Abstract
1. An homogenate of bovine adrenal medulla contains significant amounts of six acid hydrolases: acid ribonuclease, acid deoxyribonuclease, cathepsin, acid phosphatase, β-glucuronidase and arylsulphatase. Most of the activity of each enzyme could be sedimented in the large-granule fraction at 242,000 g-min.
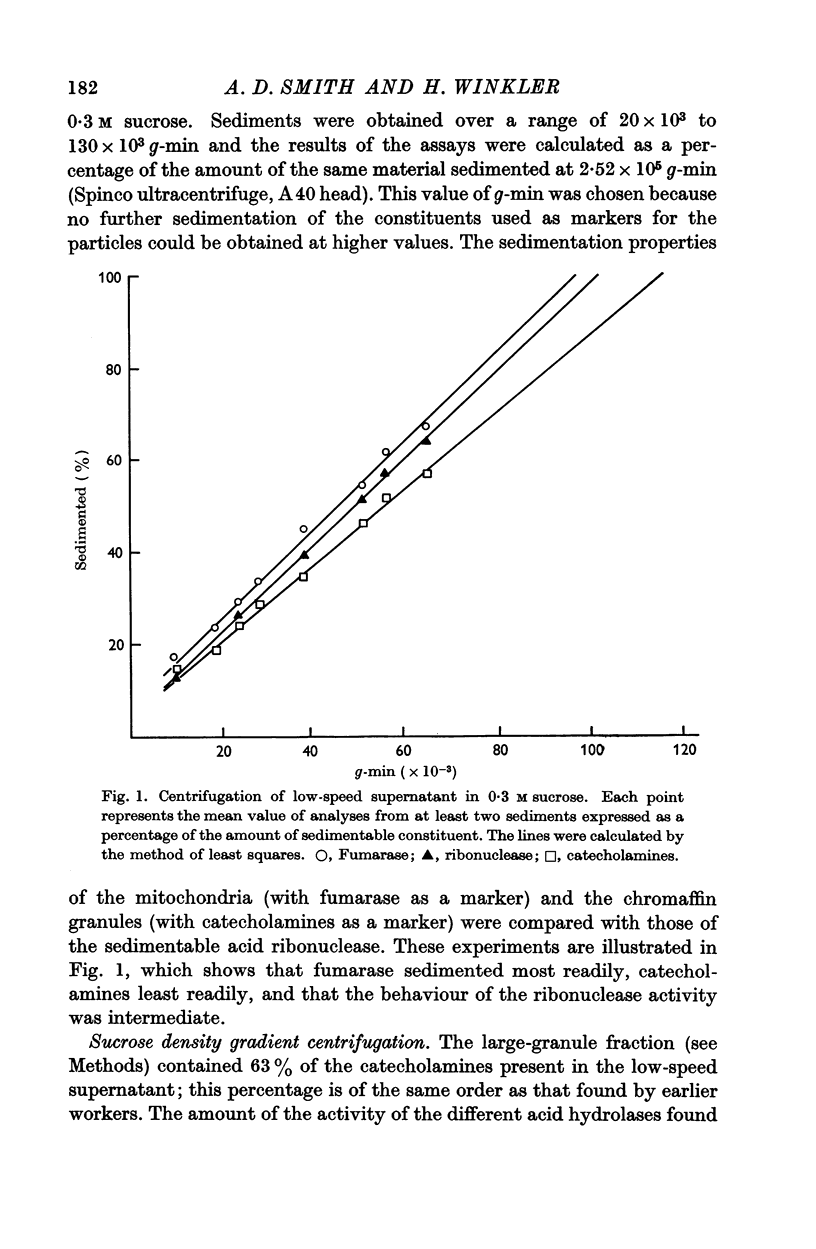
2. Differential centrifugation indicated the presence of three populations of particles, which sedimented at slightly different rates; these are, in order of decreasing sedimentation rate, mitochondria, particles containing the acid hydrolases, and chromaffin granules.
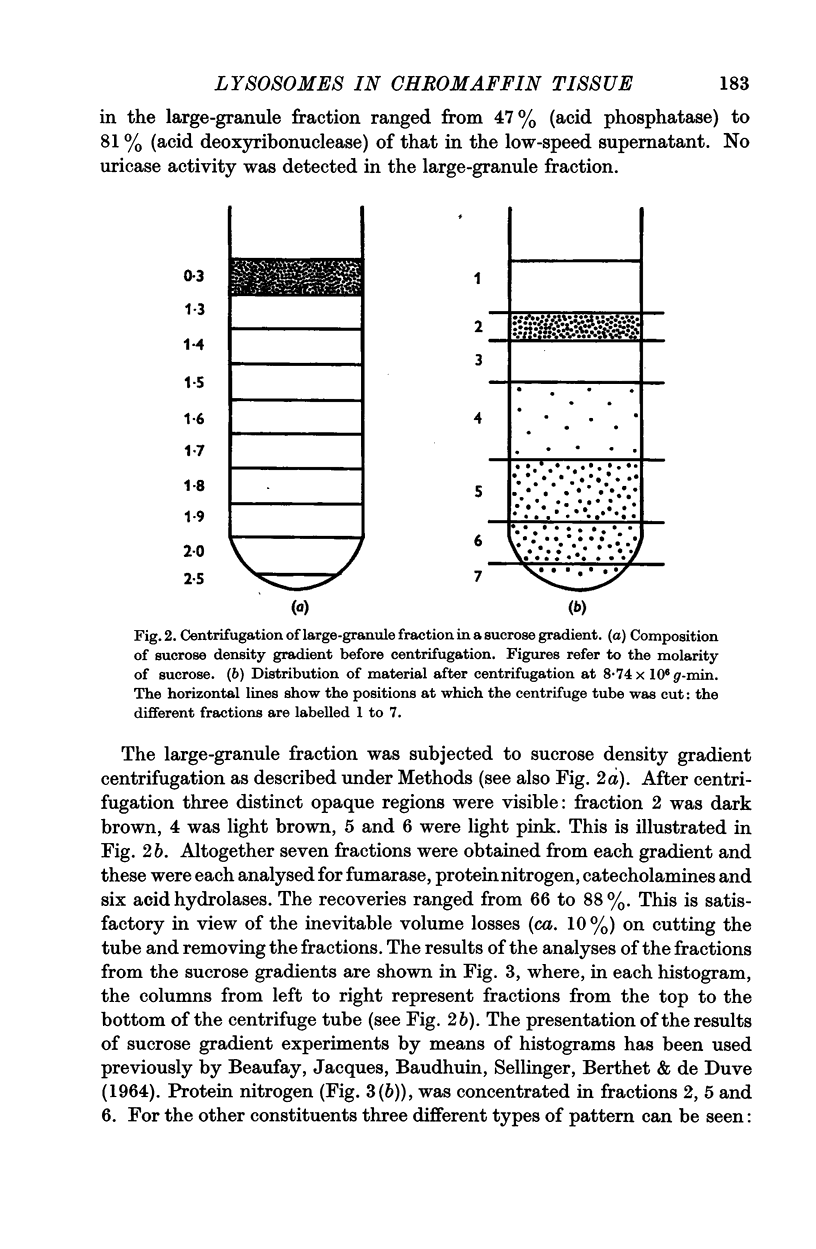
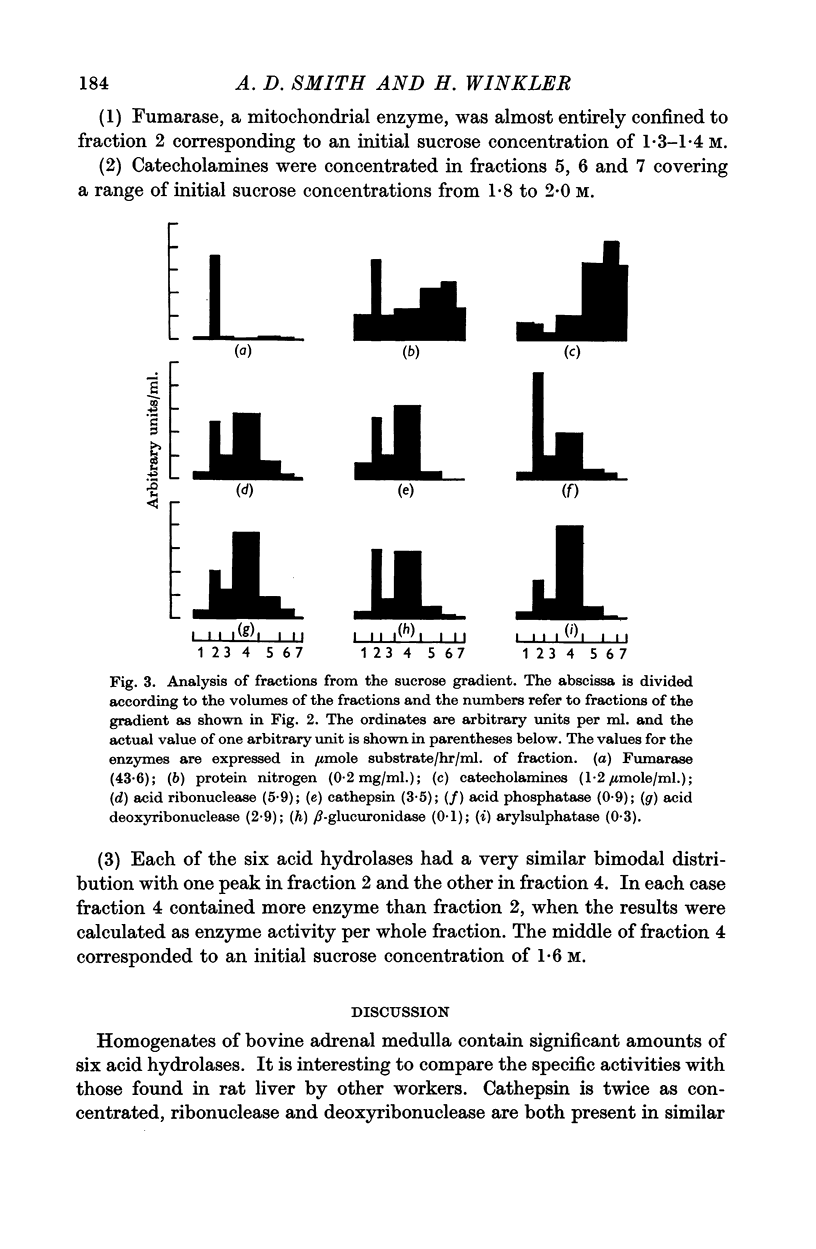
3. The three types of particle could be separated by ultracentrifuging the large-granule fraction in a sucrose density gradient. Most of the activity of each hydrolase was recovered in a layer intermediate between those formed by mitochondria and chromaffin granules.
4. The large-granule fraction therefore contains particles which are defined by their enzyme content as lysosomes.
5. Highly purified chromaffin granules, containing less than 5% of the activity of each acid hydrolase, were obtained from the gradient.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANKS P. THE ADENOSINE-TRIPHOSPHATASE ACTIVITY OF ADRENAL CHROMAFFIN GRANULES. Biochem J. 1965 May;95:490–496. doi: 10.1042/bj0950490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAUFAY H., BENDALL D. S., BAUDHUN P., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 13. Analysis of mitochondrial fractions from rat liver by density-gradient centrifuging. Biochem J. 1959 Dec;73:628–637. doi: 10.1042/bj0730628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H., BORN G. V., D'IORIO A., EADE N. R. Observations on the distribution of catechol amines and adenosinetriphosphate in the bovine adrenal medulla. J Physiol. 1956 Sep 27;133(3):548–557. doi: 10.1113/jphysiol.1956.sp005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H., HAGEN J. M., HAGEN P. Mitochondrial enzymes and chromaffin granules. J Physiol. 1957 Dec 3;139(2):316–322. doi: 10.1113/jphysiol.1957.sp005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKO H., HAGEN P., WELCH A. D. Observations on the intracellular granules of the adrenal medulla. J Physiol. 1955 Jul 28;129(1):27–49. doi: 10.1113/jphysiol.1955.sp005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUPLAND R. E. (ELECTRON MICROSCOPIC OBSERVATIONS ON THE STRUCTURE OF THE RAT ADRENAL MEDULLA. I. THE ULTRASTRUCTURE AND ORGANIZATION OF CHROMAFFIN CELLS IN THE NORMAL ADRENAL MEDULLA.) J Anat. 1965 Apr;99:231–254. [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARP N. A., FALCK B. Localization of acid phosphatase in the adrenal medullary cell. Acta Endocrinol (Copenh) 1956 Jun;22(2):95–106. doi: 10.1530/acta.0.0220095. [DOI] [PubMed] [Google Scholar]
- HILLARP N. A. Isolation and some biochemical properties of the catechol amine granules in the cow adrenal medulla. Acta Physiol Scand. 1958 Jul 17;43(1):82–96. doi: 10.1111/j.1748-1716.1958.tb01579.x. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N. Biosynthesis of adrenaline and noradrenaline. Pharmacol Rev. 1959 Jun;11(2 Pt 2):350–357. [PubMed] [Google Scholar]
- LOJDA Z., SCHREIBER V. HISTOCHEMICAL DEMONSTRATION OF THE INHIBITION OF RAT ADENOHYPOPHYSEAL ACID PHOSPHATASE BY VALINE-OXYTOCIN. J Histochem Cytochem. 1964 Nov;12:855–856. doi: 10.1177/12.11.855. [DOI] [PubMed] [Google Scholar]
- MALMSTROM B. G., GLICK D. Studies in histochemistry. XXV. Determination of phenolsulfatase in microgram quantities of tissue and its distribution in the adrenal of several species. Arch Biochem Biophys. 1952 Sep;40(1):56–67. doi: 10.1016/0003-9861(52)90073-8. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E., QUINTANA N. GOLGI APPARATUS AND LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1010–1022. [PubMed] [Google Scholar]
- Philippu A., Schümann H. J. Ribonucleaseaktivität isolierter Nebennierenmarkgranula. Experientia. 1964 Oct 15;20(10):547–548. doi: 10.1007/BF02150281. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- ROTH J. S., MILSTEIN S. W. Ribonuclease. I. A new assay method with P32 labeled yeast ribonucleic acid. J Biol Chem. 1952 May;196(2):489–498. [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. V. Sulphatase C. Biochem J. 1956 Dec;64(4):651–657. doi: 10.1042/bj0640651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of enzymes. IX. Certain purine-metabolizing enzymes. J Biol Chem. 1952 Mar;195(1):161–166. [PubMed] [Google Scholar]
- SCHREIBER V. [A hypothalamic factor activating pituitary acid phosphatases and the secretion of TSH]. Acta Univ Carol Med (Praha) 1961;7:33–87. [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Acid nucleases of the bovine adrenal medulla. Nature. 1965 Aug 7;207(997):634–634. doi: 10.1038/207634a0. [DOI] [PubMed] [Google Scholar]
- TODD P. E., TRIKOJUS V. M. Purification and properties of adrenal acid proteinase. Biochim Biophys Acta. 1960 Dec;45:234–242. doi: 10.1016/0006-3002(60)91447-5. [DOI] [PubMed] [Google Scholar]
- WETZSTEIN R. Elektronenmikroskopische Untersuchungen am Nebennierenmark von Maus, Meerschweinchen und Katze. Z Zellforsch Mikrosk Anat. 1957;46(5):517–576. [PubMed] [Google Scholar]
- WOLLMAN S. H., SPICER S. S., BURSTONE M. S. LOCALIZATION OF ESTERASE AND ACID PHOSPHATASE IN GRANULES AND COLLOID DROPLETS IN RAT THYROID EPITHELIUM. J Cell Biol. 1964 May;21:191–201. doi: 10.1083/jcb.21.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


