Abstract
The photosynthetic cyanobacterium Synechocystis sp. strain PCC6803 possesses homologs of known genes of the non-mevalonate 2-C-methyl-d-erythritol 2-phosphate (MEP) pathway for synthesis of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Isoprenoid biosynthesis in extracts of this cyanobacterium, measured by incorporation of radiolabeled IPP, was not stimulated by pyruvate, an initial substrate of the MEP pathway in Escherichia coli, or by deoxyxylulose-5-phosphate, the first pathway intermediate in E. coli. However, high rates of IPP incorporation were obtained with addition of dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GA3P), as well as a variety of pentose phosphate cycle compounds. Fosmidomycin (at 1 μM and 1 mM), an inhibitor of deoxyxylulose-5-phosphate reductoisomerase, did not significantly inhibit phototrophic growth of the cyanobacterium, nor did it affect [14C]IPP incorporation stimulated by DHAP plus GA3P. To date, it has not been possible to unequivocally demonstrate IPP isomerase activity in this cyanobacterium. The combined results suggest that the MEP pathway, as described for E. coli, is not the primary path by which isoprenoids are synthesized under photosynthetic conditions in Synechocystis sp. strain PCC6803. Our data support alternative routes of entry of pentose phosphate cycle substrates derived from photosynthesis.
Isoprenoids are synthesized in all free-living organisms. In plants, isoprenoid biosynthesis is required for the production of important compounds, including vitamins, essential oils, carotenoids, quinones, chlorophylls, rubber, and plant hormones. For these and more than 30,000 other plant isoprenoids that have been identified, biosynthesis commences with one or both of two isomeric five-carbon (C5) building blocks, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). The two major biosynthetic pathways by which IPP and DMAPP are synthesized are the mevalonic acid (MVA) pathway and the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway (also designated the non-MVA, DOXP, or 1-deoxy-d-xylulose-5-phosphate [DXP] pathway). A number of bacteria, including Escherichia coli (33), possess the MEP pathway, as apparently do the photosynthetic cyanobacterium Synechocystis sp. strain PCC6803 (homologs of MEP but not MVA pathway genes are present in the genome) (16) and plant plastids (6, 19, 20). The MEP pathway is largely defined by results from E. coli, which is a heterotrophic gram-negative bacterium.
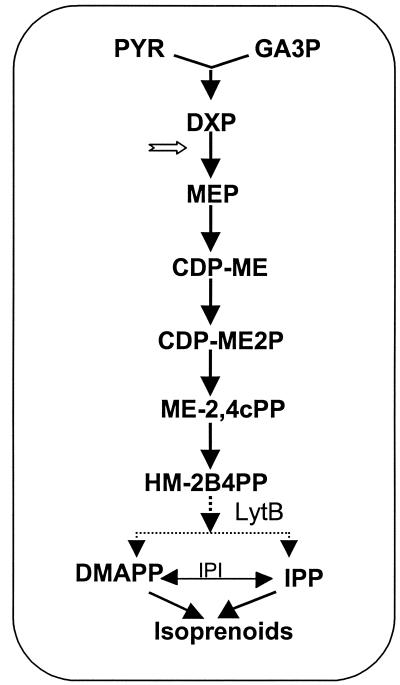
In the last few years, most of the genes of the MEP pathway have been identified in E. coli (8, 33), as illustrated in Fig. 1. In fact, this organism has become a useful host system to identify homologous genes of the MEP pathway from other organisms by complementation and to test the effectiveness of inhibitors of enzymes of the pathway (1, 15, 23). The initial precursors utilized in the pathway are pyruvate (PYR) and glyceraldehyde 3-phosphate (GA3P) and result in the production of DXP. The initial reaction is catalyzed by DXP synthase, which has been verified in vitro by using the purified enzyme from E. coli (21, 37). By a reduction and rearrangement, MEP is formed from DXP, a reaction catalyzed by 1-deoxy-d-xylulose-5-phosphate reductoisomerase. This enzyme, as indicated by analysis of the purified recombinant protein, requires divalent cations, is strictly dependent on NADPH for activity (40), and is inhibited by fosmidomycin {[3-(N-formyl-N-hydroxyamino] propylphosphonic acid} (10, 17). Subsequently, MEP is converted to 4-diphosphocytidyl-2-C-methyl-d-erythritol by the ygbP (ispD) gene product in a CTP-dependent reaction. The crude E. coli enzyme, with MEP provided as a substrate, was first shown to be effective in carotenoid synthesis in a red pepper system (32), and, subsequently, the gene was found to be essential for IPP synthesis in E. coli (18). ATP phosphorylation by a 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase (a product of the ychB [ispE] gene) results in the synthesis of 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate, as shown by Lüttgen et al. (22). The next step is thought to be the removal of CMP to give the cyclic 2-C-methyl-d-erythritol 2,4-cyclodiphosphate, a reaction catalyzed by the enzyme encoded by the ygbB (ispF) gene (12, 39). Interestingly, in a number of bacteria, 2-C-methyl-d-erythritol 2,4-cyclodiphosphate accumulates under oxidative stress (25). A conversion by the gcpE (ispG) gene product results in 1-hydroxy-2-methyl-2-butenyl 4-diphosphate (2, 4, 13, 31). The next step probably involves lytB, a gene that was first shown to be essential in Synechocystis sp. strain 6803 and which our laboratory initially proposed as an enzyme at or near the branch point leading to IPP and DMAPP synthesis (5). In E. coli, a role for lytB (ispH) in the MEP pathway has also been demonstrated (3), and Rohdich et al. (31) recently presented evidence as to how IPP and DMAPP could be formed through the involvement of this enzyme.
FIG. 1.
Pathway of isoprenoid synthesis in E. coli. The initial substrates GA3P and PYR lead to DXP and then to MEP in a reaction catalyzed by a reductoisomerase, which can be inhibited (notched arrow) by fosmidomycin. This is followed by formation of 4-diphosphocytidyl-2-C-methyl-d-erythritol (CDP-ME) and, subsequently, 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate (CDP-ME2P), 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (ME-2,4cPP), and 1-hydroxy-2-methyl-2-butenyl 4-diphosphate (HM-2B4PP). The LytB enzyme is considered to be the branch point leading to the eventual formation of IPP and DMAPP. IPP isomerase is present, but not essential. (The figure was modified from references 8, 31, and 33).
In the MVA pathway, DMAPP is synthesized from IPP via an IPP isomerase (29), and an IPP isomerase is sometimes found in cells with the MEP pathway, including plant plastids (7, 38), green algae (9), and E. coli. The IPP isomerase is not an essential enzyme in E. coli (11). We found that Synechocystis sp. strain PCC6803 and Synechococcus sp. strain PCC7942 are deficient in IPP isomerase activity (9), which is consistent with the absence in Synechocystis of an ipi gene in its genome (16). Recently, a new type of IPP isomerase (type II isomerase) was identified by Kaneda et al. (15) from Streptomyces sp. strain CL 190. The purified enzyme, expressed in E. coli, was shown to specifically require both NADPH and flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD) for the conversion of IPP to DMAPP (15). Homologs are widely found in gram-positive bacteria, archaebacteria, and Synechocystis sp. strain PCC6803, although the amino acid sequence identity of the Synechocystis and Streptomyces homology is relatively low.
The sequence of reactions of the pathway in E. coli MEP (Fig. 1) is virtually complete, but whether the same linear sequence of reactions operates in other organisms, especially in photosynthetic oxygenic organisms, remains to be tested. In this investigation, we examined Synechocystis sp. strain PCC6803 grown under phototrophic growth conditions and ascertained the utilization of photosynthate substrates, as well as the effect of a specific inhibitor (fosmidomycin) that effectively blocks the MEP pathway of E. coli.
In accordance with our data presented below, we hypothesize that in Synechocystis sp. strain PCC6803, the MEP pathway, as defined for E. coli, is not the primary pathway by which isoprenoids are synthesized under photosynthetic conditions.
MATERIALS AND METHODS
Cell culture and fractionation.
Except when indicated, the chemicals used in this study were purchased from Sigma Chemical Co. Liquid cultures of Synechocystis sp. strain PCC6803 (obtained from Wim Vermaas, Arizona State University) were grown at ca. 30°C in continuous light (15 to 20 μmol/m2 s) with shaking and slow bubbling of 5% CO2 in air. The culture medium BG-11 was supplemented with 5 mM potassium-TES [N-tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid (pH 8.3)]. The same medium was used for small volumes (2 ml/15-ml culture tube) with illumination at ca. 20 μmol/m2 s at 20°C, to test growth (measured at an optical density at 730 nm [OD730]) without and with fosmidomycin (1 μM and 1 mM). Fosmidomycin was a gift of Fujisawa Pharmaceutical Co., Ltd., or was obtained from Molecular Probes, Inc.
For in vitro fractionation studies, Synechocystis sp. strain PCC6803 cells were harvested in the log phase of growth. Cells pelleted by centrifugation were quickly rinsed in 100 mM HEPES-KOH (pH 7.7) and 1 mM dithiothreitol (DTT), treated with lysozyme (10 mg/ml, 60 min, 37°C), rinsed in the same buffer, and broken in a French pressure cell (20,000 lb/in2, 4°C). The supernatant fraction (3 to 5.5 mg of protein per ml), after centrifugation at 60,000 × g for 1 h, was stored at −80°C for use within 3 weeks.
Assay of [14C]IPP incorporation.
The reaction mixture was composed of the cell-free supernatant fraction (60,000 × g) with 100 mM HEPES-KOH, 5 mM MgCl2, 2.5 mM MnCl2, 500 μM ATP, 500 μM CTP, 100 μM thiamine-diphosphate, 5 mM glutathione,10 μM coenzyme B12 (5′-deoxyadenosyl-cobalamin), 1 mM NADPH, 500 μM NADP, and 1 mM FAD (pH 7.7). The cofactor components supplied to the reaction mixture are more extensive than those normally used for IPP isomerase assays (9, 38) in order not to limit other reactions that might be required for optimal functioning in a complex system. Each incubation was carried out in a total volume of 1 ml with a final concentration of 8.5 μM [1-14C]IPP (Amersham) (as well as 8.25 × 105 dpm/ml) at 37°C. Aliquots of 0.2 ml (0.2 to 0.46 mg of protein) were assayed for radioactivity. Except for d,l-GA3P at 1 mM, the following compounds were added at a 500 μM concentration, individually or in combination: DXP, GA3P, MEP, dihydroxyacetone phosphate (DHAP), d-erythrose 4-phosphate (ER4P), d-fructose 6-phosphate (FR6P), d-glucose 6-phosphate (GL6P), 6-phosphogluconate (6GP), phosphoenolpyruvate, d-ribulose 5-phosphate (RU5P), d-xylulose, d-erythrose, d-glucose, d-glyceraldehyde, 3-phosphoglycerate, d-mannitol, sodium ascorbate, d-sorbitol, sucrose, and d-xylose. The enzymes RU5P-3-epimerase and 6PG-dehydrogenase were added individually or in combination at 1 U/ml.
The viability of a reaction mixture or possible dilution of 14C label was routinely ascertained by addition of DMAPP after the last incubation period.
To analyze the incorporation of [14C]IPP, the allylic prenols were extracted with petroleum ether (boiling point, 55 to 110°C) after hydrolysis (0.5 N HCl, 37°C, 20 min) as described by Ershov et al. (9), and 1 ml of each extracted sample was counted in 10 ml of ScintiSafe Econo 2 cocktail (Fisher Scientific). Verification that [14C]IPP was incorporated into isoprenoids had been previously established by reversed-phase column chromatography (9). However, in these experiments, we periodically verified the incorporation. Each 0.5-ml sample of the petroleum ether extract was applied to a silica gel 6 RP-18 (EM Industries) column (24 × 1 cm) previously equilibrated in and then eluted with100% acetonitrile. The following alcohols served as calibration standards: isopentenyl alcohol (C5), geraniol (C10), linalool (C10), farnesol (C15), nerolidol (C15), and geranyl geraniol (C20) (Aldrich). DXP and MEP were obtained from Echelon Research Laboratories, Inc. (Salt Lake City, Utah).
RESULTS
Pyruvate and deoxyxylulose 5-phosphate do not stimulate isoprenoid formation.
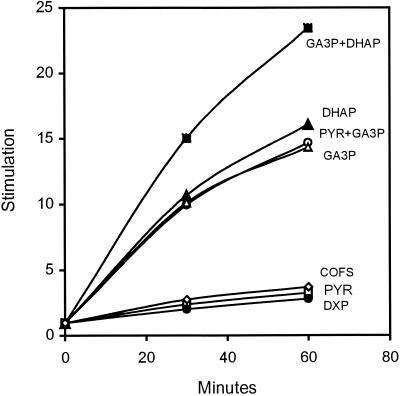
Incorporation of IPP into short- and long-chain isoprenoids requires the availability of the primer molecule DMAPP. Our previous results with cell extracts of Synechocystis sp. strain PCC6803 showed that incorporation of [14C]IPP into isoprenoids was almost completely dependent on addition of DMAPP, and IPP isomerase activity was not detected (9). Incorporation primarily into C10 to C20 isoprenoids was verified by reversed-phase column chromatography. We used the same approach in this study, but with a modified reaction mixture containing several additional cofactors (500 μM ATP, 500 μM CTP, 100 μΜ thiamine diphosphate, 1 mM NADPH, 500 μM NADP, 1 mM FAD, 5 mM glutathione, 5 mM MgCl2, 2.5 mM MnCl2, 10 μM coenzyme B12, 100 mM HEPES-KOH [pH 7.7]), so as not to limit enzymatic reactions needed for DMAPP synthesis. Since Synechocystis has homologous genes of the known MEP pathway, we began testing the initial substrates of the MEP pathway for stimulation of [14C]IPP incorporation in cell extracts. PYR and GA3P are the substrates giving rise to DXP in the MEP pathway in E. coli (8, 34). In fact, GA3P showed significant stimulation of [14C]IPP incorporation (Fig. 2) in Synechocystis, whether supplied alone or together with PYR. Stimulation with DHAP was likewise significant and to the same level as with GA3P. In vitro stimulation of IPP incorporation was even greater with GA3P plus DHAP (Fig. 2). This is not surprising, since DHAP and GA3P can be readily interconverted by triosephosphate isomerase. At least 70% of the 14C label appeared in farnesol (C15) and geranyl geraniol (C20) when analyzed on a reversed-phase column (data not shown).
FIG. 2.
GA3P and dihydroxyacetone phosphate stimulate [14C]IPP incorporation in a cell extract of Synechocystis sp. strain PCC6803 grown photoautotrophically, but 1-deoxy-d-xylulose 5-phosphate and pyruvate do not. The following compounds were added individually or in combination: GA3P (1 mM) (▵), DHAP (500 μM) (▴), DXP (500 μM) (•), PYR (500 μM) (□), GA3P plus DHAP (500 μM) (▪), and GA3P plus PYR (500 μM) (○). COFS, cofactors alone (⋄). The cell extract (60,000 × g supernatant) (pH 7.7) (100 mM HEPES-KOH), cofactor mixture (500 μM ATP, 250 μM CTP, 100 μM thiamine diphosphate, 1 mM NADPH, 500 μM NADP, 1 mM FAD, 5 mM glutathione, 5 mM MgCl2, 2.5 mM MnCl2, 10 μM coenzyme B12), and/or metabolites were incubated at 37°C. Incorporation of [14C]IPP into isoprenoids was verified by analyzing the petroleum ether extracts of the acid-hydrolyzed fraction (9).
No significant [14C]IPP incorporation was obtained with PYR above the level obtained with the cofactors alone (Fig. 2). Neither was there an additive effect when PYR was tested in combination with GA3P. This is in contrast to results expected from studies of the E. coli MEP pathway, in which a condensation of PYR and GA3P is required for DXP synthesis. Since DXP is the first product of the MEP pathway and since it stimulates isoprenoid formation in E. coli (8, 33), we tested the effect of DXP in Synechocystis sp. strain PCC6803. In our cyanobacterial system, DXP, like PYR, did not show stimulation above the level of the cofactors. These results suggest that if PYR and GA3P condensation occurs to form DXP, it is not reflected in isoprenoid biosynthesis.
Isoprenoid synthesis is stimulated by phosphorylated metabolites.
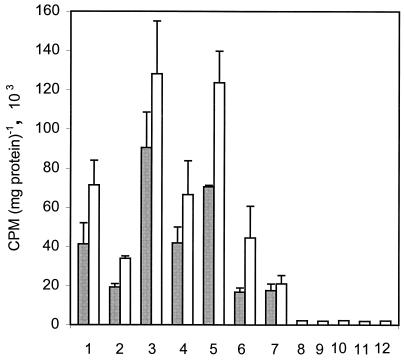
It can be presumed that, under photosynthetic conditions, the substrates for DMAPP formation, and hence isoprenoid production, may be derived from metabolite products of photosynthesis, of which GA3P is a common intermediate. Stimulation of isoprenoid synthesis was tested by adding various metabolites to the cell-free system of Synechocystis (60,000 × g supernatant with cofactors) and then monitoring the system for up to 60 min. Only phosphorylated (ER4P, RU5P, GL6P, FR6P, MEP, and GA3P) metabolites gave significant stimulation (Fig. 3). The steady increase in [14C]IPP incorporation from 0 min (data not shown) to 30 and 60 min supported by phosphorylated metabolites is indirect evidence of substrate utilization. Nonphosphorylated metabolites, such as erythrose, glucose, glyceraldehyde, sucrose, and xylulose, were virtually ineffective, even with longer incubation of up to 120 min. Equally ineffective were arabinose, 3-phosphoglycerate, fructose, mannitol, phosphoenol pyruvate, sodium ascorbate, sorbitol, and xylose (data not shown). The most effective phosphorylated metabolites were GL6P and FRU6P, followed by ER4P and then 6PG (Fig. 3). Whereas MEP showed notable stimulation of the incorporation of [14C]IPP into isoprenoids, the effect was similar to that of GA3P or DHAP when added individually. Although some variations occurred between some preparations, the trend of the relative effectiveness of each metabolite as shown in Fig. 3 was always the same. If MEP is a key intermediate in the pathway, as in E. coli, it would be expected to show significantly more stimulation. However, to the contrary, it was less effective than most other phosphorylated sugars. It thus seems probable that it is only one of several metabolites, but is not essential under the conditions tested.
FIG. 3.
Stimulation of [14C]IPP incorporation by phosphorylated sugars in cell extract of Synechocystis sp. strain PCC6803. Column numbers specify the supernatant (60,000 × g), with the cofactors and conditions given in the legend to Fig. 2, with individual incubation of the following: 1, ER4P (500 μM); 2, RU5P (500 μM); 3, GL6P (500 μM); 4, 6PG (500 μM); 5, FR6P (500 μM); 6, MEP (500 μM); 7, GA3P (1 mM); 8, erythrose (500 μM); 9, glucose (500 μM); 10, xylulose (500 μM); 11, sucrose (500 μM); and 12, glyceraldehyde (500 μM). Incubation (37°C) was for 30 min (gray bars) and 60 min (white bars). Incorporation of [14C]IPP into isoprenoids was verified by analyzing the petroleum ether extracts of the acid-hydrolyzed fraction (9). Each value is the mean + standard deviation of two (columns 1, 3, 5, 6, and 8 to 12) or three (columns 2, 4, and 7) independent experiments.
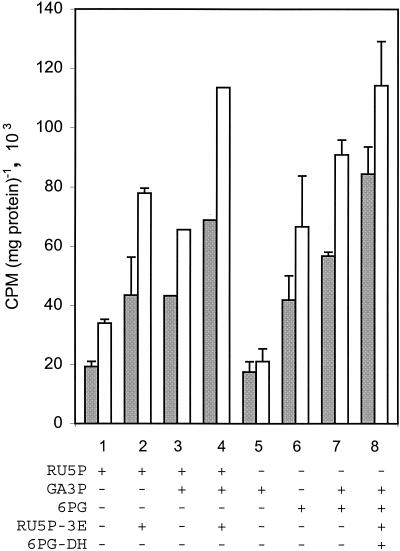
The finding that GA3P and certain phosphorylated compounds stimulate [14C]IPP incorporation suggested that photosynthetic metabolites of the pentose phosphate cycle are involved in isoprenoid synthesis. We thus considered that xylulose 5-phosphate (XY5P), or similar compounds, could be precursors leading to DMAPP synthesis (Fig. 4). Because XY5P is not commercially available, we tested substrates and enzymes that could lead to its synthesis. As seen in Fig. 4, [14C]IPP incorporation is stimulated by RU5P (column 1), and the stimulation doubles with the enzyme RU5P-3-epimerase (column 2), and is even further enhanced (ca. 50%) with GA3P (column 4). An additive effect of GA3P is also found together with 6PG (Fig. 4, compare columns 5 to 7). However, more important is the further enhanced stimulation (ca. 30%) with the addition of the enzymes 6PG-dehydrogenase and RU5P-3-epimerase (Fig. 4, column 8). This suggests greater conversion toward the production of DMAPP via XY5P in Synechocystis.
FIG. 4.
Stimulation of [14C]IPP incorporation by pentose phosphate cycle sugars in the cell-free supernatant fraction (60,000 × g) of Synechocystis PCC6803. Columns: 1, RU5P (500 μM); 2, RU5P (500 μM) plus RU5P-3-epimerase; 3, RU5P (500 μM) plus GA3P (1 mM); 4, RU5P (500 μM) plus GA3P plus RU5P-3-epimerase; 5, GA3P (1 mM); 6, 6PG (500 μM); 7, 6PG (500 μM) plus GA3P (1 mM); 8, 6PG (500 μM) plus GA3P plus 6PG-dehydrogenase plus RU5P-3-epimerase. Incubation was at 37°C for 30 min (gray bars) and 60 min (white bars) with buffer and cofactors, and analysis was performed as described in the legend to Fig. 2. Each value is the mean + standard deviation of three (columns 1, 5, and 6) or two (columns 2, 7, and 8) independent experiments, with columns 3 and 4 each representing a single experiment.
The DXR inhibitor fosmidomycin does not inhibit photosynthetic growth of Synechocystis sp. strain PCC6803.
Because cyanobacteria are regarded as progenitors of chloroplasts, presumably with a similarly functioning MEP pathway, it was of interest to test the effect of a well-known inhibitor of the pathway on the growth of Synechocystis under light in a mineral medium in which the organism is dependent on photosynthesis for substrates. Fosmidomycin is an herbicide that inhibits plant growth (19), and its effect eliminates isoprene emission in oak leaves (36). Growth of E. coli is inhibited by fosmidomycin, and it has been shown that this compound inhibits the activity of the recombinant DXR enzyme (17), thereby preventing the conversion of DXP to MEP.
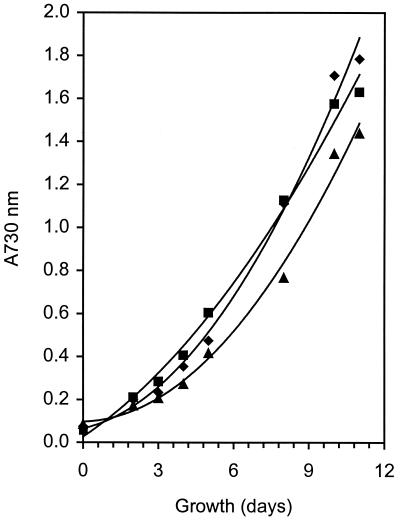
Fosmidomycin was not an effective inhibitor of growth for cultures of Synechocystis grown for at least 10 days under photoautotrophic conditions in a mineral medium (BG-11). As seen in Fig. 5, cells with 1 μM fosmidomycin grew at about the same rate and reached the same final cell density as cultures lacking the inhibitor. Even at a concentration of fosmidomycin 1,000 times higher (1 mM), there was no significant inhibition of growth (Fig. 5). As expected, we found that fosmidomycin (0.5 to 1.0 μM) completely inhibited growth of E. coli. To address the possibility that fosmidomycin might be inactivated during the culturing of the cyanobacteria, the “conditioned medium” was collected after 10 days of growth. Upon a 100-fold dilution of the “conditioned medium,” growth of E. coli was still inhibited. Thus, inactivation of fosmidomycin during the culturing of Synechocystis was not a major factor. In all cell-free experiments, fosmidomycin also did not notably affect the [14C]IPP incorporation stimulated by GA3P or DHAP (data not shown). A lack of uptake of fosmidomycin into cells, therefore, is not likely responsible for lack of growth inhibition in Synechocystis. These combined observations raise the question of the role of DXP reductoisomerase as an essential enzyme under photosynthetic conditions in this organism.
FIG. 5.
Growth of Synechocystis sp. strain PCC6803 in the presence of the MEP synthesis inhibitor fosmidomycin. Results are shown for growth in BG-11 growth medium alone (♦), BG-11 medium plus 1 μM fosmidomycin (▪), and BG-11 medium plus 1 mM fosmidomycin (▴). Cultures were grown at 20°C with 20 μM/m2 s light in air and monitored at A730. The data represent the average of three separate experiments.
Synechocystis sp. strain PCC6803 is deficient in IPP isomerase activity.
IPP isomerase is found widely distributed in plants, animals, and microorganisms. Generally, in organisms with the MVA pathway, an IPP isomerase is essential for interconversion of IPP to DMAPP (29). However, in E. coli, which also contains an IPP isomerase, the IPP isomerase is not essential (11), strongly suggesting that the MEP pathway provides for IPP and DMAPP by separate branches (5, 9, 30). Nevertheless, increasing the copy number of the ipi gene can be effective in enhancing carotenoid (a C40 isoprenoid) accumulation in E. coli (5, 14).
Recently, an unusual IPP isomerase (type II) was reported in an MVA pathway gene cluster from Streptomyces (15). This type II IPP isomerase shows no apparent resemblance to the type I enzyme characteristic of E. coli or of MVA pathway organisms and requires NADPH plus FAD (or FMN). As noted by the Seto laboratory, a remote similarity (44.9%) and identity (35.1%) of the Streptomyces sequence are present in the genome of Synechocystis (15). Having previously reported IPP isomerase deficiency in Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC7942 (9) by conventional assay methods (7, 38), we reexamined cell extracts of Synechocystis under conditions appropriate for the type II isomerase. Even with the inclusion of NADPH plus FAD (or FMN) in the reaction mixture, we were unable to demonstrate any significant IPP isomerase activity. The likelihood that the various pentose phosphate cycle intermediates activate a cryptic isomerase activity seems remote. It will be of interest to see if the putative type II IPP isomerase in this cyanobacterium is essential for growth.
DISCUSSION
The MEP pathway as defined for E. coli is not the major route for isoprenoid biosynthesis in Synechocystis under photosynthetic conditions.
The involvement of the MEP pathway in isoprenoid synthesis is well established in E. coli, where PYR and GA3P serve as the initial substrates (8, 33). Our data indicate that it is not the major pathway in Synechocystis sp. strain PCC6803 grown under photosynthetic conditions. We suggest that in this cyanobacterium, the products of photosynthesis (i.e., metabolites of the pentose phosphate cycle), lead to the DMAPP synthesis, which is required, together with IPP, for synthesis of isoprenoids. Interconversion of IPP and DMAPP is unlikely, because this cyanobacterium is deficient in IPP isomerase activity, as shown previously (9) and further confirmed in this study (see the Results section above).
If the MEP pathway, as defined in E. coli, were the predominant pathway in Synechocystis, then stimulation of [14C]IPP incorporation by PYR and DXP should have been observed, but these compounds gave virtually no stimulation (Fig. 2). This is in contrast to the role of PYR in E. coli, in which 13C-labeled PYR was shown to provide the C2 subunit, which together with GA3P (C3) accounts for the C5 of the basic isoprenic unit (33, 34). Also, in E. coli and other MEP pathway-containing organisms, DXP or its nonphosphorylated form was readily incorporated into isoprenoids (33).
MEP in Synechocystis stimulated [14C]IPP incorporation (Fig. 3), indicating that it is a useable substrate. However, its effect is much lower than expected if it were an intermediate in a linear pathway for the formation of DMAPP. There is also the lack of apparent inhibition of DXR by fosmidomycin (Fig. 5), a highly effective growth inhibitor of E. coli that had little if any effect on the growth of Synechocystis. It is possible that the cyanobacterial cells are impermeant to fosmidomycin, but this is considered unlikely, because we were also unable to demonstrate fosmidomycin inhibition in vitro. Whereas fosmidomycin also did not inhibit [14C]IPP incorporation into isoprenoids in isolated chromoplasts and chloroplasts (10), this is not strictly comparable to the results in Synechocystis, since plastids may readily interconvert IPP and DMAPP with an IPP isomerase (7, 38).
A possible pathway to DMAPP in PCC6803 under photosynthetic conditions.
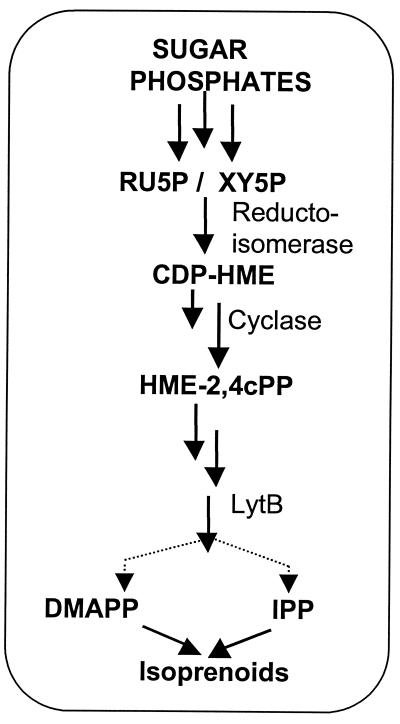
The concept of a linear, vectorial type of MEP pathway as proposed for E. coli may be oversimplistic for photosynthetic organisms. Our data suggest that there are alternative sites for entry of substrates, as shown in the hypothetical pathway for Synechocystis under photosynthetic conditions (Fig. 6). It is not yet clear at which point or points this might occur, but entry is likely to be prior to the reaction catalyzed by the lytB gene product, which is probably at a pathway branch point for DMAPP and IPP formation (5, 31). In considering the metabolic economy of a cell, the utilization of readily available substrates seems most efficient. Growth under photosynthetic autotrophic conditions leads to synthesis of pentose phosphate cycle substrates, and indeed a number of such compounds (ER4P, RU5P, FR6P, GA3P, and DHAP) and those readily converted to pentose phosphate cycle substrates (GL6P and 6PG) stimulate the apparent synthesis of DMAPP (Fig. 3 and 4). Although the route of entry into isoprenoids remains to be determined, we consider entry via XY5P a strong possibility. Our data are consistent with XY5P as a precursor for DMAPP synthesis under photosynthetic conditions (Fig. 4 and 6). The XY5P formation could proceed via one of a number of reactions, including (i) aldolase condensation of DHAP plus GA3P to give fructose 1, 6-diphosphate, followed by hydrolysis of the 1-phosphate and a transketolase reaction with a second GA3P to give ER4P plus XY5P; (ii) a GA3P transketolase reaction with sedoheptulose 7-phosphate to give ribose 5-phosphate and XY5P; and (iii) oxidation of 6PG to RU5P, which is readily converted to XY5P by RU5P-3-epimerase. Before entry into isoprenoids, XY5P could be enzymatically rearranged to form 2-C-hydroxymethyl-erythritol 4-phosphate, which would be analogous to the reductase formation of MEP (2-C-methyl-d-erythritol 4-phosphate) from DXP in E. coli.
FIG. 6.
Hypothetical pathway for the biosynthesis of the isoprenoid precursors IPP and DMAPP in Synechocystis sp. strain PCC6803 under photosynthetic conditions. The carbon atoms originate from photosynthetically produced phosphorylated sugars (e.g., GA3P, DHAP, GL6P, FR6P, 6PG, ER4P, and RU5P). These are shown in the study to stimulate isoprenoid synthesis in a cell-free system and could enter primarily via RU5P and/or XY5P, and after a reductoisomerase reaction, they could be converted to diphosphocytidyl hydroxymethyl d-erythritol (CDP-HME), followed by phosphorylation and cyclization to give 2,4 hydroxymethyl cyclodiphosphate (HME-2,4 cPP). LytB is considered to be near the branch point leading to formation of IPP and DMAPP (5). Interconversion between IPP and DMAPP appears to be lacking under these conditions.
The 6PG path could be the source of XY5P in the dark. It is of interest to recall some early experiments by Pelroy and Bassham (26) in which cyanobacteria were given 14CO2 in light for 10 min and were then switched to darkness. Within 8 min in darkness, there was a rapid loss of 14C-labeled GL6P and ribulose-1,5-bisphosphate, while 6PG rapidly rose, followed by fructose 1,6-bisphosphate, both having started from virtually zero at the time the light was turned off.
It is not unusual to find isoprenoid synthesis tightly linked to carbon cycle metabolism through photosynthesis. For example, in spinach chloroplasts, the synthesis of β-carotene, a C40 isoprenoid, was found to be directly associated with CO2 incorporation (35). What pathway or combination of pathways leads to isoprenoid synthesis under photosynthetic versus nonphotosynthetic conditions remains to be clarified. A number of MEP pathway genes from Synechocystis and Synechococcus strains have been cloned and expressed in E. coli (23, 24, 28), but activity of the enzymes within the cells is not known under either photosynthetic or nonphotosynthetic conditions. For example, the data from Disch et al. (6) and Proteau et al. (27) are reasonably consistent with the MEP pathway, but so far they do not rule out important possible variations that may be advantageous during photosynthesis. There can be little doubt of the importance of MEP pathway as detailed for E. coli, and in fact, it is possible that a similar pathway could be important for Synechocystis under photoheterotrophic conditions. A cell-free approach as begun here, together with the production of Synechocystis sp. strain PC6803 mutants and analysis of substrate formation and utilization of cells grown under different conditions, should be useful in elucidating the involvement of other plausible routes for isoprenoid synthesis.
Acknowledgments
This work was supported primarily by a grant from DOE (DEFG0298ER2032). We are grateful to Fujisawa Pharmaceutical Co., Ltd. (Japan), for a sample of fosmidomycin.
REFERENCES
- 1.Altincicek, B., M. Hintz, S. Sanderbrand, J. Wiesner, E. Beck, and H. Jomaa. 2000. Tools for discovery of inhibitors of the 1-deoxy-d-xylulose 5-phosphate (DXP) synthase and DXP reductoisomerase: an approach with enzymes from the pathogenic bacterium Pseudomonas aeruginosa. FEMS Microbiol. Lett. 190:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Altincicek, B., A.-K. Kollas, S. Sanderbrand, J. Wiesner, M. Hintz, E. Beck, and H. Jomaa. 2001. GcpE is involved in the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. J. Bacteriol. 183:2411-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altincicek, B., A.-K. Kollas, M. Eberl, J. Wiesner, S. Sanderbrand, M. Hintz, E. Beck, and H. Jomaa. 2001. LytB, a novel gene of the 2-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 499:37-40. [DOI] [PubMed] [Google Scholar]
- 4.Campos, N., M. Rodriguez-Concepción, M. Seemann, M. Rohmer, and A. Boronat. 2001. Identification of gcpE as a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 488:170-173. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham, F. X., Jr., T. P. Lafond, and E. Gantt. 2000. Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J. Bacteriol. 182:5841-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disch, A., J. Schwender, C. Müller, H. K. Lichtenthaler, and M. Rohmer. 1998. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC6714. Biochem. J. 333:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dogbo, O., and B. Camara. 1987. Purification of isopentenyl pyrophosphate isomerase and geranylgeranyl pyrophosphate from Capsicum chromoplasts by affinity chromatography. Biochim. Biophys. Acta 920:140-148. [Google Scholar]
- 8.Eisenreich, W., F. Rohdich, and A. Bacher. 2001. Deoxyxylulose phosphate pathway to terpernoids. Trends Plant Sci. 6:78-84. [DOI] [PubMed] [Google Scholar]
- 9.Ershov, Y., R. R. Gantt, F. X. Cunningham, and E. Gantt. 2000. Isopentenyl diphosphate isomerase deficiency in Synechocystis sp. strain PCC6803. FEBS Lett. 473:337-340. [DOI] [PubMed] [Google Scholar]
- 10.Fellermeier, M., K. Kis, S. Sagner, U. Maier, A. Bacher, and M. H. Zenk. 1999. Cell-free conversion of 1-deoxy-d-xylulose 5-phosphate and 2-C-methyl-d-erythritol 4-phosphate into β-carotene in higher plants and its inhibition by fosmidomycin. Tetrahedron Lett. 40:2743-2746. [Google Scholar]
- 11.Hahn, F. M., A. P. Hurlburt, and C. D. Poulter. 1999. Escherichia coli open reading frame 696 is idi, a nonessential gene encoding isopentenyl diphosphate isomerase. J. Bacteriol. 181:4499-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herz, S., J. Wungsintaweekul, C. A. Schuhr, S. Hecht, H. Lüttgen, S. Sagner, M. Fellermeier, W. Eisenreich, H. Zenk, A. Bacher, and F. Rohdich. 2000. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate to 2C-methyl-d-erythritol 2,4-cyclodiphosphate. Proc. Natl. Acad. Sci. USA. 97:2486-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hintz, M., A. Reichenberg, B. Altincicek, U. Bahr, R. M. Gschwind, A.-K. Kollas, E. Beck, J. Wiesner, M. Eberl, and H. Jomaa. 2001. Identification of (E)-4-hydroxy-3- but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 509:317-322. [DOI] [PubMed] [Google Scholar]
- 14.Kajiwara, S., P. D. Fraser, K. Kondo, and N. Misawa. 1997. Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem. J. 324:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneda, K., T. Kuzuyama, M. Takagi, Y. Hayakawa, and H. Seto. 2001. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc. Natl. Acad. Sci. USA 98:932-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, N. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:185-209. [DOI] [PubMed] [Google Scholar]
- 17.Kuzuyama, T., T. Shimizu, S. Takahashi, and H. Seto. 1998. Fosmidomycin, a specific inhibitor of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 39:7913-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuzuyama, T., M. Takagi, K. Kaneda, T. Dairi, and H. Seto. 2000. Formation of 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol from 2-C-methyl-d-erythritol 4-phosphate cytidyl transferase, a new enzyme in the nonmevalonate pathway. Tetrahedron Lett. 41:703-706. [Google Scholar]
- 19.Lichtenthaler, H. K. 1999. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:47-65. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenthaler, H. K., J. Schwender, A. Disch, and M. Rohmer. 1997. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 400:271-274. [DOI] [PubMed] [Google Scholar]
- 21.Lois, L. M., N. Campos, S. R. Putra, K. Danielsen, M. Rohmer, and A. Boronat. 1998. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. USA 95:2105-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lüttgen, H., F. Rohdich, S. Herz, J. Wungsintaweekul, S. Hecht, C. A. Schuhr, M. Fellermeier, S. Sanger, M. H. Zenk, A. Bacher, and W. Eisenreich. 2000. Biosynthesis of terpenoids: YchB protein of Escherichia coli phosphorylates the 2-hydroxy group of 4-diphosphocytidyl-2C-methyl-d-erythritol. Proc. Natl. Acad. Sci. USA 97:1062-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, B., T. Heuser, and W. Zimmer. 1999. A Synechocystis leopoliensis SAUG 1402-1 operon harboring the 1-deoxylulose 5-phosphate synthase gene and two additional open reading frames is functionally involved in the dimethylallyl diphosphate synthesis. FEBS Lett. 460:485-490. [DOI] [PubMed] [Google Scholar]
- 24.Miller, B., T. Heuser, and W. Zimmer. 2000. Functional involvement of a deoxy-d-xylulose 5-phosphate reductoisomerase gene harboring locus of Synechocystis leopoliensis in isoprenoid biosynthesis. FEBS Lett. 481:221-226. [DOI] [PubMed] [Google Scholar]
- 25.Ostrovsky, D., G. Diomina, E. Lysak, E. Matveeva, O. Ogrel, and S. Trutko. 1998. Effect of oxidative stress on the biosynthesis of 2-C-methyl-d-erythritol-2,4-cyclopyrophosphate and isoprenoids by several bacterial strains. Arch. Microbiol. 171:69-72. [DOI] [PubMed] [Google Scholar]
- 26.Pelroy, R. A., and J. A. Bassham. 1972. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch. Microbiol. 86:25-38. [DOI] [PubMed] [Google Scholar]
- 27.Proteau, P. J. 1998. Probing the non-mevalonate pathway to phytol in the cyanobacterium Synechocystis UTEX 2470 using deuterium-labeled glucose. Tetrahedron Lett. 39:9373-9376. [Google Scholar]
- 28.Proteau, P. J., Y.-H. Woo, R. T. Williamson, and C. Phaosiri. 1999. Stereochemistry of the reduction step mediated by recombinant 1-deoxy-d-xylulose 5-phosphate reductoisomerase. Org. Lett. 1:921-923. [DOI] [PubMed] [Google Scholar]
- 29.Ramos-Valdivia, A. C., R. van der Heijden, and R. Verpoorte. 1997. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat. Prod. Rep. 14:591-603. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Concepcion, M., N. Campos, L. Maria Lois, C. Maldonado, J. F. Hoeffler, C. Grosdemange-Billiard, M. Rohmer, and A. Boronat. 2000. Genetic evidence of branching in the isoprenoid pathway for the production of isopentenyl diphosphate and dimethylallyl diphosphate in Escherichia coli. FEBS Lett. 473:328-332. [DOI] [PubMed] [Google Scholar]
- 31.Rohdich, F., S. Hecht, K. Gärtner, P. Adam, C. Krieger, S. Amslinger, D. Arigoni, A. Bacher, and W. Eisenreich. 2002. Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. USA 99:1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohdich, F., J. Wungsintaweekul, M. Fellermeier, S. Sagner, S. Herz, K. Kis, W. Eisenreich, A. Bacher, and M. H. Zenk. 1999. Cytidine 5′-triphosphate-dependent biosynthesis of isoprenoids: YgbP protein of Escherichia coli catalyses the formation of 4-diphosphocytidyl-2-C-methylerythritol. Proc. Natl. Acad. Sci. USA 96:11758-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohmer, M. 1999. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16:565-574. [DOI] [PubMed] [Google Scholar]
- 34.Rohmer, M., M. Seemann, S. Horback, S. Bringer-Meyer, and H. Sahm. 1996. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J. Am. Chem. Soc. 118:2564-2566. [Google Scholar]
- 35.Schulze-Siebert, D., and G. Schultz. 1987. β-Carotene synthesis in isolated spinach chloroplasts: its tight linkage to photosynthetic carbon metabolism. Plant Physiol. 84:1233-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharkey, T. D., X. Chen, and S. Yeh. 2001. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 125:2001-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprenger, G. A., U. Schörken, T. Wiegert, S. Grolle, A. A. de Graaf, S. V. Taylor, T. P. Begley, S. Bringer-Meyer, and H. Sahm. 1997. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc. Natl. Acad. Sci. USA 94:12857-12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spurgeon, S. L., N. Sathyamoorthy, and J. W. Porter. 1984. Isopentenyl pyrophosphate isomerase and prenyltransferase from tomato fruit plastids. Arch. Biochem. Biophys. 230:446-454. [DOI] [PubMed] [Google Scholar]
- 39.Takagi, M., T. Kuzuyama, K. Kaneda, H. Watanabe, T. Dairi, and H. Seto. 2000. Studies on the nonmevalonate pathway: formation of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate from 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol. Tetrahedron Lett. 41:3395-3398. [Google Scholar]
- 40.Takahashi, S., T. Kuzuyama, H. Watanabe, and H. Seto. 1998. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. USA 95:9879-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]