Abstract
Epstein-Barr virus (EBV) latent infection membrane protein 1 (LMP1) has an intermediate domain between the two cytoplasmic carboxyl-terminal domains that are critical for transforming B-lymphocytes into lymphoblastoid cell lines (LCLs). The intermediate domain has been implicated in Janus kinase 3 (JAK3) association and activation. We now find that LCLs transformed by EBV recombinants that express Flag-LMP1 with the putative JAK3 binding and activating intermediate domain deleted and LCLs transformed by Flag-LMP1 EBV recombinants have similar levels of phosphotyrosine-activated JAK3, signal transducer and activator of transcription 3 (STAT3), or STAT5 and similar very low levels of JAK3 associated with LMP1. Further, transient Flag-LMP1 expression in a B-lymphoma cell line transduces signals that upregulate TRAF1 levels but does not alter JAK3 levels or activation state. Although these data indicate that the LMP1 putative JAK3 binding and activating intermediate domain does not mediate JAK3 association or activation in B-lymphocytes, JAK3 association with LMP1 could be significant, particularly in cells in which LMP1, JAK3, or a JAK3-associated protein is expressed at high levels.
Epstein-Barr virus (EBV) is associated with several human malignancies, including posttransplant and AIDS-related lymphoproliferative disease, Hodgkin’s disease, Burkitt lymphoma (BL), and nasopharyngeal carcinoma (for a review, see reference 37). In vivo and in vitro, EBV infects and efficiently transforms resting primary B-lymphocyte growth into indefinitely proliferating lymphoblastoid cell lines (LCLs) (for a review, see reference 28). Latent infection membrane protein 1 (LMP1) is expressed in these cells and in most EBV-associated malignancies and is essential for EBV-induced LCL outgrowth (24). LMP1 is an integral plasma membrane protein that consists of a 24-amino-acid (-aa) cytoplasmic N terminus, six hydrophobic membrane-spanning domains separated by five short reverse turns, and a 200-aa cytoplasmic carboxyl terminus (Fig. 1) (12). The 24-aa cytoplasmic N terminus tethers the first membrane-spanning domain to the cytoplasm but otherwise is not critical for LCL outgrowth (21), whereas the six membrane-spanning domains mediate LMP1 association with plasma membrane microdomain lipid rafts (1, 17). The membrane-spanning domains also constitutively aggregate and activate the LMP1 cytoplasmic carboxyl-terminal signaling domains (13, 16). Recombinant EBV genetic analyses reveal that the carboxyl terminus has two transformation effector sites, transformation effector site 1 (TES1) and TES2, that are essential for B-lymphocyte growth transformation (20, 22, 25, 26). These sites are C-terminal NF-κB activating region 1 (CTAR1) and CTAR2, and NF-κB activation is critical for LCL survival (2, 4, 18, 27, 32). TES1/CTAR1 recruits tumor necrosis factor (TNF) receptor-associated factor 1 (TRAF1), TRAF2, TRAF3, and TRAF5 (3, 5, 33, 38), whereas TES2/CTAR2 recruits TNF receptor-associated death domain protein (TRADD) and TNF receptor interacting protein (RIP) (8, 20, 22). LMP1 constitutively recruits these proteins to transduce signals that activate NF-κB (15, 30), c-Jun N-terminal kinase (JNK) (11, 29), and p38 mitogen-activated protein kinase (MAPK) (9).
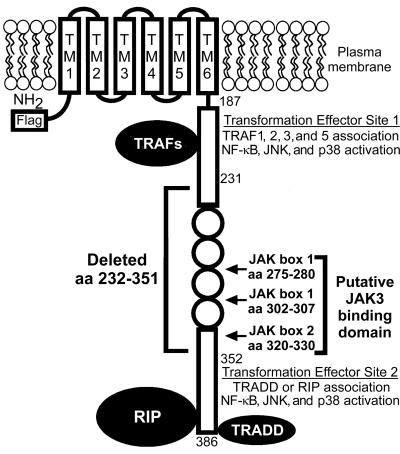
FIG. 1.
Diagram of LMP1. The Flag epitope was inserted at the amino terminus (NH2). The putative JAK3 binding domain consists of JAK boxes located at the specified amino acids. Flag-LMP1Δ232–351 EBV recombinants express LMP1 with aa 232 to 351 deleted, which includes the putative JAK3 binding domain. Residues 187 to 231 include TES1, which interacts with TRAF1, -2, -3, and -5. Residues 352 to 386 include TES2, which interacts with TRADD and RIP. The six transmembrane domains (TM) constitutively aggregate LMP1 in the plasma membrane and enable LMP1 to transduce signals via TRAFs, TRADD, or RIP that activate NF-κB, JNK, and p38 MAPK and thereby alter B-lymphocyte growth.
The experiments reported here focus on LMP1 carboxyl-terminal residues 232 to 351, which are an intermediate domain between TES1/CTAR1 and TES2/CTAR2 that has been implicated in binding and activating Janus kinase 3 (JAK3) (Fig. 1) (14). The intermediate domain includes four imperfect repeats of a conserved PQDPDNTDDNG sequence (aa 253 to 301), a PPQLT sequence (aa 320 to 324) that resembles but does not function as a TRAF binding site, 19 potential serine or threonine phosphorylation sites, two putative JAK box 1 docking sites (aa 275 to 280 and aa 302 to 307), and a putative JAK box 2 activation domain (aa 320 to 330) (12, 14, 34). Regulated expression of LMP1 in LCLs may correlate with JAK3, signal transducer and activator of transcription 1 (STAT1), or STAT3 activation, whereas transient overexpression of LMP1 and JAK3 in 293 human embryonic kidney cells may activate JAK3, STAT1, or STAT3. These effects may depend on the intermediate domain (14). JAK3 activation by LMP1 could be important in B-lymphocyte transformation because JAK3 activates STAT3 or STAT5 in response to interleukin-2 (IL-2), IL-4, IL-7, IL-9, or IL-15 stimulation (19, 31, 39, 40). However, EBV recombinants that express Flag-LMP1 with aa 232 to 351 deleted (Flag-LMP1Δ232–351) are indistinguishable from EBV recombinants that express Flag-LMP1 in efficiently transforming primary B-lymphocytes into LCLs (23). Further, EBV transforms primary B-lymphocytes from JAK3-deficient individuals (36). To resolve the conundrum surrounding the potential significance of the LMP1 putative JAK3 binding domain, we have investigated the role of the LMP1 intermediate domain in mediating JAK3 association, JAK3 activation, and JAK3 activation of STAT3 or STAT5 in EBV-transformed LCLs and in B-lymphoma cells.
LCLs transformed by recombinant EBV that express wt LMP1, Flag-LMP1, or Flag-LMP1Δ232–351 have similar levels of JAK3 and PY-JAK3.
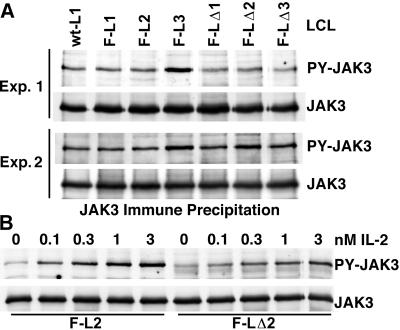
The role of the LMP1 putative JAK3 binding domain was first evaluated by comparing JAK3 and phosphotyrosine (PY)-activated JAK3 (PY-JAK3) levels in LCLs transformed by EBV recombinants that express fully wild-type (wt) LMP1 (wt-L1 LCL), Flag-LMP1 (F-L1, F-L2, and F-L3 LCLs), and Flag-LMP1Δ232–351 (F-LΔ1, F-LΔ2, and F-LΔ3 LCLs). Ten million cells were Dounce homogenized in a buffer that consists of 1% NP-40, 25 mM Tris (pH 7.2), 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg of aprotinin per ml, and 1 mM sodium orthovanadate. The lysates were cleared by centrifugation (450 × g, 5 min), and JAK3 was specifically precipitated with antibody (C-21; Santa Cruz Biotechnology) and protein G-Sepharose (Amersham Pharmacia Biotech). Precipitated proteins were resolved by electrophoresis in polyacrylamide gels containing sodium dodecyl sulfate (SDS), blotted to nitrocellulose membranes (Bio-Rad), and probed with antibody to PY residues (PY99; Santa Cruz Biotechnology). JAK3 and PY-JAK3 levels were similar in all the LCLs examined in two independent assays (Fig. 2A). To confirm the sensitivity and specificity of this method for assaying JAK3 activation, LCLs were stimulated with 0.1, 0.3, 1.0, or 3.0 nM IL-2 for 5 min at 37°C, and the effects on JAK3 were evaluated. IL-2 stimulation did not affect JAK3 levels, whereas PY-JAK3 levels directly correlated with the IL-2 dose. IL-2 had similar effects in LCLs transformed by recombinant EBV that express Flag-LMP1 or Flag-LMP1Δ232–351 (Fig. 2B). Western immunoblot analysis of proteins from untreated LCLs with Flag antibody (M5; Sigma) revealed that the levels of Flag-LMP1 and Flag-LMP1Δ232–351 proteins were similar among the cell lines tested (data not shown). Thus, the putative JAK3 binding domain does not affect JAK3 levels, JAK3 tyrosine phosphorylation, or JAK3 tyrosine phosphorylation in response to IL-2 stimulation.
FIG. 2.
(A) JAK3 and PY-JAK3 levels in LCLs transformed by recombinant EBV that express wt LMP1, Flag-LMP1 (F-L1, F-L2, and F-L3), or Flag-LMP1Δ232–351 (F-LΔ1, F-LΔ2, and F-LΔ3). JAK3 was immune precipitated and analyzed by Western blotting with antibody to PY residues (PY-JAK3) or with antibody to JAK3 (JAK3). Two experiments are shown (Exp. 1 and Exp. 2). (B) IL-2 activates tyrosine phosphorylation of JAK3. LCLs F-L2 and F-LΔ2 were treated with the indicated doses of IL-2 for 5 min. PY-JAK3 was then analyzed as described.
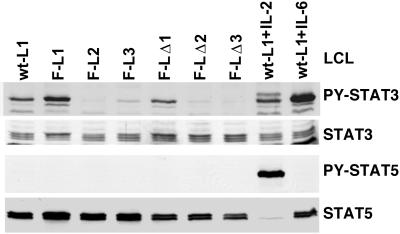
Since activated JAK3 tyrosine phosphorylates and thereby activates STAT3 and STAT5, the levels of PY-STAT3 and PY-STAT5 were also compared in LCLs transformed with recombinant EBV that express wt LMP1, Flag-LMP1, or Flag-LMP1Δ232–351 (Fig. 3). Proteins from two million cells were solubilized in buffer containing SDS, resolved by gel electrophoresis, blotted to filters, and probed with antisera specific for STAT3 (H-190; Santa Cruz Biotechnology), PY-STAT3 (New England Biolabs), STAT5 (ST5-8F7; Zymed), or PY-STAT5 (Zymed). To verify the sensitivity of the assay, wt-L1 LCL was stimulated with 10 nM IL-2 or 10 nM IL-6 (R&D Systems). Total cellular STAT3 and STAT5 levels were similar among all the LCLs examined, and IL-2 or IL-6 treatment did not affect their levels in the wt-L1 LCL (Fig. 3). The apparent STAT5 decrease in the IL-2-stimulated wt-L1 LCL is due to blockade by residual PY-STAT5 antibody. In the wt-L1 LCL, IL-6 induced STAT3 phosphorylation and IL-2 strongly induced STAT5 phosphorylation. Whereas the levels of PY-STAT3 in LCLs transformed with EBV recombinants that express Flag-LMP1 or Flag-LMP1Δ232–351 varied among individual cell lines, the levels were similar overall. Surprisingly, PY-STAT5 was not detected in nonstimulated LCLs. This was not due to constitutive dephosphorylation of STAT5, since IL-2 induced STAT5 phosphorylation. These results indicate that PY-STAT3 and PY-STAT5, which are phosphorylated by activated JAK3, are similar in LCLs transformed by recombinant EBV that express Flag-LMP1 or Flag-LMP1Δ232–351. Thus, the putative JAK3 binding domain does not affect tyrosine phosphorylation of JAK3, STAT3, or STAT5 in LCLs.
FIG. 3.
Tyrosine phosphorylation of STAT3 and STAT5 in LCLs. LCLs transformed by recombinant EBV that expresses wt LMP1, Flag-LMP1 (F-L1, F-L2, and F-L3), or Flag-LMP1Δ232–351 (F-LΔ1, F-LΔ2, and F-LΔ3) were analyzed by Western blotting with antibody to PY-activated STAT3 (PY-STAT3), all forms of STAT3, PY-STAT5, or all forms of STAT5. As a control, wt-L1 LCL was treated with either 10 nM IL-2 or 10 nM IL-6 and then analyzed by Western blotting as described.
LMP1 does not activate JAK3 in non-EBV-infected BL lymphoblasts.
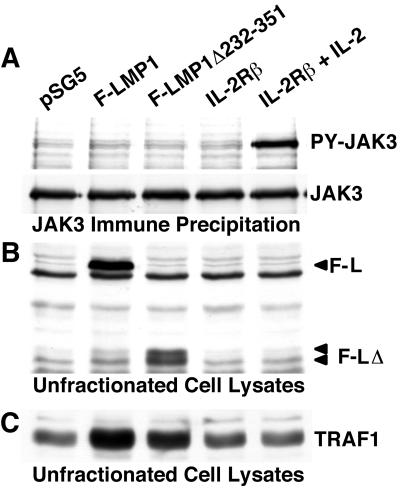
The potential effect of LMP1 expression on JAK3 activation was next investigated in BJAB, a non-EBV-infected BL lymphoblast. Ten million cells were transfected with 20 μg of empty vector pSG5 (Stratagene), pSG5 Flag-LMP1, or pSG5 Flag-LMP1Δ232–351 and cultured for 24 h. Since expression of the endogenous IL-2 receptor (IL-2R) β chain is low in BJAB cells (data not shown) and IL-2R activation of JAK3 is a useful control in these experiments, some of the cells were transfected with 20 μg of human IL-2R β chain expression vector pSRB5. The IL-2R β chain vector-transfected cells were further cultured without or with 3 nM human IL-2 to mimic constitutive receptor activation. After 24 h, 10% of the cells were solubilized in buffer containing SDS. The remaining cells were solubilized in buffer containing NP-40, JAK3 was immune precipitated, and PY-JAK3 levels were determined by Western immunoblotting as described above. Western-blotted proteins were further analyzed with Flag antibody (M5; Sigma) to detect LMP1 or with TRAF1 antibodies (H-126 and H-132; Santa Cruz Biotechnology) to detect the positive signaling effects of LMP1 on TRAF1 expression (6, 33). Flag-LMP1 or Flag-LMP1Δ232–351 vectors did not activate tyrosine phosphorylation of JAK3, whereas IL-2R β vector transfection and treatment of cells with IL-2 strongly activated JAK3 phosphorylation (Fig. 4A). Total cellular JAK3 levels were similar in all of the transfected cells (Fig. 4A). Flag-LMP1 and Flag-LMP1Δ232–351 were expressed at similar levels (Fig. 4B), and these proteins transduced activating signals that upregulated TRAF1 expression similarly (Fig. 4C). Thus, in BJAB BL lymphoblasts, ectopic expression of Flag LMP1 or Flag-LMP1Δ232–351 at levels that upregulate TRAF1 expression has no effect on JAK3 levels or on tyrosine phosphorylation of JAK3.
FIG. 4.
(A) JAK3 and PY-JAK3 levels in BL cells expressing LMP1. BJAB cells were transfected with pSG5 (empty vector), pSG5 Flag-LMP1, or pSG5 Flag-LMP1Δ232–351 DNA. As a control, cells were transfected with IL-2 β chain receptor vector DNA pSRB5 and some were further treated with 3 nM IL-2. After 24 h, cells were analyzed for PY-JAK3 as described in the legend to Fig. 2. (B) Transfected cell lysates analyzed by Western blotting with Flag antibody M5. The positions of Flag-LMP1 (F-L) and Flag-LMP1Δ232–351 (F-LΔ) proteins are marked. (C) Transfected cell lysates analyzed by Western blotting with TRAF1 antibody.
JAK3 associates weakly with both Flag-LMP1Δ232–351 and Flag-LMP1.
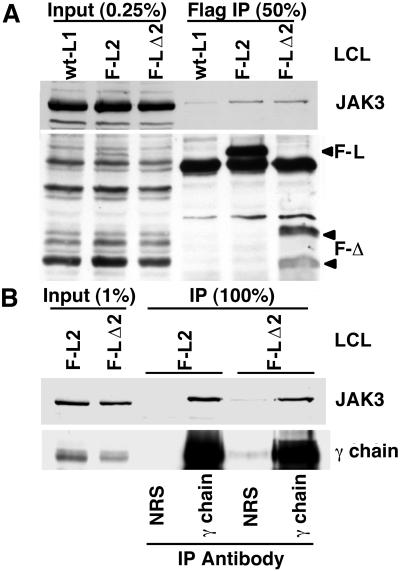
We next investigated the potential association of JAK3 with Flag-LMP1 or Flag-LMP1Δ232–351 in LCLs. One hundred fifty million cells were Dounce homogenized in buffer with detergent (0.5% [wt/vol] Brij 58 [Sigma], 50 mM Tris [pH 7.2], 100 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 20 μg of aprotinin per ml), and the lysate was cleared by centrifugation. Proteins were immune precipitated with Flag antibody (M2 affinity gel; Sigma), resolved in denaturing gels, blotted to filters, and probed with JAK3 antibody. Flag antibody specifically coprecipitated JAK3 with Flag-LMP1 and with Flag-LMP1Δ232–351 at levels that were higher than with LMP1 that lacks the Flag epitope (Fig. 5A). Taking into account an 8% Flag-LMP1 or Flag-LMP1Δ232–351 immune precipitation efficiency, 0.22 and 0.39% of JAK3 is estimated to be associated with Flag-LMP1 and Flag-LMP1Δ232–351 in LCLs, respectively. Thus, very low levels of JAK3 associate with LMP1, but the LMP1 intermediate domain does not mediate this JAK3 association.
FIG. 5.
(A) JAK3 association with Flag-LMP1Δ232–351 and Flag-LMP1. LCLs transformed by recombinant EBV, the express wt LMP1, Flag-LMP1 (F-L2), or Flag-LMP1Δ232–351 (F-LΔ2) were lysed in buffer containing Brij 58. Solubilized proteins were then subjected to M2 antibody precipitation. Unfractionated cell lysate inputs and Flag antibody immunoprecipitates (Flag IP) were analyzed by Western blotting with JAK3 antibody or with Flag antibody M5. (B) As described, solubilized proteins from LCL F-L2 or F-LΔ2 were subjected to precipitation with either normal rabbit serum (NRS) or common γc chain antibody. Unfractionated cell lysate inputs and immunoprecipitates (IP) were analyzed by Western blotting with JAK3 antibody or with common γ chain antibody.
As a control, JAK3 association with the common γ chain was assessed. Thirty million nonstimulated LCLs were lysed in buffer with detergent, and the proteins were precipitated as described but with γc chain antibody (C-20; Santa Cruz Biotechnology) and protein G-Sepharose. Precipitated proteins were analyzed by probing Western blots with JAK3 or γc chain antibody. Similar large amounts of JAK3 were coprecipitated with γc chains from LCLs transformed by recombinant EBV that express Flag-LMP1 or Flag-LMP1Δ232–351 (Fig. 5B).
In summary, LCLs transformed by recombinant EBV that express Flag-LMP1 or Flag-LMP1 that is deleted for the putative JAK3 binding and activating intermediate domain are similar in PY-JAK3, PY-STAT3, or PY-STAT5 levels; in JAK3, STAT3, or STAT5 levels; and in very low-level JAK3 association with LMP1. Transiently expressed Flag-LMP1 and Flag-LMP1 with the intermediate domain deleted were similarly ineffective at altering JAK3 levels or activation state. Thus, the LMP1 putative JAK3 binding and activating intermediate domain does not mediate JAK3 association or activation in BL lymphoblasts or LCLs.
The low-level JAK3 association with LMP1 in LCLs could result from JAK3 or a JAK3 binding protein interacting with an LMP1 domain other than the intermediate domain. The association could even be mediated by a known LMP1-associated protein such as TRAFs, TRADD, or RIP; by one of their associated signaling proteins; or by membrane lipids. The association could be sufficient to enable or prolong JAK3 activation under some circumstance of LMP1 and JAK3 expression in an EBV-infected cell. Although the significance of this interaction in LCLs is bounded in part by the very-low-level association of JAK3 with LMP1 described here, an effect may be evident in other EBV-infected cells, such as Hodgkin’s disease Reed-Sternberg cells, where higher levels of LMP1 and a JAK3-associated protein, such as CD40, are expressed (7). LMP1 aggregates in plasma membrane lipid rafts and could trap proteins that transduce signals through JAK3, such as the common γ chain of interleukin receptors or TNF receptor family protein CD40 or CD30. The latter proteins are abundantly expressed in LCLs, in B-lymphoblasts, and in Hodgkin’s disease Reed-Sternberg cells (for a review, see reference 37). Further, EBV infection, LMP1 TES1/CTAR1, and TES2/CTAR2 activate NF-κB, JNK, and p38 MAPK and induce expression of cytokines—including IL-6, IL-8, and IL-10 (19, 31)—that could activate JAK3 and mediate effects on cell gene expression, growth, and survival (9, 10, 35) (for a review, see references 28 and 37).
Acknowledgments
The pSRB5 vector of the IL-2R β chain was a gift from Kazuo Sugamura, Tohoku University. Naruhiko Takasawa contributed advice and technical assistance.
This research was supported by grants CA085180 and CA047006 from the National Cancer Institute of the USPHS.
REFERENCES
- 1.Ardila-Osorio, H., B. Clausse, Z. Mishal, J. Wiels, T. Tursz, and P. Busson. 1999. Evidence of LMP1-TRAF3 interactions in glycosphingolipid-rich complexes of lymphoblastoid and nasopharyngeal carcinoma cells. Int. J. Cancer 81:645–649. [DOI] [PubMed] [Google Scholar]
- 2.Asso-Bonnet, M., J. Feuillard, V. Ferreira, P. Bissieres, N. Tarantino, M. Korner, and M. Raphael. 1998. Relationship between IκBα constitutive expression, TNFα synthesis, and apoptosis in EBV-infected lymphoblastoid cells. Oncogene 17:1607–1615. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur, S. R., G. Cheng, D. Baltimore, and D. A. Thorley-Lawson. 1997. Localization of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777–19784. [DOI] [PubMed] [Google Scholar]
- 4.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 97:6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16:7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devergne, O., E. C. McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolcetti, R., M. Quaia, A. Gloghini, V. De Re, P. Zancai, R. Cariati, L. Babuin, A. M. Cilia, S. Rizzo, A. Carbone, and M. Boiocchi. 1999. Biologically relevant phenotypic changes and enhanced growth properties induced in B lymphocytes by an EBV strain derived from a histologically aggressive Hodgkin’s disease. Int. J. Cancer 80:240–249. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos, A. G., S. M. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085–16096. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene 14:2899–2916. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., and L. S. Young. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731–1742. [DOI] [PubMed] [Google Scholar]
- 12.Fennewald, S., V. van Santen, and E. Kieff. 1984. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J. Virol. 51:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floettmann, J. E., and M. Rowe. 1997. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C terminus and requires oligomerisation for NF-κB activation. Oncogene 15:1851–1858. [DOI] [PubMed] [Google Scholar]
- 14.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarskjöld, M.-L., and M. C. Simurda. 1992. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-κB activity. J. Virol. 66:6496–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J. Immunol. 160:1116–1121. [PubMed] [Google Scholar]
- 17.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549–560. [PubMed] [Google Scholar]
- 19.Imada, K., and W. J. Leonard. 2000. The Jak-STAT pathway. Mol. Immunol. 37:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 94:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1994. Epstein-Barr virus recombinant molecular genetic analysis of the LMP1 amino-terminal cytoplasmic domain reveals a probable structural role, with no component essential for primary B-lymphocyte growth transformation. J. Virol. 68:4369–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi, K. M., E. C. McFarland, E. A. Riley, D. Rizzo, Y. Chen, and E. Kieff. 1999. The residues between the two transformation effector sites of Epstein-Barr virus latent membrane protein 1 are not critical for B-lymphocyte growth transformation. J. Virol. 73:9908–9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye, K. M., K. M. Izumi, H. Li, E. Johannsen, D. Davidson, R. Longnecker, and E. Kieff. 1999. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. J. Virol. 73:10525–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye, K. M., K. M. Izumi, G. Mosialos, and E. Kieff. 1995. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J. Virol. 69:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney, J. L., M. E. Guinness, T. Curiel, and J. Lacy. 1998. Antisense to the Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP-1) suppresses LMP-1 and bcl-2 expression and promotes apoptosis in EBV-immortalized B cells. Blood 92:1721–1727. [PubMed] [Google Scholar]
- 28.Kieff, E., and A. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511–2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Virology, 4th ed. Lippincott, Williams, and Wilkins, Philadelphia, Pa.
- 29.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 267:24157–24160. [PubMed] [Google Scholar]
- 31.Liu, K. D., S. L. Gaffen, and M. A. Goldsmith. 1998. JAK/STAT signaling by cytokine receptors. Curr. Opin. Immunol. 10:271–278. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell, T., and B. Sugden. 1995. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389–399. [DOI] [PubMed] [Google Scholar]
- 34.Murakami, M., M. Narazaki, M. Hibi, H. Yawata, K. Yasukawa, M. Hamaguchi, T. Taga, and T. Kishimoto. 1991. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc. Natl. Acad. Sci. USA 88:11349–11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagomi, H., R. Dolcetti, M. T. Bejarano, P. Pisa, R. Kiessling, and M. G. Masucci. 1994. The Epstein-Barr virus latent membrane protein-1 (LMP1) induces interleukin-10 production in Burkitt lymphoma lines. Int. J. Cancer 57:240–244. [DOI] [PubMed] [Google Scholar]
- 36.Oakes, S. A., F. Candotti, J. A. Johnston, Y. Q. Chen, J. J. Ryan, N. Taylor, X. Liu, L. Hennighausen, L. D. Notarangelo, W. E. Paul, R. M. Blaese, and J. J. O’Shea. 1996. Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity 5:605–615. [DOI] [PubMed] [Google Scholar]
- 37.Rickinson, A., and E. Kieff. 2001. Epstein-Barr virus, p. 2573–2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Virology, 4th ed. Lippincott, Williams, and Wilkins, Philadelphia, Pa.
- 38.Sandberg, M., W. Hammerschmidt, and B. Sugden. 1997. Characterization of LMP-1’s association with TRAF1, TRAF2, and TRAF3. J. Virol. 71:4649–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugamura, K., H. Asao, M. Kondo, N. Tanaka, N. Ishii, K. Ohbo, M. Nakamura, and T. Takeshita. 1996. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 14:179–205. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O’Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94:13850–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]