Abstract
1. In conscious lactating goats a significant correlation was found between blood—milk potential difference (p.d.) and milk [lactose] such that in goats with a lower milk [lactose], milk was more negative with respect to blood.
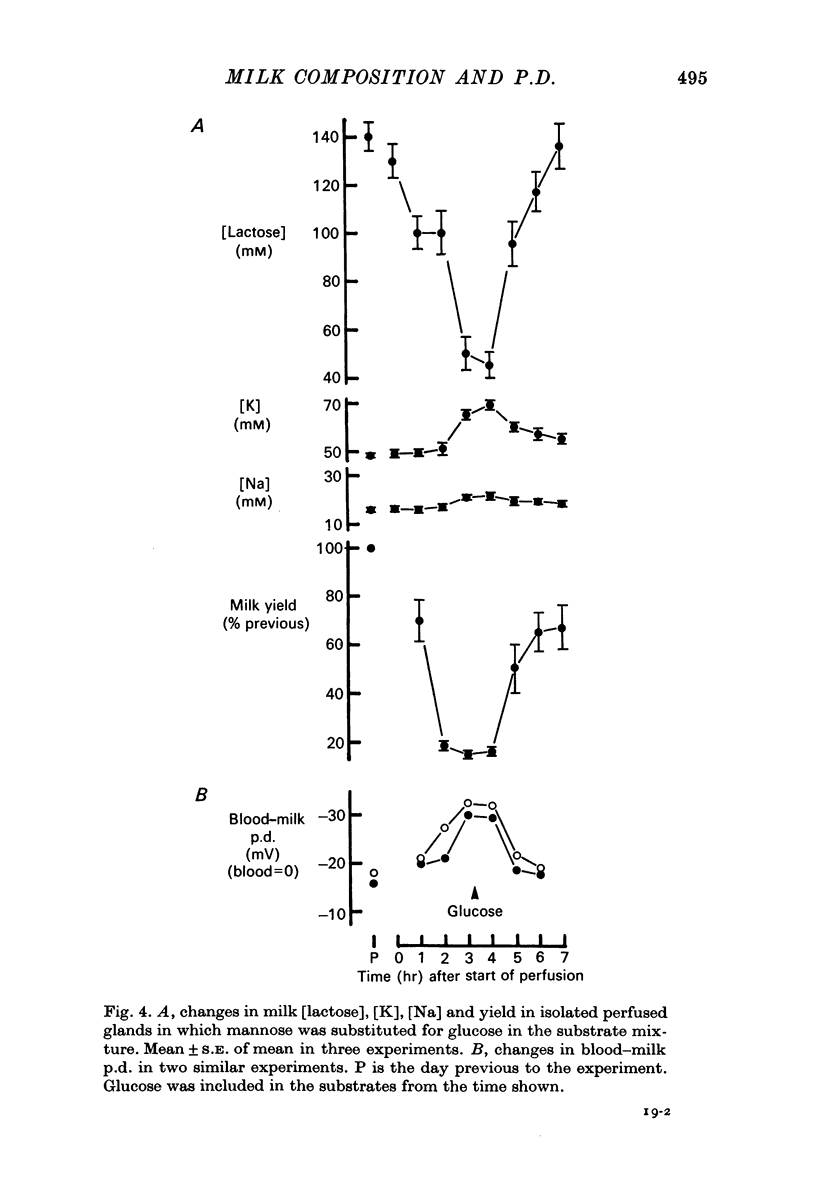
2. When mannose was substituted for glucose in the substrate mixture of isolated perfused goat mammary glands, milk yield and milk [lactose] fell while milk [Na] and [K] increased; in parallel experiments the blood—milk p.d. changed such that milk became more negative with respect to blood. These changes were reversed following the addition of glucose.
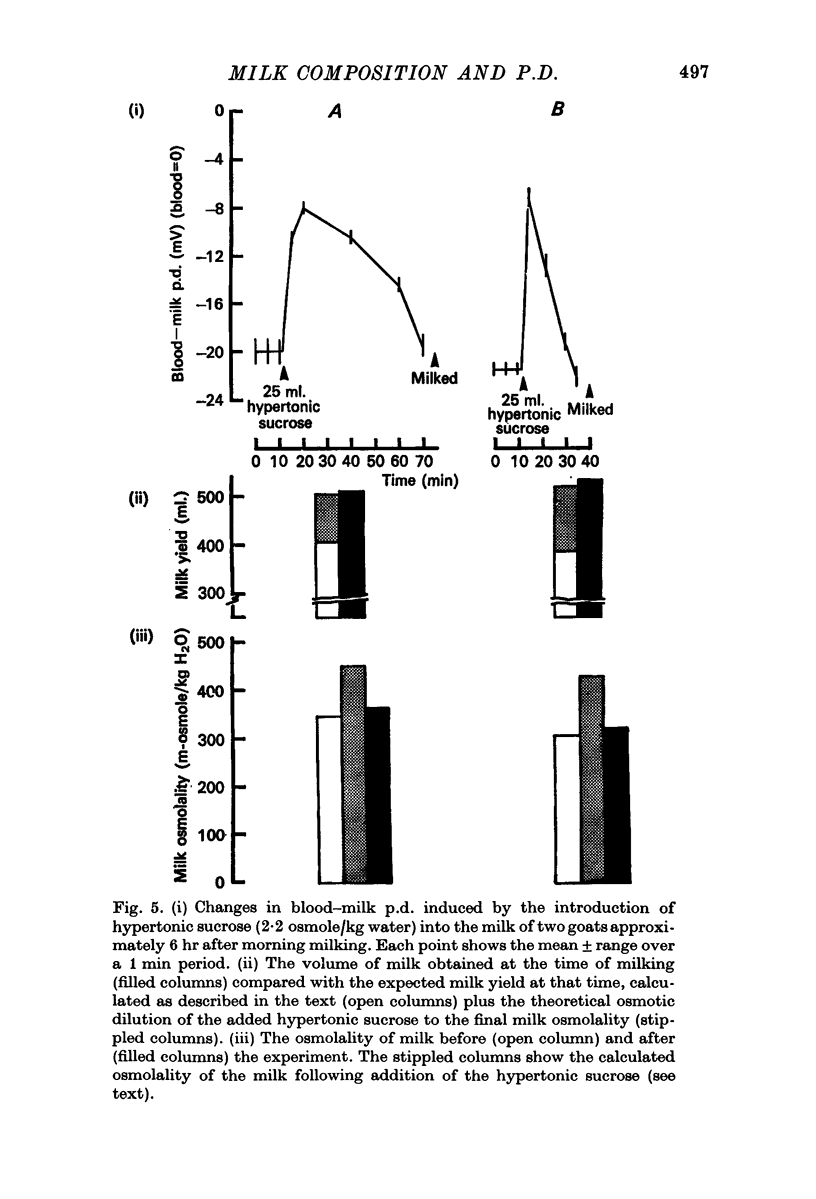
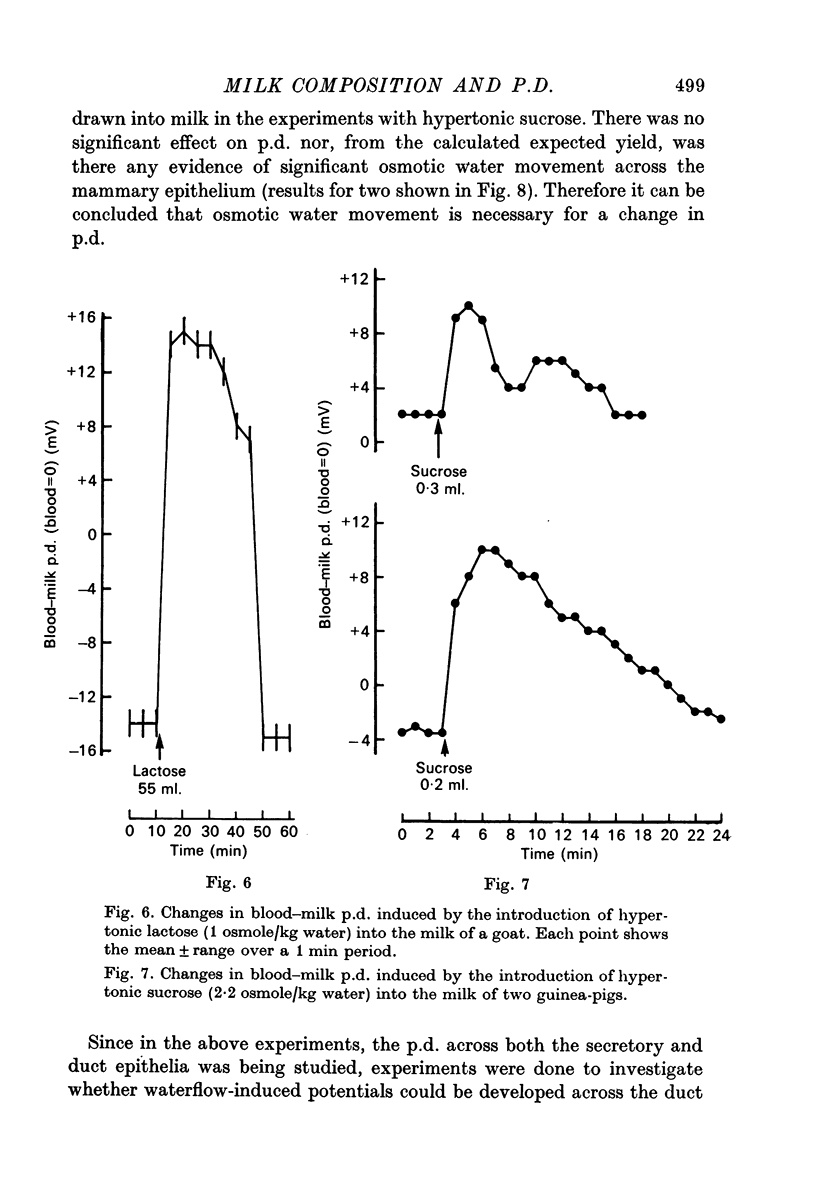
3. When milk was made hypertonic by the addition of hyperosmotic sucrose or lactose solutions, water entered milk osmotically and milk became electrically less negative or even positive with respect to blood in goats, cows and guinea-pigs.
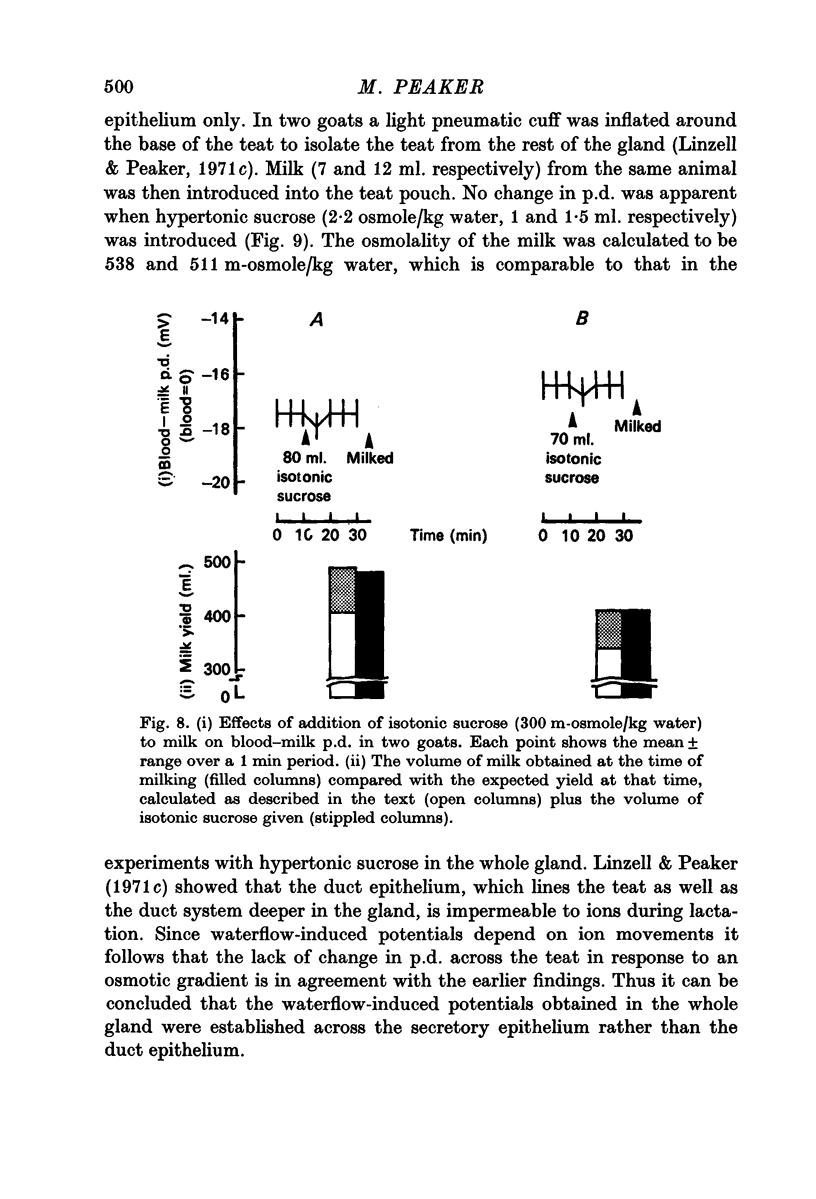
4. No effect on p.d. was apparent following the addition of isosmotic sucrose to milk in goats.
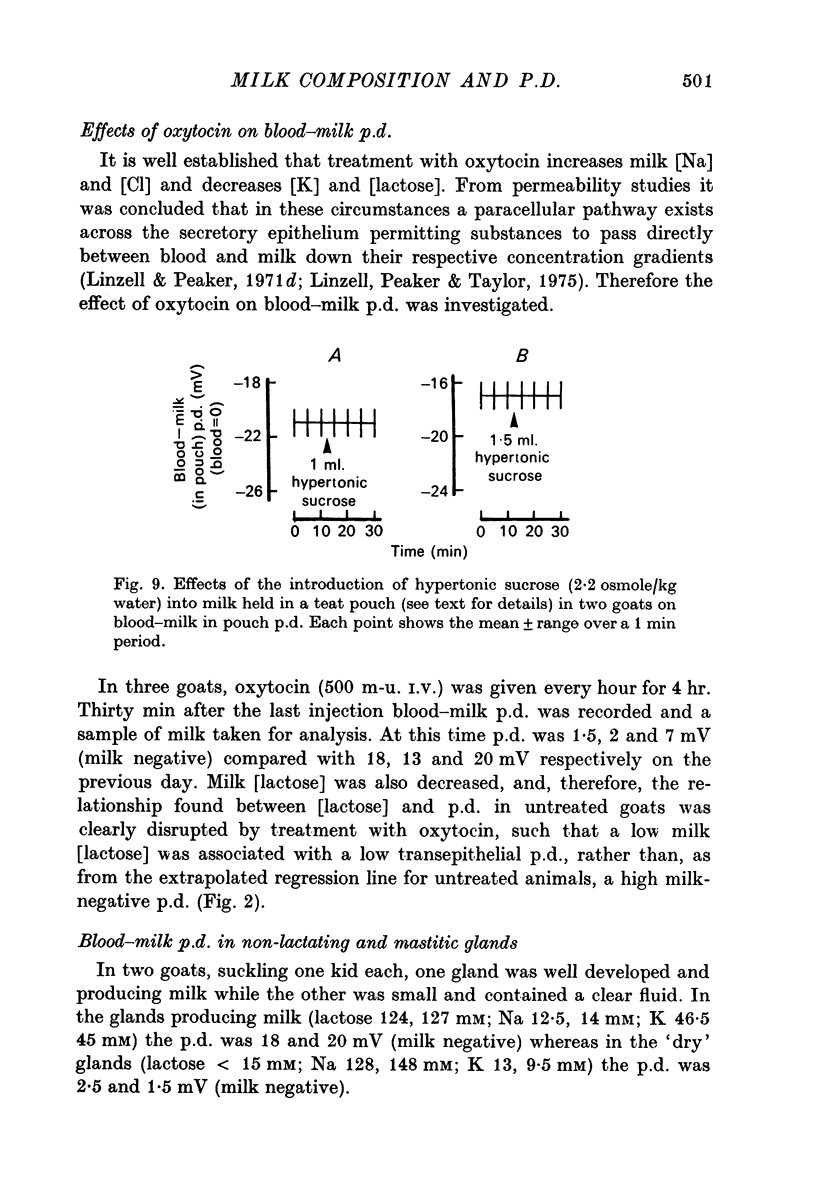
5. When milk was held in the teat of goats by a pneumatic cuff around the base of the teat, no effect on p.d. was apparent when hyperosmotic sucrose was introduced into this teat pouch.
6. It is suggested that waterflow-induced potentials (the streaming potential and the transport number effect) can be induced across the mammary epithelium.
7. In goats exogenous oxytocin lowered milk [lactose] and blood—milk p.d. became less negative with respect to blood.
8. In non-lactating and mastitic glands of goats the blood—milk p.d. was within 0·5-2·5 mV of zero.
9. The effects of oxytocin, and the low p.d. in non-lactating and mastitic glands, are compatible with the view that in such circumstances there is a paracellular pathway across the mammary epithelium which partially short-circuits the two sides.
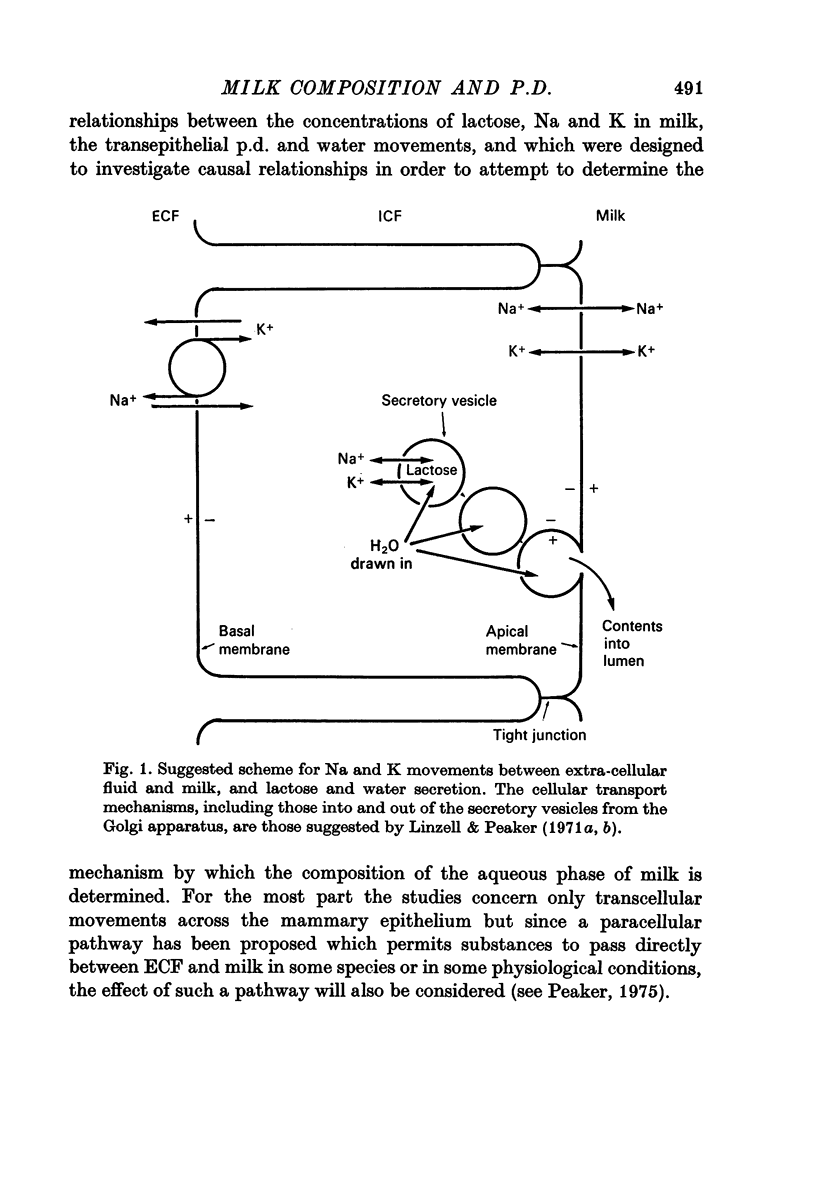
10. It is suggested that, with water being drawn osmotically into milk to dilute newly formed lactose, waterflow-induced potentials may be responsible for establishing the normal p.d. across the apical membrane of the secretary cell, thereby keeping milk [K] and [Na] lower than in intracellular fluid.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. H., Hope A. B. Electroosmosis in membranes: effects of unstirred layers and transport numbers. I. Theory. Biophys J. 1969 May;9(5):700–728. doi: 10.1016/S0006-3495(69)86413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H., Hope A. B. Electroosmosis in membranes: effects of unstirred layers and transport numbers. II. Experimental. Biophys J. 1969 May;9(5):729–757. doi: 10.1016/S0006-3495(69)86414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrucker C. R., Keenan T. W. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp Cell Res. 1975 Feb;90(2):253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- Brew K. Lactose synthetase: evolutionary origins, structure and control. Essays Biochem. 1970;6:93–118. [PubMed] [Google Scholar]
- Fleet I. R., Linzell J. L., Peaker M. The use of an autoanalyzer for the rapid analysis of milk constituents affected by subclinical mastitis. Br Vet J. 1972 Jun;128(6):297–300. doi: 10.1016/s0007-1935(17)36934-8. [DOI] [PubMed] [Google Scholar]
- HARDWICK D. C., LINZELL J. L., PRICE S. M. The effect of glucose and acetate on milk secretion by the perfused goat udder. Biochem J. 1961 Jul;80:37–45. doi: 10.1042/bj0800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDWICK D. C., LINZELL J. L. Some factors affecting milk secretion by the isolated perfused mammary gland. J Physiol. 1960 Dec;154:547–571. doi: 10.1113/jphysiol.1960.sp006595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZELL J. L. Mammary-gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. J Physiol. 1960 Oct;153:492–509. doi: 10.1113/jphysiol.1960.sp006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Fleet I. R., Mepham T. B., Peaker M. Perfusion of the isolated mammary gland of the goat. Q J Exp Physiol Cogn Med Sci. 1972 Apr;57(2):139–161. doi: 10.1113/expphysiol.1972.sp002145. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Mepham T. B., Peaker M. The secretion of citrate into milk. J Physiol. 1976 Sep;260(3):739–750. doi: 10.1113/jphysiol.1976.sp011541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Day-to-day variations in milk composition in the goat and cow as a guide to the detection of subclinical mastitis. Br Vet J. 1972 Jun;128(6):284–295. doi: 10.1016/s0007-1935(17)36932-4. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Efficacy of the measurement of the electrical conductivity of milk for the detection of subclinical mastitis in cows: detection of infected cows at a single visit. Br Vet J. 1975 Jul-Aug;131(4):447–461. doi: 10.1016/s0007-1935(17)35240-5. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Intracellular concentrations of sodium, potassium and chloride in the lactating mammary gland and their relation to the secretory mechanism. J Physiol. 1971 Aug;216(3):683–700. doi: 10.1113/jphysiol.1971.sp009547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M., Taylor J. C. The effects of prolactin and oxytocin on milk secretion and on the permeability of the mammary epithelium in the rabbit. J Physiol. 1975 Dec;253(2):547–563. doi: 10.1113/jphysiol.1975.sp011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The distribution and movements of carbon dioxide, carbonic acid and bicarbonate between blood and milk in the goat. J Physiol. 1975 Jan;244(3):771–782. doi: 10.1113/jphysiol.1975.sp010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The permeability of mammary ducts. J Physiol. 1971 Aug;216(3):701–716. doi: 10.1113/jphysiol.1971.sp009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M. Recent advances in the study of monovalent ions movements across the mammary epithelium: relation to onset of lactation. J Dairy Sci. 1975 Jul;58(7):1042–1047. doi: 10.3168/jds.s0022-0302(75)84677-7. [DOI] [PubMed] [Google Scholar]
- Peaker M., Taylor J. C. Milk secretion in the rabbit: changes during lactation and the mechanism of ion transport. J Physiol. 1975 Dec;253(2):527–545. doi: 10.1113/jphysiol.1975.sp011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]


