAC construct. FLAG immunoprecipitates and a fraction (3%) of the whole-cell lysates (WCL) were resolved by SDS-PAGE. To detect Runx2 and the HDACs, membranes were immunoblotted with GAL and FLAG monoclonal antibodies, respectively. (B) HDAC6 specifically interacts with the Runx2 carboxy terminus. COS7 cells were cotransfected with pCMV5-FLAG-HDAC6 and the indicated pCMV5-M2-Runx2 construct. (C) Runx2 residues (383-414) are sufficient to interact with HDAC6. (D) Runx2 residues (461-513) do not interact with HDAC6. In panels C and D, COS cells were transfected as described for panel B. Proteins were detected as described above for panel A. Many of the GAL-Runx2 expression plasmids produce doublet proteins. The upper band represents the protein of the correct molecular weight.
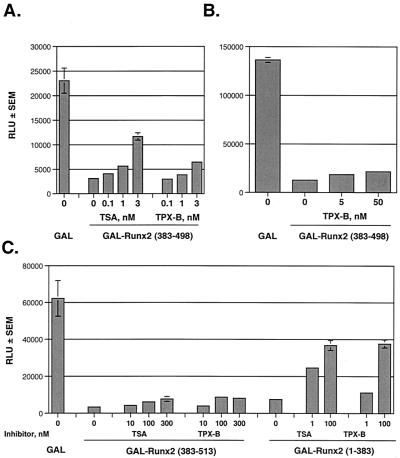
Repression by the Runx2 carboxy terminus is sensitive to the HDAC inhibitor TSA.
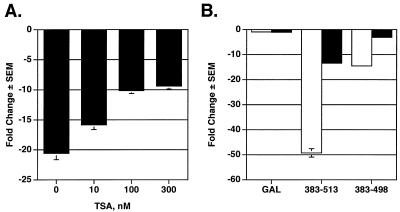
Because the Runx2 carboxy terminus is essential for p21 repression and is a very potent portable repression domain, we focused on understanding its mechanism of action in the remainder of this study. HDAC-containing complexes commonly associate with transcription factors to facilitate site-specific chromatin condensation and transcriptional repression. To determine whether deacetylase activity was associated with repression by any of the Runx2 proteins, we added the HDAC inhibitor TSA to our repression assays. TSA inhibited repression by Runx2 (383-513) in a concentration-dependent manner (Fig. 3A). Interestingly, however, TSA never completely prevented the repressive activity of this protein (Fig. 3A and B). In contrast, TSA completely inhibited the activity of GAL-Runx2 (383-498) (Fig. 3B). These data suggest that the Runx2 carboxy terminus may use HDAC-dependent and HDAC-independent mechanisms to repress transcription and that TSA sensitivity lies within residues 383 to 498.
FIG. 3.
Repression by the Runx2 carboxy terminus is sensitive to the HDAC inhibitor TSA. (A) The complete Runx2 carboxy terminus containing the TLE binding region and a second repression domain is partially sensitive to TSA. NIH-3T3 cells were transfected with GAL-TK-Luc, pCMV-SEAP, and 700 ng of pCMV5-Runx2 (383-513) in the presence or absence of the indicated concentrations of TSA. Luciferase values were corrected for transfection efficiency with SEAP values. The change in repression (n-fold) was calculated relative to that in cells expressing nonfused GAL (i.e., those transfected with pCMV-M2). (B) Runx2 (383-498) is completely sensitive to TSA. NIH-3T3 cells were transfected with the indicated pCMV5-M2-Runx2 constructs as described above and cultured in the presence (black bars) or absence (white bars) of 300 nM TSA.
Runx2 interacts with HDAC6.
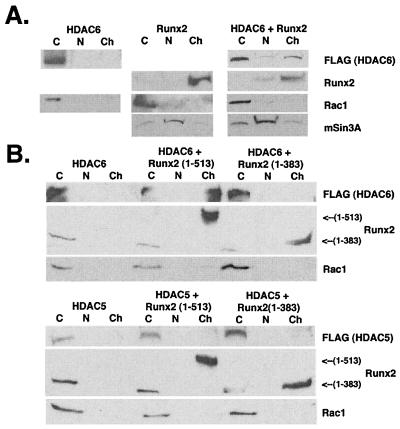
The sensitivity of Runx2 (383-498) to TSA led to the hypothesis that this region may interact directly with an HDAC. In coimmunoprecipitation-immunoblot assays, HDAC6 interacted with GAL-Runx2 (383-513) (Fig. 4A). HDACs 2, 4, and 5 did not interact with this region of Runx2. The Runx2 amino terminus [GAL-Runx2 (1-383)] and GAL did not interact with HDAC6 (Fig. 4B). These results suggested that the Runx2 carboxy terminus contained the HDAC6 binding domain. Additional GAL-Runx2 fusion proteins were tested to more precisely define the binding site. GAL-Runx2 (383-498) interacted with HDAC6, as did GAL-Runx2 (383-414) (Fig. 4C). GAL-Runx2 (461-513), however, did not coimmunoprecipitate with HDAC6 (Fig. 4D). These data are consistent with the functional activity of these proteins (Fig. 2F) and suggest that direct interactions between HDAC6 and Runx2 may contribute to Runx2-mediated repression.
Repression by the Runx2 carboxy terminus is insensitive to the HDAC6 inhibitor TPX-B.
To confirm that HDAC6 contributed to repression by the Runx2 carboxy terminus, we tested a second HDAC inhibitor, TPX-B, in our repression assays. TPX-B inhibits all HDACs, but HDAC6 is 300- to 400-fold less sensitive to TPX-B than are the other HDACs (16). In contrast, low concentrations of TSA inhibit all HDACs, including HDAC6 (16). Thus, if HDAC6 mediates repression by the Runx2 carboxy terminus, then the repression by this region would be reversed by TSA but not by TPX-B. TSA significantly reversed repression by GAL-Runx2 (383-498) (Fig. 5A) and GAL-Runx2 (1-383) (Fig. 5C) but only modestly affected GAL-Runx2 (383-513) (Fig. 3 and 5C). GAL-Runx2 (1-383) was also sensitive to TPX-B, but GAL-Runx2 (383-513) and (383-498) were not (Fig. 5). These data further demonstrate that HDAC-independent mechanisms contribute to repression by the complete carboxy terminus. They also provide functional support for physical interaction between HDAC6 and Runx2.
FIG. 5.
Runx2 repression domains are differentially sensitive to TSA and TPX-B. (A and B) The second Runx2-carboxy-terminal repression domain is sensitive to TSA but not to TPX-B. NIH-3T3 cells were cotransfected with pCMV5-GAL-TK-Luc, pCMV5-SEAP, and pCMV5-M2 or pCMV-M2-Runx2 (383-498). Transfected cells were cultured in the presence or absence of the indicated concentrations of TSA or TPX-B. (C) The complete Runx2 carboxy terminus (383-513) is partially insensitive to both TSA and TPX-B, but the remainder of Runx2 (1-383) is sensitive to both HDAC inhibitors. NIH-3T3 cells were transfected and cultured as described above. RLU, relative light units.
Runx2 recruits HDAC6 to the nucleus.
The interactions between HDAC6 and the Runx2 carboxy terminus suggested that these proteins could associate in vivo to regulate transcription of Runx2 target genes. One assumption of this hypothesis is that HDAC6 and Runx2 must interact in the nucleus. Runx2 is a nuclear transcription factor and interacts with the nuclear matrix (50). HDAC6, in contrast, shuttles between the nucleus and cytoplasm (52). Biochemical fractionation of COS cells overexpressing either FLAG-HDAC6 or Runx2 confirmed these results (Fig. 6). Thus, FLAG-HDAC6 was found only in the 0.5% NP-40-soluble, cytoplasmic fraction. Runx2 was exclusively expressed in the insoluble chromatin-containing fraction. No endogenous Runx2 or HDAC6 was detected in COS cells (data not shown). When FLAG-HDAC6 and Runx2 were coexpressed, a fraction of the total HDAC6 protein was detected in the insoluble chromatin-containing fraction (Fig. 6A and B). Runx2 localization was unaltered. HDAC6 was exclusively cytoplasmic when coexpressed with a truncated Runx2 (1-383) that lacks the HDAC6 interaction domain (Fig. 6B, top panel). Runx2 (1-383) contains the DNA binding domain, however, and thus associates with chromatin. Runx2 did not affect the localization of HDAC5 (Fig. 6B, bottom panel), which is also a class II HDAC but does not interact with Runx2 (Fig. 4). These data indicate that Runx2 and HDAC6 can coassociate with chromatin and that the Runx2 carboxy terminus, which contains an HDAC6 interaction domain, is required for this association.
FIG. 6.
Runx2 and HDAC6 coassociate on chromatin. (A) Runx2 recruits HDAC6 to chromatin. COS7 cells were transfected with pCMV5-FLAG-HDAC6, pCMV5-Runx2, or both plasmids. Cells were lysed as described in Materials and Methods to collect cytoplasmic (C), nuclear (N), or chromatin-containing (Ch) fractions. Proteins were resolved by SDS-PAGE and detected by immunoblotting with FLAG or Runx2 antibodies. Blots were also incubated with antibodies specific for Rac1 and mSinA, the cytoplasmic and nuclear proteins, respectively, to monitor the fractionation procedure. (B) The Runx2 carboxy terminus is required for HDAC6 association with chromatin. COS7 cells were transfected with pCMV5-FLAG-HDAC6 (top panel) or pCMV5-FLAG-HDAC5 (bottom panel) in the absence or presence of pCMV5-Runx2 (1-513) (full-length) or pCMV5-Runx2 (1-383). Proteins were extracted and detected as described for panel A.
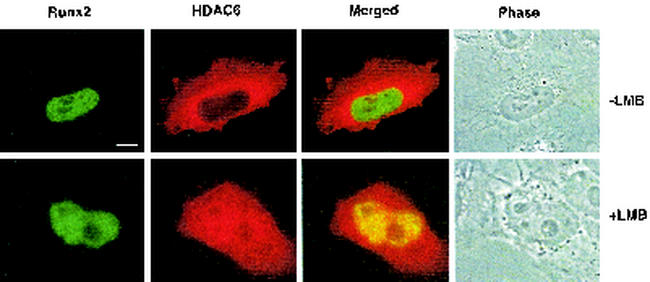
The biochemical fractionation data were confirmed by in situ immunofluorescence (Fig. 7). HeLa cells were cotransfected with HA-Runx2 and FLAG-HDAC6. Runx2 was localized to distinct subnuclear domains (Fig. 7A) as previously described (7, 25, 50, 57). HDAC6 was predominantly cytoplasmic. The addition of the nuclear export inhibitor, LMB, to the culture medium increased nuclear HDAC6 levels as previously reported (52). When trapped in the nucleus, a significant portion of HDAC6 colocalized with Runx2. These data indicate that HDAC6 can interact with Runx2 in the nucleus to regulate its transcriptional activity.
FIG. 7.
Runx2 and HDAC6 coassociate in the nucleus. HeLa cells were cotransfected with pCMV5-Runx2 and pCMV5-FLAG-HDAC6 and cultured in the presence or absence of the nuclear export inhibitor LMB prior to immunofluorescence detection. Bar, 10 μm.
HDAC6 is expressed in osteoblasts during differentiation.
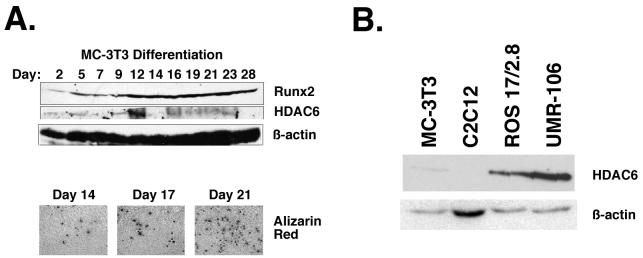
Runx2 is expressed in osteoblasts, regulates many osteoblast-specific genes, and is required for osteoblast differentiation (10, 28, 42). HDAC6 is expressed in some but not all tissues; moreover, its expression may be dependent on cellular differentiation (19, 53). Thus, if interactions between HDAC6 and Runx2 were physiologically relevant, then it would be expected that HDAC6 would be expressed in osteoblasts. To determine whether and when HDAC6 is expressed during osteoblast differentiation, we analyzed HDAC6 protein levels in the murine calvarial cell line MC3T3. This cell line is a well-studied model of osteoblast differentiation and proceeds through well-defined, successive stages of growth, maturation, and mineralization (6, 43, 49). Runx2 was detected in MC3T3 cells through all stages of differentiation and increased during differentiation (Fig. 8A). HDAC6 was also expressed in differentiating osteoblasts. Interestingly, HDAC6 levels fluctuated early in differentiation but stabilized during the mineralization stage. HDAC6 was also detected in osteosarcoma cell lines ROS17/2.8 and UMR-106, which express markers of mature osteoblasts (Fig. 8B). HDAC6 was expressed at significantly lower levels in preosteoblast and osteoblast precursor cell lines, MC-3T3-E1 and C2C12, respectively. These results demonstrate that HDAC6 is coexpressed with Runx2 in differentiating and mature osteoblasts.
FIG. 8.
HDAC6 is expressed in differentiating osteoblasts. (A) MC3T3 cells were differentiated in ascorbic acid and β-glycerolphosphate for the indicated number of days. Total cell lysates (100 μg) were resolved by SDS-PAGE. Runx2, HDAC6, and β-actin were detected by immunoblotting with protein-specific antisera. Calcium matrix production in differentiating osteoblasts was determined in parallel cultures of MC-3T3 cells by staining with Alizarin Red on days 14, 17, and 21 of differentiation. Calcium nodules are indicative of localized matrix production and differentiation. (B) HDAC6 is expressed in mature osteoblast lineage cell lines. Twenty micrograms of lysates from MC3T3-E1 (preosteoblast), C2C12 (osteoblast/myocyte precursor), ROS17/2.8, and UMR-106 (osteosarcomas with a mature osteoblast phenotype) cells were resolved by SDS-PAGE. HDAC6 and β-actin were detected by immunoblotting.
Runx2 interacts with HDAC6 in osteoblasts in vivo.
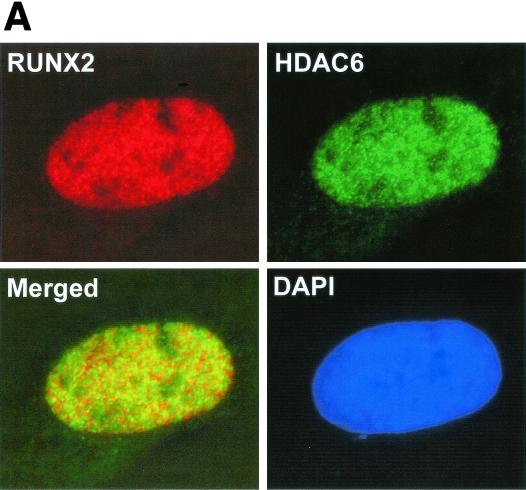
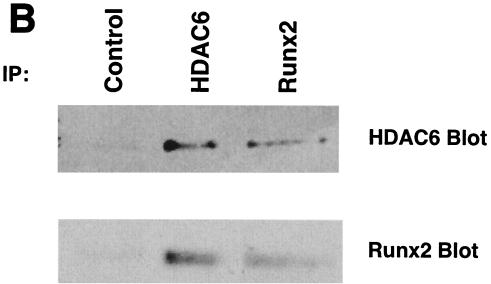
Because ROS17/2.8 cells express Runx2 and HDAC6 at high levels, they were used to confirm interactions between Runx2 and HDAC6 in vivo. Surprisingly, HDAC6 was readily detected in the nuclei of ROS17/2.8 cells as well as in the cytoplasm by in situ immunofluorescence in the absence of LMB (Fig. 9A). The presence of HDAC6 in both cytoplasmic and nuclear compartments was confirmed by biochemical fractionation (data not shown). A significant portion of the HDAC6 present in the nuclei colocalized with Runx2 (Fig. 9A). HDAC6 and Runx2 complexes were also collected by immunoprecipitation with HDAC6 antibodies and reciprocally with Runx2 antisera and detected with immunoblot analysis (Fig. 9B). These data demonstrate that Runx2 and HDAC6 form complexes in osteoblasts.
FIG. 9.
Runx2 and HDAC6 complexes are present in osteoblast lineage cells. (A) Runx2 and HDAC6 colocalize in ROS17/2.8 cell nuclei. ROS17/2.8 cells were grown on gelatin-coated coverslips, fixed, lysed, and incubated with Runx2- and HDAC6-specific antisera and DAPI. Proteins were detected by in situ immunofluorescence microscopy. Digital images were analyzed and deconvolved with the Metamorph software program. (B) HDAC6 and Runx2 coimmunoprecipitate in vivo. ROS17/2.8 lysates were incubated with HDAC6 (Cell Signaling Technology) or Runx2 (S-19; Santa Cruz) antibodies or no antibody (Control). Protein A Sepharose beads were added to all reactions. Proteins bound to the beads were resolved by SDS-PAGE and immunoblotted with HDAC6 and Runx2 antisera.
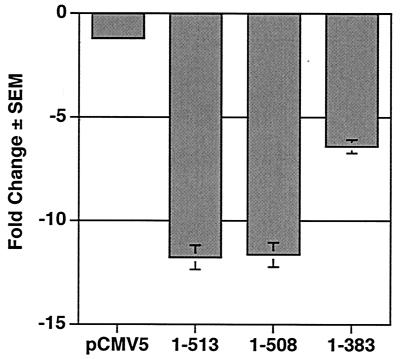
Runx2 repressed a p21-Luc reporter in ROS17/2.8 cells (Fig. 1C). To determine whether the HDAC6 interaction domain is required for repression in osteoblasts, we tested the effects of Runx2 and Runx2 truncation mutants on transcription of the p21 promoter in MC3T3 cells, which express low but detectable levels of HDAC6. p21 levels decrease in MC-3T3 cells as well as in primary osteoblasts during differentiation (9, 46). Full-length Runx2 repressed the p21 promoter in MC3T3 cells by more than 10-fold (Fig. 10). Runx2 (1-508) also repressed p21 transcription, indicating that the TLE binding domain did not contribute to this repression. Runx2 (1-383) inhibited the p21 promoter by approximately fivefold. Thus, deletion of the HDAC6 and TLE binding domains together reduced repression by 50%. We did not test HDAC inhibitors in this assay because they drastically increase p21 transcription (44, 47, 55; J. J. Westendorf, unpublished data). HDAC6 represses the cytomegalovirus and simian virus 40 intermediate-early promoters, thus decreasing Runx2 protein levels and preventing the interpretation of its effects on Runx2-dependent repression of p21 (Fig. 4A). Our data nevertheless indicate that Runx2 interacts with HDAC6 in vivo and suggest that this may contribute to p21CIP1/WAF1 regulation in differentiating osteoblasts.
FIG. 10.
The second repression domain in the Runx2 carboxy terminus is required for maximal repression of the p21 promoter in preosteoblasts. MC3T3 cells were transfected with p21-Luc, pCMV5-SEAP, and pCMV5-M2 or the indicated pCMV-M2-Runx2 expression plasmid. Values represent the means of triplicate samples.
DISCUSSION
We show that Runx2 contains multiple regions that contribute to repression, each of which may utilize distinct mechanisms to contribute to the regulation of Runx2 target genes. In this study, we focused on the Runx2 carboxy terminus because this region is a very potent transcriptional repressor and because it is required for skeletal development (7). We determined that the amino acids carboxy terminal to the activation domain of Runx2 contain two repression domains. Our results are in agreement with those of Thirunavukkarasu et al. (51), who showed that progressive deletions of the carboxy terminus increased Runx2 (OSF2/Til1 isoform) transcriptional activity. One of these domains was defined as the TLE binding residues VWRPY at the end of the protein (25, 51). In this report, we show that amino acids between the Runx2 activation domain and the TLE binding region (residues 383 to 498) are sufficient to repress transcription of a heterologous reporter. This protein domain is also necessary for maximal repression of the p21 promoter. Identical results were observed when a similar Runx2 truncation mutant was tested on the bone sialoprotein promoter (24, 25). Thus, Runx2 contains two functional repression domains carboxy terminal to the activation domain.
We identified a mechanism of repression for the second repression domain of Runx2. The carboxy terminus of Runx2 interacted specifically with HDAC6 and was required for HDAC6 interactions with chromatin. Runx2 and HDAC6 also colocalized in the nucleus. Runx2 is the first transcription factor to be identified as a HDAC6-interacting protein. In fact, only a few proteins, the transcriptional corepressor, ETO-2 (1), two ubiquitin binding proteins, p97/VCP/Cdc48p and PLAP/UFD3 (45), and the recently identified HDAC11 (17) are known to interact with HDAC6. It remains to be determined whether these proteins are components of a HDAC6 complex that interacts with Runx2. Runx factors are ubiquitinated prior to proteasome degradation (22). This suggests an interesting model where interactions with HDAC6 may precede Runx2 ubiquitination and where Runx2-mediated transcription repression is ultimately controlled by protein degradation pathways.
Runx2 residues that are carboxy terminal to the activation domain are functionally and developmentally very important. This region contains the nuclear matrix-targeting signal, which is required for proper subnuclear localization and transcriptional activation (57, 58), and two repression domains. Runx2 amino acids 383 to 414 were sufficient for interaction with HDAC6. This region also encompasses the nuclear matrix-targeting signal. It will be important to determine whether these two activities are functionally related or distinct. Point mutations that lead to premature termination of Runx2 translation were identified in a patient with cleidocranial dysplasia (59). Moreover, mice in which Runx2 alleles were targeted by homologous recombination with a construct containing a stop codon after the activation domain developed skeletal abnormalities similar to those seen in Runx2+/− and Runx2−/− mice (7, 28, 42). Thus, one or more of the functional activities of the Runx2 carboxy terminus is crucial for development.
The activities of HDAC6 and other class II HDACs are tightly regulated. They are predominantly cytoplasmic and are shuttled to the nucleus. The shuttling of HDAC4, HDAC5, and HDAC7 is regulated by calcium/calmodulin protein kinase- and 14-3-3-dependent phosphorylation for transport to and from the nucleus, respectively (20, 27, 35). HDAC6 is also transported to nuclei, but the stimuli regulating its movement are unknown (52). It will be important to identify these factors in osteoblasts, as our studies suggest that HDAC6 shuttling may be regulated by CRM1-dependent and -independent transport mechanisms in cell-type-dependent manners. CRM1-regulated and nonregulated mechanisms were demonstrated to cooperate with epidermal growth factor-stimulated mechanisms in the nuclear accumulation of MEK1 (56). Interestingly, HDAC6 nuclear accumulation has been detected in cell cycle-arrested B16 cells (52). Thus, signals that induce osteoblast differentiation and cell cycle arrest may potentially regulate Runx2 activity by inducing HDAC6 nuclear translocation. The osteosarcoma cell line ROS17/2.8 may produce autocrine factors that induce HDAC6 nuclear translocation and thus may be a good model for studying these events. HDAC6 is additionally regulated by tissue-restricted and differentiation-dependent expression (19, 53). We show that HDAC6 is coexpressed with Runx2 in osteoblasts. HDAC6 protein levels fluctuated during early osteoblast differentiation but were stable in later stages of differentiation. The observations suggest that HDAC6 expression and subcellular localization are factors in the regulation of Runx2 activity in osteoblasts.
Runx2 uses multiple mechanisms to repress transcription. We show that HDAC6 interacts with the carboxy terminus of Runx2 but that HDAC2, HDAC4, and HDAC5 do not. HDAC5 is also not recruited to the chromatin by Runx2. These data do not exclude the possibility that other HDACs interact with Runx2 or that HDAC2 and HDAC4 may interact with other regions of Runx2. In fact, our data suggest that at least one additional region present within the first 383 residues of Runx2 contributes to repression (Fig. 2D). Repression by Runx2 (1-383) is sensitive to the HDAC inhibitors TSA and TPX-B (Fig. 5), perhaps indicating that a class I HDAC complex also interacts with Runx2. Runx2 has previously been shown to interact with mSin3A (33), which is a component of HDAC1 and HDAC2 complexes (29). The mSin3A interaction domain in Runx2 has not yet been defined, but it was mapped to 30 amino acids between the DNA binding domain and activation domain of Runx1 (33). mSin3A interacts with Runx2 (1-383) (Westendorf, unpublished) and thus may mediate the repression by a third repression domain in Runx2. Although it is important to identify all repression domains in Runx2 and to determine their mechanisms of action, our data clearly indicate that it will also be important to determine how the functional domains affect each other.
Acknowledgments
We thank Scott W. Hiebert and Bart Lutterbach for generously providing the HDAC6 antisera.
The University of Minnesota Cancer Center, the Vikings Children's Fund (J.J.W.), and the National Institutes of Health (grant AR45689 to S.K.Z., A.J.V.W., J.B.L., and G.S.S.) financially supported these studies. J.E.C. was a participant in the University of Minnesota Undergraduate Life Sciences Summer Research Program.
REFERENCES
- 1.Amann, J. M., J. Nip, D. K. Strom, B. Lutterbach, H. Harada, N. Lenny, J. R. Downing, S. Meyers, and S. W. Hiebert. 2001. ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol. Cell. Biol. 21:6470-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson, B. D., A. L. Fisher, K. Blechman, M. Caudy, and J. P. Gergen. 1997. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol. Cell. Biol. 17:5581-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, J., J. Hauber, R. Hauber, R. Geiger, and B. R. Cullen. 1988. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 66:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Bertos, N. R., A. H. Wang, and X. J. Yang. 2001. Class II histone deacetylases: structure, function, and regulation. Biochem. Cell Biol. 79:243-252. [PubMed] [Google Scholar]
- 5.Blyth, K., A. Terry, N. Mackay, F. Vaillant, M. Bell, E. R. Cameron, J. C. Neil, and M. Stewart. 2001. Runx2: a novel oncogenic effector revealed by in vivo complementation and retroviral tagging. Oncogene 20:295-302. [DOI] [PubMed] [Google Scholar]
- 6.Choi, J. Y., B. H. Lee, K. B. Song, R. W. Park, I. S. Kim, K. Y. Sohn, J. S. Jo, and H. M. Ryoo. 1996. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J. Cell. Biochem. 61:609-618. [DOI] [PubMed] [Google Scholar]
- 7.Choi, J. Y., J. Pratap, A. Javed, S. K. Zaidi, L. Xing, E. Balint, S. Dalamangas, B. Boyce, A. J. van Wijnen, J. B. Lian, J. L. Stein, S. N. Jones, and G. S. Stein. 2001. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc. Natl. Acad. Sci. USA 98:8650-8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daga, A., C. A. Karlovich, K. Dumstrei, and U. Banerjee. 1996. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 10:1194-1205. [DOI] [PubMed] [Google Scholar]
- 9.Drissi, H., D. Hushka, F. Aslam, Q. Nguyen, E. Buffone, A. Koff, A. van Wijnen, J. B. Lian, J. L. Stein, and G. S. Stein. 1999. The cell cycle regulator p27kip1 contributes to growth and differentiation of osteoblasts. Cancer Res. 59:3705-3711. [PubMed] [Google Scholar]
- 10.Ducy, P., R. Zhang, V. Geoffroy, A. L. Ridall, and G. Karsenty. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747-754. [DOI] [PubMed] [Google Scholar]
- 11.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto, H., M. Enomoto-Iwamoto, M. Iwamoto, S. Nomura, M. Himeno, Y. Kitamura, T. Kishimoto, and T. Komori. 2000. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 275:8695-8702. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, D. D., R. Cai, U. Bhatia, F. A. Asselbergs, C. Song, R. Terry, N. Trogani, R. Widmer, P. Atadja, and D. Cohen. 2002. Isolation and characterization of a novel class II histone deacetylase, HDAC10. J. Biol. Chem. 277:6656-6666. [DOI] [PubMed] [Google Scholar]
- 14.Fischle, W., S. Emiliani, M. J. Hendzel, T. Nagase, N. Nomura, W. Voelter, and E. Verdin. 1999. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J. Biol. Chem. 274:11713-11720. [DOI] [PubMed] [Google Scholar]
- 15.Fischle, W., V. Kiermer, F. Dequiedt, and E. Verdin. 2001. The emerging role of class II histone deacetylases. Biochem. Cell Biol. 79:337-348. [PubMed] [Google Scholar]
- 16.Furumai, R., Y. Komatsu, N. Nishino, S. Khochbin, M. Yoshida, and S. Horinouchi. 2001. Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc. Natl. Acad. Sci. USA 98:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, L., M. A. Cueto, F. Asselbergs, and P. Atadja. 2002. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 277:25748-25755. [DOI] [PubMed] [Google Scholar]
- 18.Gray, S. G., and T. J. Ekstrom. 2001. The human histone deacetylase family. Exp. Cell Res. 262:75-83. [DOI] [PubMed] [Google Scholar]
- 19.Grozinger, C. M., C. A. Hassig, and S. L. Schreiber. 1999. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 96:4868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grozinger, C. M., and S. L. Schreiber. 2000. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 97:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guardiola, A. R., and T. P. Yao. 2001. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem. 277:3350-3356. [DOI] [PubMed] [Google Scholar]
- 22.Huang, G., K. Shigesada, K. Ito, H. J. Wee, T. Yokomizo, and Y. Ito. 2001. Dimerization with PEBP2β protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 20:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai, Y., M. Kurokawa, K. Tanaka, A. D. Friedman, S. Ogawa, K. Mitani, Y. Yazaki, and H. Hirai. 1998. TLE, the human homolog of groucho, interacts with AML1 and acts as a repressor of AML1-induced transactivation. Biochem. Biophys. Res. Commun. 252:582-589. [DOI] [PubMed] [Google Scholar]
- 24.Javed, A., G. L. Barnes, B. O. Jasanya, J. L. Stein, L. Gerstenfeld, J. B. Lian, and G. S. Stein. 2001. runt homology domain transcription factors (Runx, Cbfa, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins. Mol. Cell. Biol. 21:2891-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javed, A., B. Guo, S. Hiebert, J. Choi, J. Green, S. Zhao, M. A. Osborne, S. Stifani, J. L. Stein, J. B. Lian, A. J. van Wijnen, and G. S. Stein. 2000. Groucho/TLE/R-esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J. Cell Sci. 113:2221-2231. [DOI] [PubMed] [Google Scholar]
- 26.Kao, H. Y., C. H. Lee, A. Komarov, C. C. Han, and R. M. Evans. 2002. Isolation and characterization of mammalian HDAC10, a novel histone deacetylase. J. Biol. Chem. 277:187-193. [DOI] [PubMed] [Google Scholar]
- 27.Kao, H. Y., A. Verdel, C. C. Tsai, C. Simon, H. Juguilon, and S. Khochbin. 2001. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J. Biol. Chem. 276:47496-47507. [DOI] [PubMed] [Google Scholar]
- 28.Komori, T., H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R. T. Bronson, Y. H. Gao, M. Inada, M. Sato, R. Okamoto, Y. Kitamura, S. Yoshiki, and T. Kishimoto. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755-764. [DOI] [PubMed] [Google Scholar]
- 29.Laherty, C. D., W. M. Yang, J. M. Sun, J. R. Davie, E. Seto, and R. N. Eisenman. 1997. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell 89:349-356. [DOI] [PubMed] [Google Scholar]
- 30.Levanon, D., R. E. Goldstein, Y. Bernstein, H. Tang, D. Goldenberg, S. Stifani, Z. Paroush, and Y. Groner. 1998. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc. Natl. Acad. Sci. USA 95:11590-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund, A. H., and M. van Lohuizen. 2002. RUNX: a trilogy of cancer genes. Cancer Cell. 1:213-215. [DOI] [PubMed] [Google Scholar]
- 32.Lutterbach, B., and S. W. Hiebert. 2000. Role of the transcription factor AML-1 in acute leukemia and hematopoietic differentiation. Gene 245:223-235. [DOI] [PubMed] [Google Scholar]
- 33.Lutterbach, B., J. J. Westendorf, B. Linggi, S. Isaac, E. Seto, and S. W. Hiebert. 2000. A mechanism of repression by acute myeloid leukemia-1, the target of multiple chromosomal translocations in acute leukemia. J. Biol. Chem. 275:651-656. [DOI] [PubMed] [Google Scholar]
- 34.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2000. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97:14400-14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers, S., J. R. Downing, and S. W. Hiebert. 1993. Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol. Cell. Biol. 13:6336-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers, S., N. Lenny, W. Sun, and S. W. Hiebert. 1996. AML-2 is a potential target for transcriptional regulation by the t(8;21) and t(12;21) fusion proteins in acute leukemia. Oncogene 13:303-312. [PubMed] [Google Scholar]
- 38.Miska, E. A., C. Karlsson, E. Langley, S. J. Nielsen, J. Pines, and T. Kouzarides. 1999. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18:5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mundlos, S., F. Otto, C. Mundlos, J. B. Mulliken, A. S. Aylsworth, S. Albright, D. Lindhout, W. G. Cole, W. Henn, J. H. Knoll, M. J. Owen, R. Mertelsmann, B. U. Zabel, and B. R. Olsen. 1997. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89:773-779. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa, E., M. Maruyama, H. Kagoshima, M. Inuzuka, J. Lu, M. Satake, K. Shigesada, and Y. Ito. 1993. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc. Natl. Acad. Sci. USA 90:6859-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otto, F., H. Kanegane, and S. Mundlos. 2002. Mutations in the RUNX2 gene in patients with cleidocranial dysplasia. Hum. Mutat. 19:209-216. [DOI] [PubMed] [Google Scholar]
- 42.Otto, F., A. P. Thornell, T. Crompton, A. Denzel, K. C. Gilmour, I. R. Rosewell, G. W. Stamp, R. S. Beddington, S. Mundlos, B. R. Olsen, P. B. Selby, and M. J. Owen. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765-771. [DOI] [PubMed] [Google Scholar]
- 43.Quarles, L. D., S. R. Siddhanti, and S. Medda. 1997. Developmental regulation of osteocalcin expression in MC3T3-E1 osteoblasts: minimal role of the proximal E-box cis-acting promoter elements. J. Cell. Biochem. 65:11-24. [PubMed] [Google Scholar]
- 44.Richon, V. M., T. W. Sandhoff, R. A. Rifkind, and P. A. Marks. 2000. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. USA 97:10014-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seigneurin-Berny, D., A. Verdel, S. Curtet, C. Lemercier, J. Garin, S. Rousseaux, and S. Khochbin. 2001. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol. 21:8035-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, E., R. A. Redman, C. R. Logg, G. A. Coetzee, N. Kasahara, and B. Frenkel. 2000. Glucocorticoids inhibit developmental stage-specific osteoblast cell cycle. Dissociation of cyclin A-cyclin-dependent kinase 2 from E2F4-p130 complexes. J. Biol. Chem. 275:19992-20001. [DOI] [PubMed] [Google Scholar]
- 47.Sowa, Y., T. Orita, S. Hiranabe-Minamikawa, K. Nakano, T. Mizuno, H. Nomura, and T. Sakai. 1999. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Ann. N. Y. Acad. Sci. 886:195-199. [DOI] [PubMed] [Google Scholar]
- 48.Stewart, M., A. Terry, M. Hu, M. O'Hara, K. Blyth, E. Baxter, E. Cameron, D. E. Onions, and J. C. Neil. 1997. Proviral insertions induce the expression of bone-specific isoforms of PEBP2αA (CBFA1): evidence for a new myc collaborating oncogene. Proc. Natl. Acad. Sci. USA 94:8646-8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sudo, H., H. A. Kodama, Y. Amagai, S. Yamamoto, and S. Kasai. 1983. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 96:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, L., B. Guo, A. Javed, J. Y. Choi, S. Hiebert, J. B. Lian, A. J. van Wijnen, J. L. Stein, G. S. Stein, and G. W. Zhou. 1999. Crystal structure of the nuclear matrix targeting signal of the transcription factor acute myelogenous leukemia-1/polyoma enhancer-binding protein 2αB/core binding factor α2. J. Biol. Chem. 274:33580-33586. [DOI] [PubMed] [Google Scholar]
- 51.Thirunavukkarasu, K., M. Mahajan, K. W. McLarren, S. Stifani, and G. Karsenty. 1998. Two domains unique to osteoblast-specific transcription factor Osf2/Cbfa1 contribute to its transactivation function and its inability to heterodimerize with Cbfβ. Mol. Cell. Biol. 18:4197-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdel, A., S. Curtet, M. P. Brocard, S. Rousseaux, C. Lemercier, M. Yoshida, and S. Khochbin. 2000. Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr. Biol. 10:747-749. [DOI] [PubMed] [Google Scholar]
- 53.Verdel, A., and S. Khochbin. 1999. Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. J. Biol. Chem. 274:2440-2445. [DOI] [PubMed] [Google Scholar]
- 54.Westendorf, J. J., C. M. Yamamoto, N. Lenny, J. R. Downing, M. E. Selsted, and S. W. Hiebert. 1998. The t(8;21) fusion product, AML-1-ETO, associates with C/EBP-α, inhibits C/EBP-α-dependent transcription, and blocks granulocytic differentiation. Mol. Cell. Biol. 18:322-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao, H., T. Hasegawa, and K. Isobe. 1999. Both Sp1 and Sp3 are responsible for p21waf1 promoter activity induced by histone deacetylase inhibitor in NIH3T3 cells. J. Cell Biochem. 73:291-302. [PubMed] [Google Scholar]
- 56.Yao, Z., I. Flash, Z. Raviv, Y. Yung, Y. Asscher, S. Pleban, and R. Seger. 2001. Non-regulated and stimulated mechanisms cooperate in the nuclear accumulation of MEK1. Oncogene 20:7588-7596. [DOI] [PubMed] [Google Scholar]
- 57.Zaidi, S. K., A. Javed, J. Y. Choi, A. J. van Wijnen, J. L. Stein, J. B. Lian, and G. S. Stein. 2001. A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin gene. J. Cell Sci. 114:3093-3102. [DOI] [PubMed] [Google Scholar]
- 58.Zeng, C., S. McNeil, S. Pockwinse, J. Nickerson, L. Shopland, J. B. Lawrence, S. Penman, S. Hiebert, J. B. Lian, A. J. van Wijnen, J. L. Stein, and G. S. Stein. 1998. Intranuclear targeting of AML/CBFα regulatory factors to nuclear matrix-associated transcriptional domains. Proc. Natl. Acad. Sci. USA 95:1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, Y. W., N. Yasui, K. Ito, G. Huang, M. Fujii, J. Hanai, H. Nogami, T. Ochi, K. Miyazono, and Y. Ito. 2000. A RUNX2/PEBP2α A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc. Natl. Acad. Sci. USA 97:10549-10554. [DOI] [PMC free article] [PubMed] [Google Scholar]