Abstract
Syphilis is a chronic sexually transmitted disease caused by Treponema pallidum subsp. pallidum. Clinical manifestations separate the disease into stages; late stages of disease are now uncommon compared to the preantibiotic era. T. pallidum has an unusually small genome and lacks genes that encode many metabolic functions and classical virulence factors. The organism is extremely sensitive to environmental conditions and has not been continuously cultivated in vitro. Nonetheless, T. pallidum is highly infectious and survives for decades in the untreated host. Early syphilis lesions result from the host's immune response to the treponemes. Bacterial clearance and resolution of early lesions results from a delayed hypersensitivity response, although some organisms escape to cause persistent infection. One factor contributing to T. pallidum's chronicity is the paucity of integral outer membrane proteins, rendering intact organisms virtually invisible to the immune system. Antigenic variation of TprK, a putative surface-exposed protein, is likely to contribute to immune evasion. T. pallidum remains exquisitely sensitive to penicillin, but macrolide resistance has recently been identified in a number of geographic regions. The development of a syphilis vaccine, thus far elusive, would have a significant positive impact on global health.
INTRODUCTION
Since its recognition in 15th-century Europe as a new disease, syphilis has been the subject of great mystery and legend. Speculation abounds, often with scant evidence, about famous persons who may have had syphilis—the implicated include political figures (165, 172, 210), musicians (87, 114, 254), and literary greats (113, 187, 258, 262). Many believe that syphilis was brought to Europe by Columbus and his sailors, although there is no objective proof of this theory (176). John Hunter, the eminent mid-18th-century Scottish physician and venereologist, in order to test whether syphilis and gonorrhea were the same or different diseases, is said to have inoculated himself with pus from an individual infected with a sexually transmitted disease (46, 84, 159). Unfortunately for Hunter, the patient was infected with the etiologic agents of both diseases. This was also unfortunate for the medical community, as it caused decades of medical and scientific confusion. Finally Philippe Ricord's studies, published in 1838, clarified that syphilis and gonorrhea are indeed distinct infections (253). The greatest mystery of syphilis, however, is how the spirochete Treponema pallidum subsp. pallidum causes the many clinical features of disease. Despite the inherent difficulties in investigating this organism, researchers have been successful in uncovering some of the secrets of T. pallidum's biology and the pathogenesis of syphilis, but much remains to be discovered. In previous issues of this journal, a 1999 review focused on the epidemiological and clinical aspects of syphilis (290) and a 2005 review provided a detailed description of secondary syphilis (20). Here, we address research on the biology of T. pallidum and syphilis pathogenesis, highlighting investigative efforts over the last five years.
Syphilis is a chronic disease, and T. pallidum's only known natural host is the human. Syphilis is acquired by direct contact, usually sexual, with active primary or secondary lesions. Studies have shown that 16 to 30% of individuals who have had sexual contact with a syphilis-infected person in the preceding 30 days become infected (205, 274); actual transmission rates may be much higher (6, 106, 271). Infection also occurs when organisms cross the placenta to infect the fetus in a pregnant woman. In the United States, the incidence of syphilis during the Second World War was over 500,000 infections per year. Between the years 1945 and 2000, syphilis declined to 31,575 reported infections, with alternating peaks and troughs of infectious cases. Since 2000, there has been an increase in the number of syphilis cases in the United States, mainly among men who have sex with men (MSM) (47-50, 52); these outbreaks have been reported along the west coast of the United States and in New York. Similar increases in syphilis in MSM have been reported in western Europe and the United Kingdom (111, 145, 166, 288, 321). Outbreaks among MSM are associated with a rise in unsafe sexual behavior, perhaps a consequence of improved antiretroviral treatment for human immunodeficiency virus (HIV); in recent surveys, 37% to 52% of MSM reported multiple risk behaviors (69, 145, 146).
Compared to syphilis rates in developed countries, the worldwide burden of syphilis is formidable. The World Health Organization estimates that 12 million new cases of syphilis occur each year (107). The vast majority of these are seen in developing nations, but an increase in new cases has also been noted in eastern Europe since the dissolution of the Soviet Union (264, 336). Congenital syphilis is of particular concern in developing nations, where the lack of prenatal testing and antibiotic treatment of infected pregnant women results in congenital infection of the fetus. Congenital syphilis causes spontaneous abortion, stillbirth, death of the neonate, or disease in the infant; a recent report from Tanzania estimates that up to 50% of stillbirths are caused by syphilis (330). Of particular importance to worldwide health is the recognition that syphilis infection greatly increases the transmission and acquisition of HIV (115, 300). These factors, along with the highly destructive nature of late disease, make syphilis an important public health concern.
OVERVIEW OF THE NATURAL HISTORY OF SYPHILIS
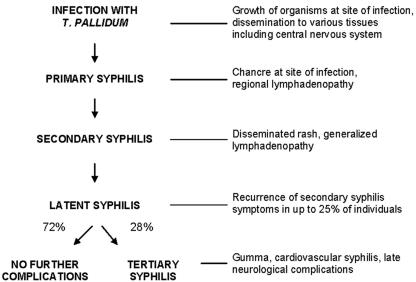
Syphilis is a multistage disease with diverse and wide-ranging manifestations; the distinct stages of syphilis were first described in detail by Philippe Ricord in the mid-1800s (253). A schematic diagram of untreated syphilis is shown in Fig. 1.
FIG. 1.
Natural history of untreated syphilis, according to Gjestland (112).
Primary Syphilis
Infection is initiated when T. pallidum penetrates dermal microabrasions or intact mucous membranes, typically resulting in a single chancre at the site of inoculation. Moderate regional lymphadenopathy is associated with the primary stage. The chancre usually becomes indurated and will progress to ulceration but typically is not purulent. In heterosexual men, primary chancres most commonly occur on the penis, but 32 to 36% of homosexual men have primary chancres in other sites, including the rectum, anal canal, and oral cavity (146, 204). In women, the primary chancre usually occurs on the labia or cervix. Because the chancre is painless and may be located in an inconspicuous anatomical site, diagnosis of syphilis in women and homosexual men is sometimes delayed until later disease manifestations become apparent. Clinical evaluation can also be complicated by the fact that the appearance of primary chancres in some individuals does not fit the classic description. Nonindurated lesions with irregular borders have been observed (67, 85), as have multiple and/or painful lesions, especially in the anal area and in individuals who are infected with HIV (146, 259).
The primary chancre develops an average of 3 weeks after exposure; the incubation period ranges between 10 and 90 days (320). Based on intradermal inoculation of human volunteers with graded doses of T. pallidum, the 50% infectious dose is estimated to be 57 organisms (188); lesions develop more rapidly when the inoculum size is larger (189). The primary chancre heals spontaneously within 4 to 6 weeks, but may still be discernible in about 15% of patients at the onset of secondary syphilis (150, 204, 259). HIV-infected individuals are reported to be two to three times more likely to have concurrent primary and secondary disease than HIV-uninfected individuals (150, 259).
Secondary Syphilis
Within hours of inoculation, and during the evolution of the primary stage, T. pallidum disseminates widely and organisms are deposited in a variety of tissues. Manifestations of secondary syphilis usually occur within 3 months of initial infection and are sometimes quite subtle. A detailed description of secondary syphilis can be found in the review by Baughn and Musher (20). Sore throat, muscle aches, malaise, and weight loss are examples of variable systemic symptoms of secondary syphilis. Generalized nontender lymphadenopathy occurs in up to 85% of cases (66, 141). The most common manifestation of secondary syphilis is a disseminated mucocutaneous rash. Pale and discrete macular lesions appear initially on the trunk and proximal extremities; these are followed by lesions of various morphologies among patients. At diagnosis, the most common types of secondary syphilis lesions are maculopapular (in 50% to 70% of patients), papular (12%), macular (10%), and annular papular (6% to 14%) (66, 141). In rare individuals, lesions may become necrotic, a condition called lues maligna (86); more often, secondary lesions are inconspicuous and may go undetected (66). Rash is frequently found on the palms of the hands and soles of the feet and, in 4% to 11% of patients, T. pallidum infection of hair follicles results in alopecia of the scalp (141, 204). There have also been infrequent reports of facial and body hair loss (1, 239).
Concurrent with the appearance of secondary lesions, about 10% of patients develop condylomata lata (66, 204). These enlarged lesions, appearing in warm and moist areas including the perineum and anus, are highly infectious. Localized inflammation of the oral cavity, tongue, and genital mucous membranes can cause mucous patches (66, 204); infrequently, secondary syphilis can be accompanied by gastric and renal involvement and hepatitis (118, 213). T. pallidum has been found in liver biopsy samples taken from patients with secondary syphilis (92, 170), while glomerulonephritis resulting from immunoglobulin-treponeme antigen complexes deposited in the glomeruli appears to cause kidney damage (17, 224, 315). Nephrotic syndrome may also be present. Approximately 5% of individuals with secondary syphilis experience the early manifestations of neurosyphilis, including meningitis and ocular disease (66, 301).
CNS Involvement
Classically, neurological complications of syphilis have been associated with tertiary disease, but studies have demonstrated that penetration of the central nervous system (CNS) by T. pallidum occurs during earlier stages of disease. Neurosyphilis is defined by increased protein and leukocyte levels in the cerebrospinal fluid (CSF) or a reactive CSF-VDRL (Venereal Disease Research Laboratory) test. T. pallidum can be detected in the CSF of many patients with syphilis, sometimes so early after CNS invasion that pleocytosis and other CSF abnormalities may have not yet appeared. About 40% of early syphilis patients and 25% of individuals with latent infection meet at least one of the diagnostic criteria for neurosyphilis (183, 193). The prognostic implications of CNS invasion are difficult to determine. The majority of persons with CNS invasion by T. pallidum appear to resolve or control CNS treponemes; however, there are no known indicators for subsequent development of symptomatic neurosyphilis. A recent large neurosyphilis study indicates that a serum rapid plasma reagin (RPR) titer greater than or equal to 1:32 is significantly associated with neurosyphilis, regardless of syphilis stage or concurrent HIV infection (193).
Early symptoms of neurosyphilis may occur concurrently with primary or secondary syphilis or may follow resolution of secondary syphilis. Typical symptoms of acute early meningitis include fever, headache, nausea, vomiting, and stiff neck. Cranial nerve involvement may result in visual disturbances, including blurred vision and photophobia, hearing loss, and facial weakness (143). Ocular inflammation (uveitis) and numbness and pain in the extremities can also occur. Rarely, individuals with early neurosyphilis report memory loss and mental confusion. In the decade after the recognition of HIV infection, some investigators claimed that neurosyphilis symptoms are exacerbated in HIV-infected individuals; however, that conclusion has not been supported by more-recent large studies (193, 259). Also, HIV infection was not more likely to result in increased CSF pleocytosis (193). Despite the lack of association between HIV status and severity of CNS disease, standard benzathine penicillin therapy, particularly in those with concurrent HIV infection, may not be sufficient to resolve infection. Thus, neurosyphilis therapy is warranted in all patients with CSF abnormalities consistent with neurosyphilis (194).
Latent Syphilis
Disseminated lesions and other manifestations of secondary syphilis usually resolve spontaneously within 3 months of appearance, and symptoms are absent for a variable period of time in untreated individuals. Latent syphilis is divided into two stages, based upon an approximation of the time of infection. For the first year after infection, patients are considered to have early latent syphilis; up to 25% may have recurrent secondary manifestations (112). Late latent syphilis is defined as asymptomatic infection of longer than one year or unknown duration. Serologic testing during the late latent stage is positive, but sexual transmission is unlikely. Organisms may seed the bloodstream intermittently during latent syphilis and can infect the developing fetus during pregnancy. Latent syphilis ends when curative antibiotic therapy is administered or when manifestations of tertiary disease develop.
Tertiary Syphilis
Today, when coincidental antibiotic therapy is common, late manifestations of syphilis are rarely seen. In contrast, the historic Oslo (112) and Tuskegee (161) studies help to provide an understanding of the late manifestations of disease. In the retrospective Oslo study (112), conducted in the preantibiotic era, approximately one-third of individuals with latent syphilis infection developed clinical manifestations of tertiary syphilis. These syndromes often did not appear until 20 to 40 years after the onset of infection. Progressive inflammation caused gumma (late benign syphilis), a localized form of tissue and bone destruction, in 15% of patients with untreated syphilis. Cardiovascular syphilis, usually seen as aortic insufficiency or aneurysm, was observed in 10% of untreated patients. Symptomatic late neurosyphilis was recognized in 6.5% of untreated patients. Although the Tuskegee study (161), in which 408 African-American men with untreated latent syphilis in Macon County, Alabama, were observed prospectively between the 1932 and 1972, provided information about late disease manifestations, the motivations of the investigators have been questioned by ethicists and human interest groups. Informed consent was not obtained from subjects and, regrettably, penicillin treatment was not offered to the subjects after its advent as syphilis therapy in the 1950s, leading to preventable morbidity and mortality.
Gumma.
Granulomatous, nodular lesions with variable central necrosis may develop as early as 2 years after initial infection, although they usually appear much later. These destructive lesions most commonly affect the skin and bones, although they may also occur in the liver, heart, brain, stomach, and upper respiratory tract. T. pallidum can occasionally be identified in gummatous lesions (122, 301); lesions rarely heal spontaneously but resolve rapidly with appropriate antibiotic therapy. Unless they affect a critical organ, gummas usually do not cause serious complications, thus the term “late benign syphilis.”
Cardiovascular syphilis.
Prior to the advent of penicillin therapy, the majority of deaths due to syphilis were thought to result from cardiovascular involvement (260). Typically, syphilitic aortitis involves the ascending aorta; in most cases, aortitis is uncomplicated and asymptomatic. Complications occur in approximately 10% of individuals with untreated syphilis (160), the most common being aortic regurgitation. Other complications are coronary ostial stenosis and saccular aneurysm (153). Recently, PCR methods have detected T. pallidum DNA in an aortic aneurysm (223), confirming that damage results from actual infection of the aorta.
Late neurological complications.
Within 5 to 10 years of untreated initial infection, early invasion of the CNS may progress to meningovascular syphilis, with complications due to cerebrovascular accident. Prior to this event, individuals may experience vertigo, insomnia, and personality changes; these symptoms are followed by arterial involvement that can be widespread or focal (201). Loss of consciousness and seizures may also be present.
Late parenchymatous syphilis, presenting as general paresis or tabes dorsalis, appears two to three decades after infection. Symptoms of general paresis include personality changes, emotional instability, memory impairment, hallucinations, and hyperactive reflexes. Involvement of spinal cord posterior columns and dorsal root ganglia causes tabes dorsalis, which presents as sensory ataxia in the lower extremities, paresthesia, and sudden-onset vomiting or abdominal pain. Wide-based gait often results from the loss of position and vibratory senses. Loss of temperature and deep pain sensations can also occur; the latter may result in Charcot's joints (trophic lesions in ankles, knees, and hips). Optic nerve damage occurs in about 20% of cases of tabes (289), and both paresis and tabes can present with papillary abnormalities.
Congenital Syphilis
T. pallidum can be transmitted from the bloodstream of the infected woman to her developing fetus at any time during pregnancy, although risk of fetal infection is much higher during early maternal syphilis (the first year of infection) than during later stages (284). Antibiotic treatment of the mother during the first two trimesters is usually sufficient to prevent negative outcomes, but later treatment or lack of treatment may result in fetal death, fetal damage, or birth of an infected infant. Destructive effects are thought to depend upon the immune response of the fetus and include spontaneous abortion, stillbirth, and premature delivery. Affected infants typically have low weight at birth, and infants with congenital syphilis may be underweight even relative to other infants of the same gestational age (196). Pulmonary hemorrhage, secondary bacterial infection, and severe hepatitis cause death of approximately 4% of T. pallidum-infected neonates soon after delivery (65, 104).
Like adult disease, congenital syphilis is divided into stages: early manifestations appearing in the first 2 years of life, late manifestations appearing after 2 years, and residual stigmata. Early manifestations are infectious and resemble severe symptoms of adult secondary syphilis; they usually become apparent 2 to 10 weeks after delivery. The first symptom seen in up to 50% of newborns with congenital syphilis is “snuffles.” Invasion of T. pallidum into the nasal mucosa causes persistent rhinitis with a whitish discharge that is sometimes tinged with blood. T. pallidum can further invade the bones and cartilage of the nose and palate, leading to gummatous destruction later in life. Infants with early congenital syphilis commonly have skin lesions resembling those of adult secondary disease, sometimes accompanied by desquamation of the skin of palms and soles (68), condylomata lata, and mucous patches. Other evidence of infection includes anemia, hepatosplenomegaly, renal involvement, and jaundice. Osteochondritis of the long bones can lead to pain and lack of movement of the upper and lower extremities (Parrot's pseudoparalysis); radiographs of the long bones can be helpful in establishing the diagnosis.
Late manifestations of congenital syphilis occur after two years of life. Between the ages of 5 and 25, interstitial keratitis may cause damage to the cornea and iris, and eighth-nerve deafness may be apparent. Asymptomatic and symptomatic neurosyphilis, arthropathy, bilateral effusions of knees and elbows (Clutton's joints), and gummatous periostitis of the palate and nasal septum may also occur. Many of these manifestations progress despite treatment. Hutchinson's teeth, peg-shaped notched upper incisors, is another characteristic late stigma of congenital syphilis. Other tooth deformities, saddle nose, and saber shins may also occur.
BIOLOGY OF T. PALLIDUM
T. pallidum subsp. pallidum belongs to a family of spiral-shaped bacteria, the Spirochaetaceae (spirochetes), and is related to other pathogenic treponemes that cause nonvenereal diseases. T. pallidum subsp. endemicum (bejel), T. pallidum subsp. pertenue (yaws), and T. carateum (pinta) can be differentiated from T. pallidum by the clinical manifestations of their respective diseases and, more recently, by genetic differences (55, 58). T. pallidum varies from 6 to 15 μm in length and is 0.2 μm in diameter. In both clinical and laboratory settings, dark-field microscopy is used for visualization of this small organism. The spiral-shaped body of T. pallidum is surrounded by a cytoplasmic membrane, which is enclosed by a loosely associated outer membrane. A thin layer of peptidoglycan between the membranes provides structural stability. Endoflagella, organelles that allow for the characteristic corkscrew motility of T. pallidum, are located in the periplasmic space (155).
Limited Metabolic Capacity
The T. pallidum genome, known to be small (327, 328), was confirmed by the Genome Sequencing Project to be 1.14 Mb and to encode 1,041 putative proteins (105). Few bacteria have genomes smaller than that of T. pallidum; indeed, the genomes of many conventional gram-negative (e.g., Escherichia coli K-12, 4.6 Mb) and gram-positive (e.g., Bacillus subtilis, 4.2 Mb) bacteria are several times larger. The T. pallidum Genome Sequencing Project also confirmed what had already been suggested by ongoing experimental efforts: that T. pallidum has a striking lack of metabolic capabilities. The organism is able to carry out glycolysis (105, 217, 270) but lacks tricarboxylic acid cycle enzymes and an electron transport chain (105). Analysis of the T. pallidum genome suggests an absence of pathways for the use of alternative carbon sources for energy and for de novo synthesis of enzyme cofactors and nucleotides (105). Amino acid and fatty acid synthesis pathways are also lacking (105), but T. pallidum does carry enzymes for the interconversion of amino acids and fatty acids. With this dearth of biosynthetic pathways, it is suspected that T. pallidum derives most essential macromolecules from the host, using interconversion pathways to generate others.
To take up macromolecules from the host environment, specific transporters may be utilized by T. pallidum. Homologs of transporters for a variety of amino acids are found in the T. pallidum genome (105). The lipoprotein TpN32 is homologous to a member of the newly identified methionine uptake transporter family in E. coli (337), and the methionine-binding properties of TpN32 were recently described (83), suggesting that these are part of a methionine transport system in T. pallidum. Six T. pallidum transporters have specificity for cations (105). The most extensively studied of these is the ATP-binding cassette (ABC) transporter encoded by the tro operon. TroA (alternatively termed TROMP1), the cation-binding protein of the Tro complex, binds zinc (82, 105, 171) and manganese (137), trace metals that may be required for enzyme function in T. pallidum.
T. pallidum homologs to dct (Rhodobacter) and y4o (Rhizobium) encode proteins that are likely to transport multiple sugars across the cytoplasmic membrane. In classical gram-negative organisms, the ABC transporter MglABC has specificity for galactose (126, 212). The homologous Mgl system in T. pallidum (21, 236, 299) may also bind galactose, but it has been speculated that, because of its inability to utilize galactose as a carbon source, T. pallidum may utilize MglABC as a glucose transporter (81). Ribose may be taken into the cell via an ABC transporter with homology to the RbsAC transporter system in the related spirochete Borrelia burgdorferi; however, carbon utilization studies demonstrated that ribose is not degraded by T. pallidum (217). Thus, the RbsAC homolog may function to transport other sugars or may be nonfunctional in T. pallidum.
Because the T. pallidum genome encodes no known homologs to porin proteins, it is unclear how nutrients are moved across the outer membrane into the periplasmic space. A recent study (135) suggests that Tp0453, a putative outer membrane protein, may perturb the outer membrane by insertion into its inner leaflet, allowing nonselective diffusion of nutrients into the periplasm.
In Vivo and In Vitro Growth of T. pallidum
Although T. pallidum was one of the first infectious agents to be associated with a human syndrome (269), efforts to elucidate molecular mechanisms of T. pallidum virulence have been hampered by certain characteristics of the organism. T. pallidum does not survive outside the mammalian host; infectious capability is lost within a few hours or days of harvest. To obtain sufficient organisms for experimental manipulation, T. pallidum must be propagated in rabbits (317). As yet, researchers have been unable to propagate T. pallidum in tissue culture more than 100-fold, an equivalent of about seven generations (95, 96, 220). With the expectation that providing fresh media and removing toxins from the culture system would extend its metabolic capabilities, Norris and Edmondson attempted to subculture T. pallidum in vitro (221) but were unable to maintain viable organisms. This inability of T. pallidum to survive and multiply outside the mammalian host is perhaps the greatest impediment to syphilis research.
The generation time of T. pallidum is unusually slow. Inoculation studies determined that T. pallidum doubles every 30 to 33 h in vivo (77, 189); extrapolating generation time from the number of replication cycles in tissue culture yields an in vitro generation time of 30 to 50 h (95, 220). Several biological factors may contribute to T. pallidum's sluggish replication rate. Because it lacks a tricarboxylic acid cycle and an electron transport chain (105), T. pallidum depends upon glycolysis as the sole pathway for the synthesis of ATP. In fact, the theoretical energy yield of an organism that undergoes aerobic respiration, such as E. coli, is 38 ATP, 19 times greater than the 2 ATP synthesized from glycolysis alone. E. coli doubles approximately every 20 min, at least 90 times faster than T. pallidum, suggesting that low energy production is not the only factor that inhibits T. pallidum replication. Because of a lack of enzymes such as catalase and oxidase that detoxify reactive oxygen species (105), the in vitro survival of T. pallidum is prolonged by low oxygen concentrations. Genes for several other oxygen-protective enzymes have been identified in the T. pallidum genome. Neelaredoxin (Tp0823) is hypothesized to convert superoxide to peroxide, which is then reduced to water by hydroperoxide reductase C (Tp0509). Each of these enzymes is regenerated by other T. pallidum proteins (136).
In addition to its sensitivity to oxygen, T. pallidum may have a limited stress response. The typical heat shock response regulated by σ32 is lacking (105, 296), possibly reflecting the sensitivity of the organism to growth temperature (95). At least one T. pallidum enzyme is unstable at normal body temperature (22), suggesting that the heat lability of enzymes may also contribute to the slow growth of the organism. Heat therapy for late neurosyphilis was introduced in 1918 by the Viennese psychiatrist Julius Wagner von Jauregg (326), a discovery for which he later won the Nobel Prize in Medicine. The regimen consisted of inoculating patients with malaria-infected blood and, 10 to 12 febrile episodes later, treating them with quinine. The high temperatures induced by this regimen, along with other methods of raising body temperature that were later introduced, presumably killed T. pallidum in the CNS. Doctors reported high percentages of complete or partial remission of general paresis symptoms (although the “treatment” killed an estimated 10% of patients) (301), causing heat therapy to be in vogue for more than a quarter of a century. It is clear that T. pallidum has limited heat tolerance; this, along with oxygen sensitivity and possibly other as-yet-unrecognized factors, may hinder the replication of T. pallidum both in vivo and in vitro.
Genetic intractability, related to the inability to grow the organism, is another hindrance to T. pallidum molecular research. Unlike the related spirochetes Treponema denticola (110) and B. burgdorferi (89, 268, 291), no system for genetic manipulation of T. pallidum yet exists. Because of the fragility of its outer membrane, genetic manipulation of T. pallidum may prove impossible. Heterologous expression in related organisms such as T. denticola (70) may be the most practical way to study T. pallidum genes and advance our understanding of this enigmatic organism.
Paucity of Recognized Virulence Factors
Despite its fragility to environmental factors, T. pallidum readily causes chronic infection and varied disease manifestations in the host. The T. pallidum genome sequence does not reveal any obvious classical virulence factors that could account for syphilis signs and symptoms. T. pallidum lacks lipopolysaccharide (LPS) (105), the endotoxin found in the outer membranes of many gram-negative bacteria that causes fever and inflammation. However, T. pallidum does produce a number of lipoproteins which may induce expression of inflammatory mediators via toll-like receptor 2 (TLR2) recognition (38, 173). Many gram-negative pathogens utilize type III secretion systems to insert virulence-related proteins into the cytoplasm of host cells (335); T. pallidum lacks homologs for the recognized type III components (105). Cytolytic enzymes or other cytotoxins have not been shown to play a role in syphilis pathogenesis, and a cytopathic effect of treponemes on cells in culture requires extraordinarily high numbers of bacteria (103). Therefore, the identification of several putative hemolysins in the annotated T. pallidum genome was unexpected. These predicted proteins, however, have only weak similarity to known cytolysins, and recombinant preparations of these proteins showed no hemolytic activity. Thus, the true function of these proteins is unknown. Nonetheless, experimental studies and exploration of the T. pallidum genome have revealed significant new information about many aspects of syphilis pathogenesis.
INVASION
Despite a striking lack of metabolic capabilities, sensitivity to oxygen, and decreased viability in an environment warmer than body temperature, T. pallidum is able to invade and survive in a wide variety of tissues and organs. The detection of T. pallidum in the CSF of a high proportion of individuals with early syphilis, as well as the disseminated clinical manifestations of secondary, tertiary, and congenital syphilis, provide evidence of the highly invasive capabilities of the organism. Typical of other pathogenic spirochetes, including Borrelia and Leptospira species, dissemination is not only widespread, it is rapid. In rabbit infection, T. pallidum enters the bloodstream within minutes of intratesticular or intradermal inoculation (77, 301), and organisms applied to mucosa are found in deeper tissues within hours (190).
Direct evidence for the ability of T. pallidum to invade many different tissue types is provided by numerous studies of experimental infection. Blood from mice, monkeys, and rabbits during early stages of infection is infectious to naïve animals, indicating the ability of the organism to gain access to the bloodstream. Infectivity tests have also been performed on various tissues from these animals. The liver and lymph nodes of intradermally infected macaques were shown to contain live T. pallidum bacteria (317) and, after 45 days of mouse infection, the skin, brain, spleen, and lymph nodes were infectious to rabbits (261).
The rabbit is the most extensively studied animal model for syphilis. Rabbits are less expensive and easier to work with than monkeys, and unlike mice, which do not develop any signs of infection, rabbits produce disease manifestations that are similar clinically and histologically to human primary and secondary disease (9, 276). After intratesticular infection in rabbits, early researchers noted skin and bone lesions (317), indications of the presence of virulent T. pallidum. T. pallidum was demonstrated by microscopy in rabbit skin, testes, spleen, and lymph nodes after intradermal infection (276). After testicular infection of rabbits, treponemes were visible in the lymph nodes, brain, and aqueous humor, and in the CSF as early as 18 h postinoculation (73). T. pallidum RNA has also been detected by reverse transcription-PCR in the CSF of intravenously infected animals (306), and Marra et al. found that 6% of rabbits inoculated by the intrathecal method developed ocular syphilis (192), demonstrating that organisms are able to travel from the CSF to the eye. Not only do treponemes disseminate to distant sites but they also can persist in distant tissues during chronic infection. Unapparent or latent infection in experimental animals is routinely demonstrated by the ability of lymph node extracts to infect naïve animals (63, 138, 317); infectivity testing has also demonstrated T. pallidum in the blood and liver of intratesticularly infected rabbits (73).
The diverse manifestations of human syphilis also demonstrate the invasiveness of T. pallidum. Humans are initially infected with syphilis at anogenital and, more rarely, oral and nongenital dermal sites, yet the rash of secondary syphilis is a clear indication that organisms disseminate widely from the primary site of contact. T. pallidum has been detected directly in tissues and fluids far from the initial site of infection. Using PCR and infectivity testing, T. pallidum is routinely found in the CSF of individuals with early and latent syphilis (183, 193). T. pallidum has been detected decades after initial infection in tertiary gummatous lesions of the skin by silver staining and immunofluorescence microscopy (122, 160, 301) and by PCR (338).
Attachment
As with many bacterial pathogens, the first step in T. pallidum invasion is the attachment of organisms to host cells. T. pallidum has been shown to attach to a wide variety of cell types including epithelial, fibroblastlike, and endothelial cells of rabbits and humans (99, 134, 168, 311). A reduction in attachment occurs when either bacterial or eukaryotic cells in culture are not viable (99). Organisms are also able to adhere to isolated capillary (241), kidney (102), and abdominal wall tissues (257) ex vivo. Microscopic examination of T. pallidum associated with cell cultures (98, 99) reveals that large numbers of organisms attach to cells by one or the other end. Some researchers have speculated that specialized T. pallidum adhesins are located at the tips of the organisms, but treponemes have also been observed to be attached to host cells along their length. It has been speculated that initial attachment can occur at multiple sites along the length of the bacterium but that the active motility of the attached treponemes serves to cause the bacterial adhesin molecules to migrate through the somewhat fluid outer membrane toward the tips, in a “capping” phenomenon. The number of treponemes that can attach to each host cell appears to be limited: the addition of virulent T. pallidum bacteria to cells with organisms already attached does not increase the total number of adherent T. pallidum bacteria per cell (134). This limitation of binding may occur because all of the receptors on the host cell are occupied, implying a specific adhesin-ligand interaction; integrins have been implicated as host cell receptors for T. pallidum (168). The nonpathogenic treponemal species T. phagedenis, T. refringens, and T. vincentii do not adhere to cultured cells (98, 99, 134), indicating that attachment is specific to pathogenic treponemes. Heat-killed T. pallidum organisms do not attach to cells (100), nor do T. pallidum organisms that have been rendered nonmotile by 22 to 23 h of incubation at 37°C (99). Immune rabbit (99, 134) or human (322) serum prevents the adherence of viable T. pallidum organisms to cell cultures, suggesting that specific surface antigens of T. pallidum may mediate attachment to host cells.
Components of host serum, cell membranes, and the extracellular matrix (ECM) have been shown to bind to T. pallidum, and ECM components have been demonstrated to be involved in mediating attachment of other pathogenic bacteria to host cells. T. pallidum attaches to fibronectin-coated coverslips (41, 101, 102, 234, 309); this attachment is enhanced by pretreatment of organisms with fibronectin (308) and is inhibited by antifibronectin antibodies (102, 309) or immune rabbit serum (102). Pretreatment of cell monolayers with antifibronectin antibodies also inhibits the attachment of T. pallidum to these cells (234, 309). Other ECM components, such as laminin, collagen I, and hyaluronic acid, have been reported to bind to T. pallidum (39, 102). Except for the latter, attachment of T. pallidum to coated coverslips is blocked by antibodies directed against each component (102).
Three treponemal proteins with fibronectin-binding properties were discovered and partially characterized over 20 years ago (18, 310), but until recently the identity of potential T. pallidum adhesins was unknown. Cameron et al. (41) used computer analysis to search the T. pallidum genome for adhesin candidates. Two genes, referred to by their genome sequence designations tp0155 and tp0483, were expressed as recombinant proteins and assayed for the ability to bind to fibronectin. Tp0155 binds to matrix fibronectin, while Tp0483 binds to both soluble and matrix forms of fibronectin (41); binding occurs in a dose-dependent manner. This finding suggests that one molecule may mediate attachment via fibronectin in the bloodstream, while the other is functional in tissues. Another recombinant T. pallidum protein, Tp0751, binds specifically to laminin (39), and antibodies against Tp0751 have been shown to inhibit the binding of T. pallidum to laminin-coated coverslips (40).
Binding to ECM components is a virulence factor in many microorganisms (97). The major sheath protein (Msp) of the related oral spirochete T. denticola binds fibronectin and laminin (94, 120), as well as other ECM components (88, 319), implicating Msp as an important molecule in colonizing host tissues. Streptococcus pyogenes binds to host ligands using a wide variety of adhesins (227). Immunization with an S. pyogenes fibronectin-binding protein, Sfb1, protects mice against intranasal challenge (119) but does not protect against subcutaneous challenge (197), suggesting that the variety of S. pyogenes adhesin molecules may allow the organism to penetrate a wide variety of tissue types. In the same way, T. pallidum adhesins that bind to different ECM components may contribute to the ability of the organism to penetrate different tissues and disseminate widely during infection.
Motility
Motility is a virulence factor for many bacterial pathogens (157, 225). T. pallidum is a highly motile organism that propels itself by rotating around its longitudinal axis. This corkscrewlike motility is common to other spirochetes (45) and allows this group of organisms to swim easily through gel-like materials that hinder most other flagellated organisms (23). This ability allows some spirochetes to occupy unique ecological niches, such as sediments in pond and lake bottoms, the guts of certain arthropods, and the rumens of cows and sheep. The arrangement of spirochete flagella is also unique among the bacteria. Early electron microscopy studies of T. pallidum revealed filamentous structures wrapped around the cytoplasmic body (209, 303). These “axial filaments,” now called endoflagella or periplasmic flagella, are located in the periplasmic space, between the cytoplasmic membrane and the outer membrane (155). Three to six fibrils insert at the ends of the organism and extend toward the center of the cell (155, 305). The fibrils have a typical flagellar structure with a long shaft and an insertion apparatus made up of a hook, collar, and basal knob (142). Unlike most bacterial flagellar filaments, which are comprised of a single protein, the shaft of the T. pallidum flagella is made up of several major filament proteins (72, 229). Three proteins, FlaB1 (34.5 kDa), FlaB2 (33 kDa), and FlaB3 (31 kDa), make up the flagellar core (64, 226), and the core is covered with a sheath made up of subunits of the 37-kDa FlaA protein (152). Motility-related proteins that make up the flagellar motor/switch and assembly apparatuses have homology to those found in other bacteria (105, 127, 128, 174). Cellular (7, 10) and humoral (31, 125, 178) immune responses to endoflagella are induced during syphilis infection, and the major flagellar proteins are recognized by antibodies from infected humans (11, 31, 124, 125, 243) and rabbits (123, 178, 181). T. pallidum endoflagellal core subunits share antigenic epitopes with endoflagella of the nonpathogenic treponeme T. phagedenis biotype Reiter (28, 31, 125, 178, 243); there are also pathogen-specific flagellar sheath epitopes in T. pallidum (28, 178, 186). Despite their strong antigenicity, immunization with flagellar subunits from T. phagedenis and T. pallidum failed to induce complete protection against T. pallidum infection (63, 140), probably because the flagella are not exposed on the surface of the organism.
Chemotaxis
Motile bacteria depend upon chemotactic responses to move toward favorable environments and away from hostile environments. The dissemination of motile bacterial pathogens in tissues may be facilitated by chemotaxis. Methyl-accepting chemotaxis transmembrane proteins (MCPs) and cytoplasmic chemotaxis proteins (Che) comprise the chemotaxis system of gram-negative bacteria, and T. pallidum has homologs for proteins of each of these systems. Four genes for MCPs are found in the T. pallidum genome (105, 116, 121). MCPs sense attractants and repellents in the environment; glucose and histidine are molecules that may have affinity for T. pallidum MCPs. Two operons in the T. pallidum genome contain genes for the Che response regulators (105, 117). Pathogenic processes such as crossing the endothelial barrier to reach the bloodstream are likely to depend upon mechanisms that allow T. pallidum to sense and respond to nutrient gradients.
INFLAMMATION AND IMMUNE RESPONSE
Compared to the wealth of information about the disease-causing mechanisms of many bacterial pathogens, little is known about how T. pallidum causes the protean manifestations of syphilis. In the absence of cytotoxins and other known virulence factors, it is probable that inflammation and the ensuing adaptive immune response to T. pallidum cause the tissue destruction characteristic of syphilis infection.
Inflammation
T. pallidum quickly gains access to deeper tissues and the bloodstream (190, 301), probably by traversing the junctions between endothelial cells (255, 311). T. pallidum has been shown to induce the production of matrix metalloproteinase-1 (MMP-1) in dermal cells (71). MMP-1 is involved in breaking down collagen, which may help T. pallidum to penetrate tissues. The presence of a pathogen signals inflammatory and immune cells to migrate from the bloodstream to the site of infection. An early step in this homing mechanism is the expression of cell adhesion molecules on capillary endothelial cells, promoting the leakage of serous fluids and the migration of leukocytes out of blood vessels into infected tissues. Virulent T. pallidum induces cultured endothelial cells to express the adhesion molecules ICAM-1, VCAM-1, and E-selectin (169, 255). These are also activated by the 47-kDa T. pallidum lipoprotein TpN47 but not by heat-killed T. pallidum or the nonpathogenic treponeme T. phagedenis (169, 255), suggesting that endothelial cell activation is a pathogen-specific, active process mediated by specific T. pallidum molecules. Preincubation with virulent T. pallidum organisms increases the ability of endothelial cells to bind to lymphocytes (256), and this binding is blocked by antibodies against the cell adhesion molecules (169), supporting a functional role for ICAM-1, VCAM-1, and E-selectin expression in the inflammation caused by T. pallidum infection.
During acute bacterial infection, polymorphonuclear lymphocytes (PMNs) are often the first cells to infiltrate the site of infection. PMNs are seen in very early syphilis lesions that are experimentally induced (214, 277, 312, 317) and naturally acquired (36), although infiltration is transient (33, 312) and the number of PMNs is low relative to that seen in other acute bacterial infections (228, 275). Dermal injection of synthetic analogs of TpN17 and TpN47 lipoproteins also induces transient infiltration by PMNs of the injection site (219, 280).
To kill ingested pathogens, phagocytic vacuoles of PMNs fuse with cytoplasmic granules that contain enzymes, superoxide radicals, and antimicrobial peptides. The antimicrobial rabbit neutrophil defensins NP-1, NP-2, and NP-5 have been detected at the site of syphilis infection within 24 h of inoculation with T. pallidum (33), and some defensins have been shown to neutralize the infectivity of T. pallidum in vitro (35). Cathelicidins are another class of antimicrobial peptides found in PMN granules, and six different cathelicidins have shown various levels of activity in T. pallidum-killing assays in vitro (76, 266). While these studies suggest that PMNs may be involved in early infection, the inability of PMNs to adequately control T. pallidum is demonstrated by the progression of infection following this mild localized early inflammatory response.
During bacterial infection, endothelial cells, dendritic cells, and macrophages recognize shared microbial patterns such as LPS, peptidoglycan, and the acylated moieties of lipoproteins. This recognition is mediated by receptors, the TLRs, found on the cell surface. The human embryonic kidney cell line HEK293, which lacks TLRs, is unresponsive to stimulation with the lipoprotein TpN47 but is activated by TpN47 when stably transfected with TLR2 (38). Stimulation is enhanced by the expression of CD14 (38, 279), a membrane protein that acts as a coreceptor to mediate lipoprotein signaling via TLR2 and LPS signaling via TLR4. The role of TLR2 in signaling the presence of T. pallidum lipoproteins was confirmed by studies with Chinese hamster ovary (CHO) cells that have a normal TLR4 receptor but whose TLR2 receptor is inactivated by a point mutation. As with HEK293 cells, it was found that stimulation by TpN47 occurred only in CHO cells that had been stably transfected with a gene that expresses a functional TLR2 receptor (173).
Dendritic Cells
Dendritic cells (DCs) are also stimulated by synthetic microbial lipopeptides through the TLR2 pathway (139, 287). Specialized DCs called Langerhans cells are found in skin, the site of the majority of primary and secondary lesions; DCs are also found in the mucosa, the intestinal wall, and the heart, all potential sites of T. pallidum infection. In many bacterial infections, the bacteria are taken up by immature DCs at the site of infection, and the DCs then migrate to lymph nodes where they activate T cells (251). It has been shown that T. pallidum interacts with and is phagocytized by immature DCs in cell culture (37, 286). As they mature, DCs produce inflammatory cytokines in response to pathogenic stimulation. Expression of the inflammatory cytokines interleukin 1β (IL-1β), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α) in DCs is stimulated by exposure to whole T. pallidum organisms or to a synthetic lipopeptide representing the lipid portion of TpN47 (37). The TpN47 synthetic lipopeptide also stimulates immature DCs grown in cell culture to express the maturation markers CD54, CD83, major histocompatibility complex class II (the molecule on which antigen is presented to CD4+ T cells), as well as other markers (37, 139, 286, 287). In an assessment of their functional activity, DCs that had been stimulated with whole T. pallidum organisms were more capable of stimulating T cells in vitro than DCs that had not been exposed to T. pallidum (286).
In a model developed to simulate human infection, the inner forearm of several volunteers was injected with TpN17 and TpN47 synthetic lipopeptides and suction was used to raise blisters at the sites (280). DCs present in blister fluids expressed the maturation marker CD83, as well as molecules involved in T-cell stimulation (280). CD83-positive DCs have also been found in disseminated skin lesions of human secondary syphilis (162).
Specific T. pallidum molecules that have been shown to stimulate DCs, the lipoproteins TpN17 and TpN47, are not surface localized. The initiation of lipoprotein signaling of DCs is not likely to occur until the organisms are being degraded, exposing the lipoproteins to the TLR2 receptors. This theory is supported by observations that longer times than usual are required for T. pallidum stimulation of DCs (37). A delay in DC maturation, resulting in a slower inflammatory response, could allow the early dissemination of T. pallidum, giving organisms the opportunity to penetrate organs and tissues before an active inflammatory response has been mounted by the host.
Cytokine Production
Endothelial cell activation and migration of inflammatory cells are further augmented by the secretion of soluble immune factors called cytokines. In an in vitro system, exposure to intact and sonicated T. pallidum induces macrophages to express TNF-α (278), a cytokine that activates a number of components of the immune response. Stimulation of macrophages by purified T. pallidum lipopeptides, or with representative synthetic lipopeptides, also induces expression of proinflammatory cytokines. TpN47 activates the expression of TNF-α, IL-1β, IL-6, IL-8, and IL-12 (38, 242, 279). TNF-α production is also induced by TpN15, TpN17, and TpN38 (2, 248), and stimulation of macrophage cell lines with TpN17 activates IL-1β production (218). Additionally, TpN47 induces expression of the T-cell chemoattractant cytokines MIP-1α and MIP-1β (281). Liver macrophages called Kupffer cells also produce TNF-α in response to stimulation with whole T. pallidum organisms or with the lipoproteins TpN47, TpN17, TpN15, and TmpA (191). Taken together, these findings implicate T. pallidum lipoproteins as potent inducers of inflammation during early syphilis infection.
Immune Clearance
DCs act as a bridge between innate and adaptive immunity by presenting specific antigens to T cells in the lymph nodes (251), stimulating them to differentiate and migrate to the site of infection where they perform their specialized functions. After intratesticular infection of rabbits with an inoculum of 108 T. pallidum organisms, splenic T cells sensitized to T. pallidum antigens could be demonstrated in these rabbits as early as 3 days following infection (180). T cells induced in response to syphilis infection are highly reactive to several of the major T. pallidum proteins, including TpN47, TpN17, and TpN15—lipoproteins embedded in the outer leaflet of the cytoplasmic membrane—and TpN37, TpN35, TpN33, and TpN30—proteins that make up the core and sheath of T. pallidum flagella (7, 10). T cells were detectable at the infection site within 3 days postinfection (179) and reached peak concentrations at days 10 to 13, roughly the same time that the number of T. pallidum organisms in testis tissue reached its maximum. Macrophages also infiltrate the site of infection after 6 to 10 days and reach maximal numbers at approximately day 13. Between days 13 and 17 postinfection, the number of detectable T. pallidum organisms sharply declines (179). These studies suggest a mechanism similar to delayed-type hypersensitivity as the mode of immune clearance in T. pallidum infection.
Similarly, T cells and macrophages are found in rabbit dermal lesions (276), as well as in human primary chancres (90, 314, 325) and secondary lesions (90, 198, 325, 333). Helper (CD4+) T cells and cytolytic (CD8+) T cells are present in primary and secondary lesions (90, 314, 325). McBroom et al. suggest that many CD4+ cells in secondary lesions are in fact macrophages expressing the CD4 receptor (198). mRNA for the cytokines gamma interferon (IFN-γ) and IL-2, which function to activate macrophages and stimulate proliferation of T cells, respectively, is also found in primary and secondary lesions (323). Both CD4+ and CD8+ T cells produce IFN-γ, and the lytic mediators granzyme B and perforin have been detected in syphilis lesions, suggesting that infiltrating CD8+ T cells are activated (325). As T. pallidum is found almost exclusively extracellularly, the role of the CD8+ lytic compounds in clearance of T. pallidum from the site of infection is unclear; however, granzyme B and perforin may be partially responsible for the tissue destruction characteristic of syphilis lesions.
A functional role for macrophages in immune clearance was first suggested by early reports that found intact and degraded T. pallidum organisms inside phagocytic vacuoles of macrophages (155, 275) and by the demonstration of phagocytosis of opsonized T. pallidum organisms in vitro by rabbit peritoneal macrophages (184). In response to cytokine signals from infiltrating T cells, macrophages migrate to the site of infection, are activated by interferon IFN-γ, and ingest and kill organisms. In vitro phagocytosis and killing of T. pallidum by macrophages has been demonstrated, and phagocytosis is increased when organisms are opsonized by preincubation with serum from rabbits infected with T. pallidum (13, 15). Opsonizing agents are serum components, antibodies, or the complement protein C3b, which make the pathogen recognizable to macrophages via specific cell surface receptors. In macrophage recognition of T. pallidum, opsonization is accomplished by antibodies, both immunoglobulin G (IgG) (283) and IgM (16). T. pallidum antigens, including Tp92 and TprK, have been shown to induce production of opsonic antibodies (44, 54, 283). Antibodies against the VDRL (Venereal Disease Research Laboratory) antigen, a complex of cardiolipin, cholesterol, and lecithin, also increase the phagocytosis of T. pallidum by macrophages (16). Interestingly, it has been shown that those few treponemes remaining at the site of intratesticular infection after most have been cleared by the immune response are able to resist ingestion by macrophages (185), suggesting that this subpopulation of organisms may be able to avoid binding by opsonic antibody and therefore persist in the face of active immune clearance. The mechanism of this resistance has not been explored.
Antibodies in Syphilis Immunity
IgM antibodies are usually the first to develop after establishment of bacterial infection, followed shortly by IgG. In the intratesticular model of syphilis infection in rabbits, anti-T. pallidum IgM and IgG are detectable as early as 6 days postinfection (123, 181, 211). Specific IgM continues to be produced in infected rabbits and humans, even after disease symptoms have subsided (11, 14, 181), suggesting that exposure to T. pallidum antigens continually stimulates B cells. IgG persists throughout late latent syphilis in humans (11, 124), and strong IgG reactivity has been demonstrated in rabbit serum up to 17 months after infection (123). The antibody response elicited during infection is specific for a broad range of T. pallidum molecules (200, 222), including lipids found on the surface of T. pallidum (26), flagellar proteins (31, 243), lipoproteins (61, 148, 272, 273, 285), and various other proteins, including the Tprs (167). Some of these proteins are cross-reactive with antigens of the cultivable Treponema species (12, 125, 178), but a number are specific for T. pallidum subspecies, indicating that there are both shared and unique antigenic determinants in pathogenic and nonpathogenic treponemes.
Besides opsonization, there are other functions of antibodies produced during T. pallidum infection. Antiserum from T. pallidum-infected rabbits, presumably the IgG component, has been shown to block organisms from binding to cells in vitro, suggesting that attachment to host cells is mediated by treponemal adhesin molecules. In the presence of complement, anti-T. pallidum antibodies immobilize organisms (30, 216) and neutralize the ability of the organisms to produce typical dermal lesions (25, 316). Passive administration of whole serum and fractionated IgG from long-term-infected rabbits delays lesion development in challenged rabbits during serum administration (24, 30, 232, 282, 313, 318, 332), but lesions develop at the inoculation site within days of discontinuing antibody administration (24, 332). This demonstrates that specific antibody alone, while inhibitory to the establishment of lesions, is not sufficient to kill T. pallidum and prevent infection.
Antibodies in Diagnostic Testing
The antibodies produced in response to syphilis infection are exploited by clinicians for diagnostic purposes. The first serologic test for syphilis, developed in the early 20th century, was the Wasserman test. This test is classified as nontreponemal because the antigen, comprised of lecithin, cholesterol, and cardiolipin, is not unique to T. pallidum. These lipids are thought to be derived from the host and incorporated into the membrane of the metabolically limited T. pallidum (105), producing a configuration that is antigenic. Decades later, the antilipoidal antibody VDRL and rapid plasma reagin (RPR) tests were developed (132, 237). Automation, antigen stability, the ability to use plasma rather than serum, and macroscopic observation (199) make the RPR test more conducive for use in clinical laboratories. The VDRL test continues to be used in some settings, although it has no advantages over the RPR for diagnosis of syphilis.
The sensitivities of the RPR and VDRL syphilis diagnostic tests depend upon the stage of disease. In disease of short duration, i.e., when the primary chancre has just appeared, antilipoidal antibody tests are often negative; after several weeks of infection, however, the tests are usually positive. Accordingly, the mean sensitivities during primary syphilis of the RPR and VDRL tests are 86% and 78%, respectively, while the sensitivities of both tests during secondary syphilis are 100% (164). Antilipoidal antibody reactivity can arise as a result of tissue damage from recent or concurrent infectious diseases such as hepatitis or underlying autoimmune diseases such as rheumatoid arthritis or systemic lupus erythematosus; these antibodies can cause a false-positive RPR or VDRL test. Because autoantibodies increase as a result of aging, elderly people are also at risk for a false-positive result.
The T. pallidum immobilization (TPI) test, developed as a result of the discovery that serum from syphilis-infected patients inhibits treponemal mobility in the presence of active complement (216), was the first test to be specific for antitreponemal antibodies. Within a decade of its discovery, the TPI test was replaced by the more sensitive fluorescent treponemal antibody (FTA) test (80), later refined by an absorption step to the FTA-ABS test (149). These tests use anti-human Ig labeled with fluorescein to detect antibodies bound to T. pallidum organisms on slides. Hemagglutination, a technically simpler test developed in the same era as the FTA-ABS test, detects reactive antibody that agglutinates red blood cells sensitized with T. pallidum antigen (263). The T. pallidum particle agglutination assay (235) uses biologically inert gel particles in place of red blood cells and has fewer equivocal reactions than the hemagglutination test.
To diagnose neurosyphilis, the CSF can also be tested for antibodies induced in response to infection with T. pallidum. While insensitive, the CSF-VDRL test is very specific for neurosyphilis (133). The FTA-ABS CSF test has also been examined in neurosyphilis diagnosis (164) and, while more sensitive than the CSF-VDRL test, may not be specific for neurosyphilis (91), possibly because of the passive transfer of serum-derived antibodies to the CSF. Thus, some investigators suggest that the use of the FTA-ABS CSF test should be limited to ruling out neurosyphilis in cases with a negative test result (154, 195).
In an attempt to develop a test with excellent sensitivity, especially during the earliest syphilis stages, several laboratories have explored the use of recombinant T. pallidum antigens in either enzyme immunoassay (108, 151, 245, 267, 324) or immunoblot (8, 233) format. Several antigens that elicit high antibody titers during syphilis infection and are not cross-reactive with serum from patients with other common spirochetal diseases have been identified (108, 324). One antigen in particular, Tp0453, was shown to be highly sensitive in detecting infection during primary syphilis (324); additionally, Tp0453 did not react with serum from uninfected individuals or from individuals with leptospirosis, Lyme disease, or relapsing fever. Testing with recombinant T. pallidum antigens may improve syphilis diagnosis, especially the detection of early infection.
EVASION OF THE IMMUNE RESPONSE AND CHRONIC INFECTION
In the face of a hostile host environment and a strong specific immune response, how does T. pallidum survive? There are several mechanisms the organism might use to ensure its continued existence in the host. As discussed above, T. pallidum penetrates a broad variety of anatomical sites, including the central nervous system, eye, and placenta, tissues that may be “immune privileged” in that less surveillance by the innate immune system may occur in those sites. Organisms may survive in these tissues, slowly replicating and possibly reseeding other tissues. T. pallidum may also exploit its slow metabolism to survive in tissues, even those that are not immune privileged. It has been speculated that a minimum number of organisms, or “critical antigenic mass,” is required to trigger a host response (177, 317). By maintaining infection with very few organisms in anatomical sites distant from one another, T. pallidum may prevent its clearance by failing to alert the immune response to its presence. In this scenario, T. pallidum may spend months to years in a quiescent environment, with organisms dividing very slowly. Indeed, it is quite likely that T. pallidum undergoes an even lower rate of division during latent disease, as late latent syphilis must be treated by a prolonged course of penicillin to prevent treatment failure (51). Unknown factors cause T. pallidum to begin dividing at a higher rate again in certain anatomical areas in a small percentage of individuals, leading to symptomatic late syphilis.
An important defense mechanism utilized by the host is iron sequestration. The host iron-binding proteins transferrin and lactoferrin cause free iron to be unavailable to bacteria, impairing their growth. T. pallidum has been reported to interact with both transferrin and lactoferrin (4, 5, 292), and the organism may be able to acquire iron from these host proteins (5). T. pallidum may also overcome host iron sequestration by utilizing enzymes that bind metals other than iron. Unlike many bacterial pathogens, T. pallidum lacks an electron transport chain, which is made up of enzymes that use iron as a cofactor, and T. pallidum appears to have very few other enzymes or components that require iron (105). A regulated system for the uptake of metals such as zinc and manganese has been described (171, 238), suggesting that these metals may act as iron alternatives. As mentioned above, neelaredoxin is thought to be important for protection against damage by superoxide radicals (158, 175). The neelaredoxin enzyme is found mainly among primitive species of anaerobic bacteria and appears to bind iron or zinc. Studies of neelaredoxin were conducted with recombinant protein; the metal-binding properties of the native enzyme have not been examined.
Surface of T. pallidum
Some organisms survive in infected hosts despite the presence of strongly reactive antibodies directed against a number of T. pallidum proteins (11, 222) and activated T cells and macrophages in lesions (90, 314, 325). Outer surfaces are the first bacterial component to encounter the host and are often the targets of host adaptive immunity. Early researchers noted that antibodies in serum from infected animals did not readily bind to intact treponemes (80, 202). This observation was later supported by Penn and Rhodes (231), who found that only those treponemes that had been physically disrupted reacted with anti-T. pallidum antiserum, leading them to propose that the surface of T. pallidum is nonantigenic. As a follow-up to these observations, it was noted that treponemes were able to be labeled with radioactive iodine (230) or antibodies raised against T. pallidum (298) only when the outer layer had been disrupted by detergents or by incubation that effectively “aged” the organisms. Furthermore, in experiments involving the interaction of T. pallidum-specific antibodies with viable organisms—agglutination (131), neutralization (25), and opsonization (13, 184)—organisms must be incubated with antibodies for unusually long periods before effects are observed. Taken together, these observations suggest that there are few antigenic targets on the surface of the organisms, causing the binding and aggregation of antibodies to proceed slowly. The relative antigenic inertness of the T. pallidum outer membrane was first explored in two independent studies (249, 329) in which T. pallidum, visualized by freeze fracture electron microscopy (EM), was shown to have only rare integral proteins in its outer membrane, approximately 1% of the number found in the outer membrane of E. coli. In later experiments, outer membrane vesicles isolated from T. pallidum were analyzed for integral proteins by freeze fracture EM and tested for antigens by immunoblot analysis (32, 250). These studies confirmed the paucity of integral outer membrane proteins in T. pallidum, a characteristic that may help the organism escape immune detection and that has inspired researchers to call T. pallidum “the stealth pathogen” (265). The rare T. pallidum outer membrane proteins are implicated as likely to be very important in interactions with the host; for this reason, their identity has been the subject of intense research over the last two decades.
Many techniques have been used to explore the identity of T. pallidum outer membrane proteins. Earlier studies used phase partitioning with various detergents (79, 230, 244, 298), separation of membranes with acid (293), or density gradient ultracentrifugation of organisms lysed in a hypotonic solution (3). These methods revealed a subset of proteins that had been previously discovered by immunoblotting of whole organism lysates (178). Several of these, including TpN47, were initially identified as surface-exposed proteins (3, 156), and much effort was devoted to confirming their location. However, further studies indicated that these proteins are not surface exposed but are more likely to be anchored in the inner membrane with portions extending into the periplasm (27, 147, 240, 304). By examining organisms microscopically after physical manipulations such as centrifugation and washing, or after treatment with detergents, Cox et al. substantiated the growing suspicion that the T. pallidum outer membrane is easily damaged by these procedures (75). Most bacteria with a double membrane have a peptidoglycan layer that is linked to the outer membrane by lipoprotein molecules, but in T. pallidum peptidoglycan is thought to associate with the more abundant inner membrane proteins (246). Additionally, T. pallidum lacks LPS (105, 130, 230, 247), a molecule that lends structural stability to bacterial outer membranes. The combination of these ultrastructural features is a likely explanation for the fragility of the T. pallidum outer membrane.
With conventional techniques being unable to positively identify T. pallidum outer membrane proteins, researchers turned to novel molecular (29, 129) and physical (74) methods. Two research groups used E. coli fusion vectors to screen T. pallidum genomic DNA for genes with export signals or transmembrane domains (29, 129). This method provides only indirect evidence for surface exposure and, because of their inherent differences, E. coli expression data do not always translate to biological meaning in T. pallidum. Accordingly, the TpN47 and TpN38 (MglB) lipoproteins, originally identified as localized to the outer membrane by cloning T. pallidum DNA into E. coli (62, 93), were later shown to be inner membrane localized.
In an attempt to preserve the delicate structure of T. pallidum, researchers protected treponemes by suspending them within agarose beads during immunofluorescence labeling. These studies showed that serum from syphilis-infected individuals reacts with organisms only after they are treated with detergent, increasing the permeability of the outer membrane (74). Only detergent-treated organisms were stained by specific antisera to the major membrane lipoproteins TpN47, TpN17, and TpN15 (74), confirming that they are not surface exposed. This technique has been used to identify a phosphorylcholine lipid on the surface of T. pallidum (26) but may not be sensitive enough to detect rare outer membrane-spanning proteins. For the last 25 years, techniques used to identify surface molecules have focused on proteins that are well anchored in the membrane with membrane-spanning domains. A recent report identifies a protein that, rather than spanning the outer membrane, appears to loosely associate with its inner leaflet (135). The report of small proteins secreted into the medium surrounding in vitro-cultured T. pallidum bacteria (293) also raises the possibility that some surface-exposed proteins are loosely anchored in the outer leaflet of the outer membrane by the C or N terminus or by a lipid tail. Further research may continue to reveal previously unidentified surface molecules.
Recent research efforts to identify proteins that may be exposed on the surface of the organism have utilized computer analysis of the T. pallidum genome. Several proteins that are predicted to have a cleavable signal sequence, transmembrane domains, and other characteristics of proteins that span the outer membrane have been identified (39, 54). As discussed above, three of these, Tp0155, Tp0483, and Tp0751, bind to ECM components and are candidate host-binding molecules (39, 41). Their potential involvement in T. pallidum binding to host cells strongly implicates these proteins as being outer surface localized.
Tpr Proteins
Several other genes that encode candidate outer membrane proteins belong to the tpr gene family. The twelve tpr genes are divided into three subfamilies. The tprC, tprD, tprF, and tprI genes belong to subfamily I; tprE, tprG, and tprJ belong to subfamily II; and tprA, tprB, tprH, tprK, and tprL belong to subfamily III. The proteins encoded by tprF, tprI, and tprK are predicted to be located in the outer membrane (39, 54); the subfamily I proteins and TprK are predicted to have cleavable signal sequences. Most of the proteins encoded by the tpr genes (the Tprs) elicit an immune response in experimental syphilis (167, 208, 302). Antibody responses arise at different times after infection: anti-TprK antibodies are seen as soon as 17 days postinfection and are robustly reactive at day 30 (167, 208), while antibodies against the members of subfamilies I and II often are not detectable until 45 days after infection and reach peak titers at day 60 (167, 302). The time of development of antibodies to specific Tprs may reveal the timing of expression of the proteins that induced those antibodies. Regulation of expression of related proteins is referred to as phase variation and may be utilized by T. pallidum to down-regulate the expression of those Tprs against which an immune response has been mounted, while simultaneously up-regulating the expression of new Tprs. The expression of new proteins that are not recognized by the existing immune response may help T. pallidum maintain chronic infection. Additionally, immunological studies suggest that different strains of T. pallidum express different repertoires of Tpr proteins (167).
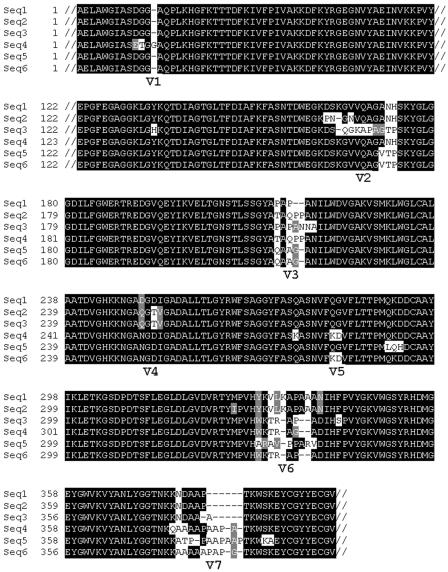
Besides its potential location in the outer membrane, other intriguing features have singled out the TprK protein as important to syphilis pathogenesis. A translational start site downstream of the site predicted in the T. pallidum genome sequence has been proposed (54) and confirmed experimentally by Hazlett et al. (138). This alternative TprK translational start introduces an N-terminal cleavable signal sequence, supporting the prediction that TprK is a membrane-localized protein (54). The tprK gene and predicted amino acid sequences are characterized by seven discrete variable (V) regions that are separated by stretches of conserved sequence; this is shown in Fig. 2. To date, diverse tprK sequences have been demonstrated in every T. pallidum strain (56, 163, 295). Syphilitic hosts are infected with multiple subpopulations of organisms, differentiated by their tprK sequences (57); Fig. 2 shows the diverse TprK sequences encoded by T. pallidum during a single infection. Diversity in tprK sequence accumulates over the course of infection (57, 163), and some V regions are more diverse than others. DNA sequence cassettes that correspond to diverse V-region sequences were discovered in an area of the T. pallidum chromosome separate from the tprK gene (57). These sequence cassettes are potential sequence donor sites and are presumed to replace small or large portions of V-region sequences in the tprK gene, thus creating new, diverse sequences by gene conversion (57). Together, the tprK expression site and the donor regions comprise approximately 4,000 bp in the minimal T. pallidum genome, suggesting that variation in tprK must play an important role in the survival of T. pallidum.
FIG. 2.
Amino acid alignment of TprK showing six different sequences in the T. pallidum Sea81-4 strain harvested from a single infection. Conserved regions are indicated by black shading; V regions have white and gray shading. The V region that exhibits the greatest diversity is V6, with six different sequences; in comparison, only two different sequences are found in V1. For ease of visualization, the conserved C- and N-terminal portions of the protein and 64 amino acids of conserved sequence between V1 and V2 are truncated (//). (The GenBank accession numbers of the sequences shown are AY346063 to AY346068.)
The TprK protein elicits both cellular and humoral immunity in infected animals. Antibodies to TprK that arise in response to T. pallidum infection are specifically targeted to the V regions (207, 208), while T cells recognize conserved TprK epitopes (208). Very slight changes in the amino acid sequence of a V region can abrogate the ability of antibodies to bind a certain V region (208), suggesting that antibody binding to V regions is highly specific. Multiple studies show that immunization with recombinant TprK protein attenuates lesion development upon challenge with virulent T. pallidum (54, 206, 207), and animals that are challenged with a T. pallidum strain expressing a TprK protein that is homologous to the immunizing protein are better protected than those challenged with a strain expressing TprK proteins with different V regions (207). These findings suggest that V-region-specific immunity is important in protection against progressive infection. Taken together, the immunologic characteristics and sequence variation of the TprK protein indicate that TprK undergoes antigenic variation, the first such system identified in T. pallidum. The host immune response may eliminate organisms that express TprK sequences against which a specific antibody response has been mounted. Generating new variation in TprK may help variant T. pallidum organisms escape immune recognition, serving as another mechanism by which T. pallidum may be able to survive the host immune response and cause chronic infection.
GENETIC VARIATION AMONG SUBSPECIES AND STRAINS
tpr Genes
As discussed in the previous section, diversity in tprK has been observed in all syphilis strains examined thus far. The heterogeneity observed in the seven variable regions of tprK arises during rabbit passage (57, 163); diversity is also seen in tprK sequenced directly from patients with primary and secondary syphilis (163, 215). Some of the other tpr genes have also displayed variation, although the diversity is of a different type. Variation of tprC and tprD has been noted, but diversity exists only among strains, not within strains as with tprK. The tprC and tprD genes are located in different loci in the T. pallidum genome but share an identical sequence in the T. pallidum Nichols type strain (54, 105). Some of the non-Nichols T. pallidum strains also have identical tprC and tprD sequences; however, some strains carry an alternative gene in the tprD locus, called tprD2, that varies in sequence from tprD (59). Strains that carry tprD2 have a slightly different gene in their tprC locus, and, unlike the identical tprC and tprD sequences, alternative tprC sequences are not identical to tprD2 (302).
The tprD2 sequence is also found in other pathogenic T. pallidum subspecies, including strains of the T. pallidum subspecies pertenue and endemicum, which cause the nonvenereal treponematoses yaws and bejel, respectively. The Gauthier strain of T. pallidum subsp. pertenue carries in its tprJ locus a gene that is a hybrid of tprG and tprJ sequences (297), as does one identified T. pallidum subsp. pallidum strain. As described above, regulation of the expression of different Tprs may help T. pallidum avoid the immune response, and sequence differences may also confer different functions to the proteins.
Other T. pallidum Genes
Other genes have been examined for differences among strains of T. pallidum subsp. pallidum, as well as among the T. pallidum subspecies. The gene that encodes the TpN15 lipoprotein is identical among T. pallidum subsp. pallidum strains and the subspecies endemicum and pertenue (53). However, a sequence difference in a region upstream of the gene introduces a site for cleavage by the restriction enzyme Eco47III (55) in T. pallidum subsp. pallidum strains but not in strains of the other subspecies. A sequence change in the gpd gene also confers a restriction enzyme cleavage site that differentiates T. pallidum subsp. pallidum from T. pallidum subsp. pertenue and endemicum (43). The pathogenic T. pallidum subspecies are morphologically identical by microscopy, and the syphilis, yaws, and bejel infections induce many cross-reactive antibodies, precluding serological differentiation. Thus, unique restriction sites such as those in the genes that encode TpN15 and Gpd provide a genetic method for differentiating the syphilis spirochete from the other pathogenic treponemes.
IMPLICATIONS FOR SYPHILIS CONTROL
Treatment of Infection
After 60 years of use, penicillin still remains the drug of choice in syphilis treatment. Several T. pallidum proteins have been shown to bind penicillin (78, 246, 331). Penicillin-binding proteins often function in cell wall synthesis pathways; thus, penicillin kills susceptible bacteria by interfering with production of cell walls. Recently, one penicillin-binding protein, TpN47, was shown to have β-lactamase activity (60); paradoxically, this activity is inhibited by the products of the reaction and the organism remains penicillin sensitive. Guidelines published by the Centers for Disease Control and Prevention (51) specify oral doxycycline or tetracycline as alternative treatments in the case of penicillin allergy (except for pregnant women).
Macrolide Resistance
In the 1990s, azithromycin emerged as a particularly attractive alternative to penicillin therapy for syphilis (144, 252). Azithromycin is given orally, in contrast to benzathine penicillin, which must be delivered intramuscularly in a large volume. Early syphilis has been shown to be successfully treated with a single dose of azithromycin (144), and large azithromycin treatment trials are ongoing. Recently, however, a study of T. pallidum samples from patients in San Francisco, Calif., Baltimore, Md., Seattle, Wash., and Dublin, Ireland, identified macrolide-resistant strains among these populations (182); this resistance was shown to be associated with a single base mutation in the 23S rRNA gene (182, 294). Macrolide resistance was particularly high in San Francisco (37% of samples collected in 2003) and Dublin (88% of samples collected in 2002) and lower, but present, in other cities (182). In cities with high rates of macrolide-resistant strains, penicillin should remain the drug of choice. In other cities, certain situations may warrant the use of azithromycin to treat syphilis; however, to ensure efficacy of treatment, it is essential that patients be monitored carefully with clinical reevaluation and serological testing.
Vaccine Development
Syphilis infection increases the risk of transmitting and acquiring HIV infection. Not only are syphilis lesions a portal of entry for HIV but the immune cells that carry and are susceptible to the virus, macrophages and T lymphocytes, are found in abundance in syphilis lesions. There is also some evidence for the direct involvement of T. pallidum in facilitating HIV infection and progression. Virulent T. pallidum and the TpN47 lipoprotein up-regulate HIV replication in monocyte precursors that are infected with HIV (307). Additionally, TpN47 stimulation of monocytes up-regulates expression of the HIV receptor CCR5 (281). Prevention of syphilis infections would help to stem the rising numbers of new HIV infections in the world and would certainly prevent congenital syphilis in developing nations. These issues, in addition to the possible serious late sequelae of untreated syphilis, provide a clear rationale for active syphilis vaccine development efforts.
Thus far, only one animal study has demonstrated complete protection against infection with T. pallidum (203). The immunization regimen, 60 intravenous inoculations with γ-irradiated T. pallidum over 37 weeks, was far too cumbersome and expensive to repeat in rabbits and too impractical to test in humans. In many other studies, immunization with T. pallidum molecules (endoflagella [63], 4D [34], Gpd [42], TmpB [334], Tp92 [44], TpN15 [53], TpN47 [19], TprF [302], TprI [109], and TprK [54, 206, 207]) was conducted and protection against infectious challenge was determined. Immunization stimulated the production of strongly reactive antibodies, some of which were shown to be opsonic (44, 54). These immunization regimens, however, induced only partial protection, shown by attenuated development and rapid healing of lesions. This lack of complete protection in the face of high antibody titers suggests that the cellular immune response is critical to early clearance of T. pallidum. The requirement for cellular immunity in syphilis protection is supported by the fact that passive transfer of antibodies against T. pallidum (24, 232, 316), or against one of its lipid components (26), does not completely protect against infection. Additionally, the recombinant molecules used for immunization in many of these studies are likely not to be folded in native conformation; thus, antibodies that bind to conformational epitopes of native antigen may not be produced by immunization. Finally, the first targets of the immune response, outer membrane proteins, are very rare in T. pallidum. The identification of molecules found on T. pallidum's surface is likely to be essential in the development of an effective syphilis vaccine. It is hoped that their discovery and use in a multivalent vaccine will lead to the production of an effective vaccine for syphilis.
REFERENCES
- 1.Abdul Gaffoor, P. M. 1990. Syphilitic alopecia. Indian J. Sex. Transm. Dis. 11:66-67. [PubMed] [Google Scholar]
- 2.Akins, D. R., B. K. Purcell, M. M. Mitra, M. V. Norgard, and J. D. Radolf. 1993. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect. Immun. 61:1202-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete, J. F., and J. B. Baseman. 1980. Surface characterization of virulent Treponema pallidum. Infect. Immun. 30:814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete, J. F., and J. B. Baseman. 1979. Surface-associated host proteins on virulent Treponema pallidum. Infect. Immun. 26:1048-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderete, J. F., K. M. Peterson, and J. B. Baseman. 1988. Affinities of Treponema pallidum for human lactoferrin and transferrin. Genitourin. Med. 64:359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander, L. J., and A. G. Schoch. 1949. Prevention of syphilis. Arch. Dermatol. Syphilol. 59:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Arroll, T. W., A. Centurion-Lara, S. A. Lukehart, and W. C. Van Voorhis. 1999. T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect. Immun. 67:4757-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backhouse, J. L., and S. I. Nesteroff. 2001. Treponema pallidum western blot: comparison with the FTA-ABS test as a confirmatory test for syphilis. Diagn. Microbiol. Infect. Dis. 39:9-14. [DOI] [PubMed] [Google Scholar]
- 9.Baker-Zander, S., and S. Sell. 1980. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am. J. Pathol. 101:387-413. [PMC free article] [PubMed] [Google Scholar]
- 10.Baker-Zander, S. A., M. J. Fohn, and S. A. Lukehart. 1988. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J. Immunol. 141:4363-4369. [PubMed] [Google Scholar]
- 11.Baker-Zander, S. A., E. W. Hook III, P. Bonin, H. H. Handsfield, and S. A. Lukehart. 1985. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J. Infect. Dis. 151:264-272. [DOI] [PubMed] [Google Scholar]
- 12.Baker-Zander, S. A., and S. A. Lukehart. 1984. Antigenic cross-reactivity between Treponema pallidum and other pathogenic members of the family Spirochaetaceae. Infect. Immun. 46:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker-Zander, S. A., and S. A. Lukehart. 1992. Macrophage-mediated killing of opsonized Treponema pallidum. J. Infect. Dis. 165:69-74. [DOI] [PubMed] [Google Scholar]
- 14.Baker-Zander, S. A., R. E. Roddy, H. H. Handsfield, and S. A. Lukehart. 1986. IgG and IgM antibody reactivity to antigens of Treponema pallidum after treatment of syphilis. Sex. Transm. Dis. 13:214-220. [DOI] [PubMed] [Google Scholar]
- 15.Baker-Zander, S. A., J. M. Shaffer, and S. A. Lukehart. 1993. Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum ssp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol. Med. Microbiol. 6:273-279. [DOI] [PubMed] [Google Scholar]
- 16.Baker-Zander, S. A., J. M. Shaffer, and S. A. Lukehart. 1993. VDRL antibodies enhance phagocytosis of Treponema pallidum by macrophages. J. Infect. Dis. 167:1100-1105. [DOI] [PubMed] [Google Scholar]
- 17.Bansal, R. C., H. Cohn, K. Fani, and Y. L. Lynfield. 1978. Nephrotic syndrome and granulomatous hepatitis in secondary syphilis. Arch. Dermatol. 114:1228-1229. [PubMed] [Google Scholar]
- 18.Baseman, J. B., and E. C. Hayes. 1980. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J. Exp. Med. 151:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baughn, R. E., A. Jiang, R. Abraham, V. Ottmers, and D. M. Musher. 1996. Molecular mimicry between an immunodominant amino acid motif on the 47-kDa lipoprotein of Treponema pallidum (Tpp47) and multiple repeats of analogous sequences in fibronectin. J. Immunol. 157:720-731. [PubMed] [Google Scholar]
- 20.Baughn, R. E., and D. M. Musher. 2005. Secondary syphilitic lesions. Clin. Microbiol. Rev. 18:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker, P. S., D. R. Akins, J. D. Radolf, and M. V. Norgard. 1994. Similarity between the 38-kilodalton lipoprotein of Treponema pallidum and the glucose/galactose-binding (MglB) protein of Escherichia coli. Infect. Immun. 62:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoit, S., J. E. Posey, M. R. Chenoweth, and F. C. Gherardini. 2001. Treponema pallidum 3-phosphoglycerate mutase is a heat-labile enzyme that may limit the maximum growth temperature for the spirochete. J. Bacteriol. 183:4702-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg, H. C., and L. Turner. 1979. Movement of microorganisms in viscous environments. Nature 278:349-351. [DOI] [PubMed] [Google Scholar]
- 24.Bishop, N. H., and J. N. Miller. 1976. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J. Immunol. 117:191-196. [PubMed] [Google Scholar]
- 25.Bishop, N. H., and J. N. Miller. 1976. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J. Immunol. 117:197-207. [PubMed] [Google Scholar]
- 26.Blanco, D. R., C. I. Champion, A. Dooley, D. L. Cox, J. P. Whitelegge, K. Faull, and M. A. Lovett. 2005. A monoclonal antibody that conveys in vitro killing and partial protection in experimental syphilis binds a phosphorylcholine surface epitope of Treponema pallidum. Infect. Immun. 73:3083-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco, D. R., C. I. Champion, M. M. Exner, E. S. Shang, J. T. Skare, R. E. Hancock, J. N. Miller, and M. A. Lovett. 1996. Recombinant Treponema pallidum rare outer membrane protein 1 (Tromp1) expressed in Escherichia coli has porin activity and surface antigenic exposure. J. Bacteriol. 178:6685-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco, D. R., C. I. Champion, J. N. Miller, and M. A. Lovett. 1988. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect. Immun. 56:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco, D. R., M. Giladi, C. I. Champion, D. A. Haake, G. K. Chikami, J. N. Miller, and M. A. Lovett. 1991. Identification of Treponema pallidum subspecies pallidum genes encoding signal peptides and membrane-spanning sequences using a novel alkaline phosphatase expression vector. Mol. Microbiol. 5:2405-2415. [DOI] [PubMed] [Google Scholar]
- 30.Blanco, D. R., J. N. Miller, and P. A. Hanff. 1984. Humoral immunity in experimental syphilis: the demonstration of IgG as a treponemicidal factor in immune rabbit serum. J. Immunol. 133:2693-2697. [PubMed] [Google Scholar]
- 31.Blanco, D. R., J. D. Radolf, M. A. Lovett, and J. N. Miller. 1986. The antigenic interrelationship between the endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum Nichols strain. I. Treponemicidal activity of cross-reactive endoflagellar antibodies against T. pallidum. J. Immunol. 137:2973-2979. [PubMed] [Google Scholar]
- 32.Blanco, D. R., K. Reimann, J. Skare, C. I. Champion, D. Foley, M. M. Exner, R. E. Hancock, J. N. Miller, and M. A. Lovett. 1994. Isolation of the outer membranes from Treponema pallidum and Treponema vincentii. J. Bacteriol. 176:6088-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borenstein, L. A., T. Ganz, S. Sell, R. I. Lehrer, and J. N. Miller. 1991. Contribution of rabbit leukocyte defensins to the host response in experimental syphilis. Infect. Immun. 59:1368-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borenstein, L. A., J. D. Radolf, T. E. Fehniger, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1988. Immunization of rabbits with recombinant Treponema pallidum surface antigen 4D alters the course of experimental syphilis. J. Immunol. 140:2415-2421. [PubMed] [Google Scholar]
- 35.Borenstein, L. A., M. E. Selsted, R. I. Lehrer, and J. N. Miller. 1991. Antimicrobial activity of rabbit leukocyte defensins against Treponema pallidum subsp. pallidum. Infect. Immun. 59:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bos, J. D., F. Hamerlinck, and R. H. Cormane. 1980. Immunoglobulin-bearing polymorphonuclear leucocytes in primary syphilis. Br. J. Vener. Dis. 56:218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouis, D. A., T. G. Popova, A. Takashima, and M. V. Norgard. 2001. Dendritic cells phagocytose and are activated by Treponema pallidum. Infect. Immun. 69:518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 39.Cameron, C. E. 2003. Identification of a Treponema pallidum laminin-binding protein. Infect. Immun. 71:2525-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron, C. E., N. L. Brouwer, L. M. Tisch, and J. M. Kuroiwa. 2005. Defining the interaction of the Treponema pallidum adhesin Tp0751 with laminin. Infect. Immun. 73:7485-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron, C. E., E. L. Brown, J. M. Kuroiwa, L. M. Schnapp, and N. L. Brouwer. 2004. Treponema pallidum fibronectin-binding proteins. J. Bacteriol. 186:7019-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 1998. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect. Immun. 66:5763-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 1999. Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect. Immun. 67:3168-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron, C. E., S. A. Lukehart, C. Castro, B. Molini, C. Godornes, and W. C. Van Voorhis. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181:1401-1413. [DOI] [PubMed] [Google Scholar]
- 45.Canale-Parola, E. 1978. Motility and chemotaxis of spirochetes. Annu. Rev. Microbiol. 32:69-99. [DOI] [PubMed] [Google Scholar]
- 46.Carter, R. 1993. John Hunter, 1728-1793. World J. Surg. 17:563-565. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. 2003. Internet use and early syphilis infection among men who have sex with men—San Francisco, California, 1999-2003. Morbid. Mortal. Wkly. Rep. 52:1229-1232. [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. 2001. Outbreak of syphilis among men who have sex with men—Southern California, 2000. Morbid. Mortal. Wkly. Rep. 50:117-120. [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. 2002. Primary and secondary syphilis among men who have sex with men—New York City, 2001. Morbid. Mortal. Wkly. Rep. 51:853-856. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. 1999. Resurgent bacterial sexually transmitted disease among men who have sex with men—King County, Washington, 1997-1999. Morbid. Mortal. Wkly. Rep. 48:773-777. [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. 2002. Sexually transmitted diseases treatment guidelines 2002. Morbid. Mortal. Wkly. Rep. 51(RR-6):1-78. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. 2004. Trends in primary and secondary syphilis and HIV infections in men who have sex with men—San Francisco and Los Angeles, California, 1998-2002. Morbid. Mortal. Wkly. Rep. 53:575-578. [PMC free article] [PubMed] [Google Scholar]
- 53.Centurion-Lara, A., T. Arroll, R. Castillo, J. M. Shaffer, C. Castro, W. C. Van Voorhis, and S. A. Lukehart. 1997. Conservation of the 15-kilodalton lipoprotein among Treponema pallidum subspecies and strains and other pathogenic treponemes: genetic and antigenic analyses. Infect. Immun. 65:1440-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centurion-Lara, A., C. Castro, L. Barrett, C. Cameron, M. Mostowfi, W. C. Van Voorhis, and S. A. Lukehart. 1999. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centurion-Lara, A., C. Castro, R. Castillo, J. M. Shaffer, W. C. Van Voorhis, and S. A. Lukehart. 1998. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J. Infect. Dis. 177:1036-1040. [DOI] [PubMed] [Google Scholar]
- 56.Centurion-Lara, A., C. Godornes, C. Castro, W. C. Van Voorhis, and S. A. Lukehart. 2000. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect. Immun. 68:824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centurion-Lara, A., R. E. LaFond, K. Hevner, C. Godornes, B. J. Molini, W. C. Van Voorhis, and S. A. Lukehart. 2004. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol. Microbiol. 52:1579-1596. [DOI] [PubMed] [Google Scholar]
- 58.Centurion-Lara, A., B. J. Molini, L. Giacani, K. Hevner, E. S. Sun, and S. A. Lukehart. 2005. Unpublished results.
- 59.Centurion-Lara, A., E. S. Sun, L. K. Barrett, C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 2000. Multiple alleles of Treponema pallidum repeat gene D in Treponema pallidum isolates. J. Bacteriol. 182:2332-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cha, J. Y., A. Ishiwata, and S. Mobashery. 2004. A novel beta-lactamase activity from a penicillin-binding protein of Treponema pallidum and why syphilis is still treatable with penicillin. J. Biol. Chem. 279:14917-14921. [DOI] [PubMed] [Google Scholar]
- 61.Chamberlain, N. R., M. E. Brandt, A. L. Erwin, J. D. Radolf, and M. V. Norgard. 1989. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect. Immun. 57:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamberlain, N. R., J. D. Radolf, P. L. Hsu, S. Sell, and M. V. Norgard. 1988. Genetic and physicochemical characterization of the recombinant DNA-derived 47-kilodalton surface immunogen of Treponema pallidum subsp. pallidum. Infect. Immun. 56:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Champion, C. I., J. N. Miller, L. A. Borenstein, M. A. Lovett, and D. R. Blanco. 1990. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect. Immun. 58:3158-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Champion, C. I., J. N. Miller, M. A. Lovett, and D. R. Blanco. 1990. Cloning, sequencing, and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5- and 31.0-kilodalton proteins. Infect. Immun. 58:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapel, T. A. 1988. Congenital syphilis. Compr. Ther. 14:25-28. [PubMed] [Google Scholar]
- 66.Chapel, T. A. 1980. The signs and symptoms of secondary syphilis. Sex. Transm. Dis. 7:161-164. [DOI] [PubMed] [Google Scholar]
- 67.Chapel, T. A. 1978. The variability of syphilitic chancres. Sex. Transm. Dis. 5:68-70. [DOI] [PubMed] [Google Scholar]
- 68.Chawla, V., K. Gupta, and M. B. Raghu. 1985. Congenital syphilis: a clinical profile. J. Trop. Pediatr. 31:204-208. [DOI] [PubMed] [Google Scholar]
- 69.Chen, S. Y., S. Gibson, M. H. Katz, J. D. Klausner, J. W. Dilley, S. K. Schwarcz, T. A. Kellogg, and W. McFarland. 2002. Continuing increases in sexual risk behavior and sexually transmitted diseases among men who have sex with men: San Francisco, Calif., 1999-2001, USA. Am. J. Public Health 92:1387-1388. [PMC free article] [PubMed] [Google Scholar]
- 70.Chi, B., S. Chauhan, and H. Kuramitsu. 1999. Development of a system for expressing heterologous genes in the oral spirochete Treponema denticola and its use in expression of the Treponema pallidum flaA gene. Infect. Immun. 67:3653-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung, K. Y., K. S. Kim, M. G. Lee, N. S. Chang, and J. B. Lee. 2002. Treponema pallidum induces up-regulation of interstitial collagenase in human dermal fibroblasts. Acta Derm. Venereol. 82:174-178. [DOI] [PubMed] [Google Scholar]
- 72.Cockayne, A., M. J. Bailey, and C. W. Penn. 1987. Analysis of sheath and core structures of the axial filament of Treponema pallidum. J. Gen. Microbiol. 133:1397-1407. [DOI] [PubMed] [Google Scholar]
- 73.Collart, P., P. Franceschini, and P. Durel. 1971. Experimental rabbit syphilis. Br. J. Vener. Dis. 47:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox, D. L., D. R. Akins, S. F. Porcella, M. V. Norgard, and J. D. Radolf. 1995. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol. Microbiol. 15:1151-1164. [DOI] [PubMed] [Google Scholar]
- 75.Cox, D. L., P. Chang, A. W. McDowall, and J. D. Radolf. 1992. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect. Immun. 60:1076-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cox, D. L., Y. Sun, H. Liu, R. I. Lehrer, and W. M. Shafer. 2003. Susceptibility of Treponema pallidum to host-derived antimicrobial peptides. Peptides 24:1741-1746. [DOI] [PubMed] [Google Scholar]
- 77.Cumberland, M. C., and T. B. Turner. 1949. Rate of multiplication of Treponema pallidum in normal and immune rabbits. Am. J. Syphilis 33:201-212. [PubMed] [Google Scholar]
- 78.Cunningham, T. M., J. N. Miller, and M. A. Lovett. 1987. Identification of Treponema pallidum penicillin-binding proteins. J. Bacteriol. 169:5298-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham, T. M., E. M. Walker, J. N. Miller, and M. A. Lovett. 1988. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J. Bacteriol. 170:5789-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deacon, W. E., V. H. Falcone, and A. Harris. 1957. A fluorescent test for treponemal antibodies. Proc. Soc. Exp. Biol. Med. 96:477-480. [DOI] [PubMed] [Google Scholar]
- 81.Deka, R. K., M. S. Goldberg, K. E. Hagman, and M. V. Norgard. 2004. The Tp38 (TpMglB-2) lipoprotein binds glucose in a manner consistent with receptor function in Treponema pallidum. J. Bacteriol. 186:2303-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deka, R. K., Y. H. Lee, K. E. Hagman, D. Shevchenko, C. A. Lingwood, C. A. Hasemann, M. V. Norgard, and J. D. Radolf. 1999. Physicochemical evidence that Treponema pallidum TroA is a zinc-containing metalloprotein that lacks porin-like structure. J. Bacteriol. 181:4420-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deka, R. K., L. Neil, K. E. Hagman, M. Machius, D. R. Tomchick, C. A. Brautigam, and M. V. Norgard. 2004. Structural evidence that the 32-kilodalton lipoprotein (Tp32) of Treponema pallidum is an L-methionine-binding protein. J. Biol. Chem. 279:55644-55650. [DOI] [PubMed] [Google Scholar]
- 84.Dempster, W. J. 1978. Towards a new understanding of John Hunter. Lancet i:316-318. [DOI] [PubMed] [Google Scholar]
- 85.DiCarlo, R. P., and D. H. Martin. 1997. The clinical diagnosis of genital ulcer disease in men. Clin. Infect. Dis. 25:292-298. [DOI] [PubMed] [Google Scholar]
- 86.Don, P. C., R. Rubinstein, and S. Christie. 1995. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int. J. Dermatol. 34:403-407. [DOI] [PubMed] [Google Scholar]
- 87.Donnenberg, M. S., M. T. Collins, R. M. Benitez, and P. A. Mackowiak. 2000. The sound that failed. Am. J. Med. 108:475-480. [DOI] [PubMed] [Google Scholar]
- 88.Edwards, A. M., H. F. Jenkinson, M. J. Woodward, and D. Dymock. 2005. Binding properties and adhesion-mediating regions of the major sheath protein of Treponema denticola ATCC 35405. Infect. Immun. 73:2891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 90.Engelkens, H. J., F. J. ten Kate, J. Judanarso, V. D. Vuzevski, J. B. van Lier, J. C. Godschalk, J. J. van der Sluis, and E. Stolz. 1993. The localisation of treponemes and characterisation of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis, or early infectious yaws. Genitourin. Med. 69:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Escobar, M. R., H. P. Dalton, and M. J. Allison. 1970. Fluorescent antibody tests for syphilis using cerebrospinal fluid: clinical correlation in 150 cases. Am. J. Clin. Pathol. 53:886-890. [DOI] [PubMed] [Google Scholar]
- 92.Feher, J., T. Somogyi, M. Timmer, and L. Jozsa. 1975. Early syphilitic hepatitis. Lancet ii:896-899. [DOI] [PubMed] [Google Scholar]
- 93.Fehniger, T. E., J. D. Radolf, A. M. Walfield, T. M. Cunningham, J. N. Miller, and M. A. Lovett. 1986. Native surface association of a recombinant 38-kilodalton Treponema pallidum antigen isolated from the Escherichia coli outer membrane. Infect. Immun. 52:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fenno, J. C., K. H. Muller, and B. C. McBride. 1996. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J. Bacteriol. 178:2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fieldsteel, A. H., D. L. Cox, and R. A. Moeckli. 1981. Cultivation of virulent Treponema pallidum in tissue culture. Infect. Immun. 32:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fieldsteel, A. H., D. L. Cox, and R. A. Moeckli. 1982. Further studies on replication of virulent Treponema pallidum in tissue cultures of Sf1Ep cells. Infect. Immun. 35:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fitzgerald, T. J., P. Cleveland, R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J. Bacteriol. 130:1333-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fitzgerald, T. J., R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect. Immun. 18:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fitzgerald, T. J., J. N. Miller, and J. A. Sykes. 1975. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect. Immun. 11:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzgerald, T. J., and L. A. Repesh. 1985. Interactions of fibronectin with Treponema pallidum. Genitourin. Med. 61:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fitzgerald, T. J., L. A. Repesh, D. R. Blanco, and J. N. Miller. 1984. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br. J. Vener. Dis. 60:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitzgerald, T. J., L. A. Repesh, and S. G. Oakes. 1982. Morphological destruction of cultured cells by the attachment of Treponema pallidum. Br. J. Vener. Dis. 58:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiumara, N. J. 1975. Syphilis in newborn children. Clin. Obstet. Gynecol. 18:183-189. [DOI] [PubMed] [Google Scholar]
- 105.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, K. Roberts, M. Sandusky, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 106.Garnett, G. P., S. O. Aral, D. V. Hoyle, W. Cates, Jr., and R. M. Anderson. 1997. The natural history of syphilis. Implications for the transmission dynamics and control of infection. Sex. Transm. Dis. 24:185-200. [DOI] [PubMed] [Google Scholar]
- 107.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 108.Gerber, A., S. Krell, and J. Morenz. 1996. Recombinant Treponema pallidum antigens in syphilis serology. Immunobiology 196:535-549. [DOI] [PubMed] [Google Scholar]
- 109.Giacani, L., V. Sambri, A. Marangoni, F. Cavrini, E. Storni, M. Donati, S. Corona, P. Lanzarini, and R. Cevenini. 2005. Immunological evaluation and cellular location analysis of the TprI antigen of Treponema pallidum subsp. pallidum. Infect. Immun. 73:3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Girons, I. S., B. Chi, and H. Kuramitsu. 2000. Development of shuttle vectors for spirochetes. J. Mol. Microbiol. Biotechnol. 2:443-445. [PubMed] [Google Scholar]
- 111.Giuliani, M., G. Palamara, A. Latini, A. Maini, and A. Di Carlo. 2005. Evidence of an outbreak of syphilis among men who have sex with men in Rome. Arch. Dermatol. 141:100-101. [DOI] [PubMed] [Google Scholar]
- 112.Gjestland, T. 1955. The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta Derm. Venereol. 35:3-368; Annex I-LVI. [DOI] [PubMed] [Google Scholar]
- 113.Gordon, A. G. 1997. The death of Edgar Allan Poe—a case of syphilis? Md. Med. J. 46:289-290. [PubMed] [Google Scholar]
- 114.Gordon, A. G. 1999. Seeking Haydn's secrets. Cerebrovasc. Dis. 9:54. [DOI] [PubMed] [Google Scholar]
- 115.Greenblatt, R. M., S. A. Lukehart, F. A. Plummer, T. C. Quinn, C. W. Critchlow, R. L. Ashley, L. J. D'Costa, J. O. Ndinya-Achola, L. Corey, A. R. Ronald, et al. 1988. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS 2:47-50. [DOI] [PubMed] [Google Scholar]
- 116.Greene, S. R., and L. V. Stamm. 1998. Molecular characterization of Treponema pallidum mcp2, a putative chemotaxis protein gene. Infect. Immun. 66:2999-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greene, S. R., L. V. Stamm, J. M. Hardham, N. R. Young, and J. G. Frye. 1997. Identification, sequences, and expression of Treponema pallidum chemotaxis genes. DNA Seq. 7:267-284. [DOI] [PubMed] [Google Scholar]
- 118.Greenstein, D. B., C. M. Wilcox, and D. A. Schwartz. 1994. Gastric syphilis. Report of seven cases and review of the literature. J. Clin. Gastroenterol. 18:4-9. [PubMed] [Google Scholar]
- 119.Guzman, C. A., S. R. Talay, G. Molinari, E. Medina, and G. S. Chhatwal. 1999. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J. Infect. Dis. 179:901-906. [DOI] [PubMed] [Google Scholar]
- 120.Haapasalo, M., K. H. Muller, V. J. Uitto, W. K. Leung, and B. C. McBride. 1992. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect. Immun. 60:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hagman, K. E., S. F. Porcella, T. G. Popova, and M. V. Norgard. 1997. Evidence for a methyl-accepting chemotaxis protein gene (mcp1) that encodes a putative sensory transducer in virulent Treponema pallidum. Infect. Immun. 65:1701-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Handsfield, H. H., S. A. Lukehart, S. Sell, S. J. Norris, and K. K. Holmes. 1983. Demonstration of Treponema pallidum in a cutaneous gumma by indirect immunofluorescence. Arch. Dermatol. 119:677-680. [PubMed] [Google Scholar]
- 123.Hanff, P. A., N. H. Bishop, J. N. Miller, and M. A. Lovett. 1983. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J. Immunol. 131:1973-1977. [PubMed] [Google Scholar]
- 124.Hanff, P. A., T. E. Fehniger, J. N. Miller, and M. A. Lovett. 1982. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J. Immunol. 129:1287-1291. [PubMed] [Google Scholar]
- 125.Hanff, P. A., J. N. Miller, and M. A. Lovett. 1983. Molecular characterization of common treponemal antigens. Infect. Immun. 40:825-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harayama, S., J. Bollinger, T. Iino, and G. L. Hazelbauer. 1983. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J. Bacteriol. 153:408-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardham, J. M., J. G. Frye, and L. V. Stamm. 1995. Identification and sequences of the Treponema pallidum fliM′, fliY, fliP, fliQ, fliR and flhB′ genes. Gene 166:57-64. [DOI] [PubMed] [Google Scholar]
- 128.Hardham, J. M., J. G. Frye, N. R. Young, and L. V. Stamm. 1997. Identification and sequences of the Treponema pallidum flhA, flhF, and orf304 genes. DNA Seq. 7:107-116. [DOI] [PubMed] [Google Scholar]
- 129.Hardham, J. M., and L. V. Stamm. 1994. Identification and characterization of the Treponema pallidum tpn50 gene, an ompA homolog. Infect. Immun. 62:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardy, P. H., Jr., and J. Levin. 1983. Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc. Soc. Exp. Biol. Med. 174:47-52. [DOI] [PubMed] [Google Scholar]
- 131.Hardy, P. H., Jr., and E. E. Nell. 1955. Specific agglutination of Treponema pallidum by sera from rabbits and human beings with treponemal infections. J. Exp. Med. 101:367-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harris, A., A. A. Rosenberg, and L. M. Riedel. 1946. A microflocculation test for syphilis using cardiolipin antigen: preliminary report. J. Vener. Dis. Inf. 27:159-172. [PubMed] [Google Scholar]
- 133.Hart, G. 1986. Syphilis tests in diagnostic and therapeutic decision making. Ann. Intern. Med. 104:368-376. [DOI] [PubMed] [Google Scholar]
- 134.Hayes, N. S., K. E. Muse, A. M. Collier, and J. B. Baseman. 1977. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect. Immun. 17:174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hazlett, K. R., D. L. Cox, M. Decaffmeyer, M. P. Bennett, D. C. Desrosiers, C. J. La Vake, M. E. La Vake, K. W. Bourell, E. J. Robinson, R. Brasseur, and J. D. Radolf. 2005. TP0453, a concealed outer membrane protein of Treponema pallidum, enhances membrane permeability. J. Bacteriol. 187:6499-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hazlett, K. R., D. L. Cox, R. A. Sikkink, F. Auch'ere, F. Rusnak, and J. D. Radolf. 2002. Contribution of neelaredoxin to oxygen tolerance by Treponema pallidum. Methods Enzymol. 353:140-156. [DOI] [PubMed] [Google Scholar]
- 137.Hazlett, K. R., F. Rusnak, D. G. Kehres, S. W. Bearden, C. J. La Vake, M. E. La Vake, M. E. Maguire, R. D. Perry, and J. D. Radolf. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J. Biol. Chem. 278:20687-20694. [DOI] [PubMed] [Google Scholar]
- 138.Hazlett, K. R., T. J. Sellati, T. T. Nguyen, D. L. Cox, M. L. Clawson, M. J. Caimano, and J. D. Radolf. 2001. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hertz, C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 140.Hindersson, P., C. S. Petersen, and N. H. Axelsen. 1985. Purified flagella from Treponema phagedenis biotype Reiter does not induce protective immunity against experimental syphilis in rabbits. Sex. Transm. Dis. 12:124-127. [DOI] [PubMed] [Google Scholar]
- 141.Hira, S. K., J. S. Patel, S. G. Bhat, K. Chilikima, and N. Mooney. 1987. Clinical manifestations of secondary syphilis. Int. J. Dermatol. 26:103-107. [DOI] [PubMed] [Google Scholar]
- 142.Holt, S. C. 1978. Anatomy and chemistry of spirochetes. Microbiol. Rev. 42:114-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hook, E. W., III, and C. M. Marra. 1992. Acquired syphilis in adults. N. Engl. J. Med. 326:1060-1069. [DOI] [PubMed] [Google Scholar]
- 144.Hook, E. W., III, D. H. Martin, J. Stephens, B. S. Smith, and K. Smith. 2002. A randomized, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis. Sex. Transm. Dis. 29:486-490. [DOI] [PubMed] [Google Scholar]
- 145.Hopkins, S., F. Lyons, C. Coleman, G. Courtney, C. Bergin, and F. Mulcahy. 2004. Resurgence in infectious syphilis in Ireland: an epidemiological study. Sex. Transm. Dis. 31:317-321. [DOI] [PubMed] [Google Scholar]
- 146.Hourihan, M., H. Wheeler, R. Houghton, and B. T. Goh. 2004. Lessons from the syphilis outbreak in homosexual men in east London. Sex. Transm. Infect. 80:509-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsu, P. L., N. R. Chamberlain, K. Orth, C. R. Moomaw, L. Q. Zhang, C. A. Slaughter, J. D. Radolf, S. Sell, and M. V. Norgard. 1989. Sequence analysis of the 47-kilodalton major integral membrane immunogen of Treponema pallidum. Infect. Immun. 57:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hubbard, C. L., F. C. Gherardini, P. J. Bassford, Jr., and L. V. Stamm. 1991. Molecular cloning and characterization of a 35.5-kilodalton lipoprotein of Treponema pallidum. Infect. Immun. 59:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hunter, E. F., W. E. Deacon, and P. E. Meyer. 1964. An improved FTA test for syphilis, the absorption procedure (FTA-ABS). Public Health Rep. 79:410-412. [PMC free article] [PubMed] [Google Scholar]
- 150.Hutchinson, C. M., E. W. Hook III, M. Shepherd, J. Verley, and A. M. Rompalo. 1994. Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann. Intern. Med. 121:94-100. [DOI] [PubMed] [Google Scholar]
- 151.Ijsselmuiden, O. E., L. M. Schouls, E. Stolz, G. N. Aelbers, C. M. Agterberg, J. Top, and J. D. van Embden. 1989. Sensitivity and specificity of an enzyme-linked immunosorbent assay using the recombinant DNA-derived Treponema pallidum protein TmpA for serodiagnosis of syphilis and the potential use of TmpA for assessing the effect of antibiotic therapy. J. Clin. Microbiol. 27:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Isaacs, R. D., J. H. Hanke, L. M. Guzman-Verduzco, G. Newport, N. Agabian, M. V. Norgard, S. A. Lukehart, and J. D. Radolf. 1989. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect. Immun. 57:3403-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jackman, J. D., Jr., and J. D. Radolf. 1989. Cardiovascular syphilis. Am. J. Med. 87:425-433. [DOI] [PubMed] [Google Scholar]
- 154.Jaffe, H. W., S. A. Larsen, M. Peters, D. F. Jove, B. Lopez, and A. L. Schroeter. 1978. Tests for treponemal antibody in CSF. Arch. Intern. Med. 138:252-255. [PubMed] [Google Scholar]
- 155.Jepsen, O. B., K. H. Hougen, and A. Birch-Andersen. 1968. Electron microscopy of Treponema pallidum Nichols. Acta Pathol. Microbiol. Scand. 74:241-258. [DOI] [PubMed] [Google Scholar]
- 156.Jones, S. A., K. S. Marchitto, J. N. Miller, and M. V. Norgard. 1984. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J. Exp. Med. 160:1404-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 158.Jovanovic, T., C. Ascenso, K. R. Hazlett, R. Sikkink, C. Krebs, R. Litwiller, L. M. Benson, I. Moura, J. J. Moura, J. D. Radolf, B. H. Huynh, S. Naylor, and F. Rusnak. 2000. Neelaredoxin, an iron-binding protein from the syphilis spirochete, Treponema pallidum, is a superoxide reductase. J. Biol. Chem. 275:28439-28448. [DOI] [PubMed] [Google Scholar]
- 159.Kampmeier, R. H. 1977. John Hunter—a man of conviction. Sex. Transm. Dis. 4:114-115. [DOI] [PubMed] [Google Scholar]
- 160.Kampmeier, R. H. 1964. The late manifestations of syphilis: skeletal, visceral and cardiovascular. Med. Clin. N. Am. 48:667-697. [Google Scholar]
- 161.Kampmeier, R. H. 1972. The Tuskegee study of untreated syphilis. South. Med. J. 65:1247-1251. [PubMed] [Google Scholar]
- 162.Koga, T., H. Duan, Y. Moroi, K. Urabe, and M. Furue. 2003. Activated and mature CD83-positive dendritic cells and interferon-gamma-positive cells in skin eruptions of secondary syphilis. Acta Derm. Venereol. 83:214-217. [DOI] [PubMed] [Google Scholar]
- 163.LaFond, R. E., A. Centurion-Lara, C. Godornes, A. M. Rompalo, W. C. Van Voorhis, and S. A. Lukehart. 2003. Sequence diversity of Treponema pallidum subsp. pallidum tprK in human syphilis lesions and rabbit-propagated isolates. J. Bacteriol. 185:6262-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Larsen, S. A., B. M. Steiner, and A. H. Rudolph. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lascaratos, J., and E. Poulakou-Rebelakou. 1999. Did Justinian the Great (527-565 CE) suffer from syphilis? Int. J. Dermatol. 38:787-791. [DOI] [PubMed] [Google Scholar]
- 166.Lautenschlager, S. 2005. Sexually transmitted infections in Switzerland: return of the classics. Dermatology 210:134-142. [DOI] [PubMed] [Google Scholar]
- 167.Leader, B. T., K. Hevner, B. J. Molini, L. K. Barrett, W. C. Van Voorhis, and S. A. Lukehart. 2003. Antibody responses elicited against the Treponema pallidum repeat proteins differ during infection with different isolates of Treponema pallidum subsp. pallidum. Infect. Immun. 71:6054-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee, J. H., H. J. Choi, J. Jung, M. G. Lee, J. B. Lee, and K. H. Lee. 2003. Receptors for Treponema pallidum attachment to the surface and matrix proteins of cultured human dermal microvascular endothelial cells. Yonsei Med. J. 44:371-378. [DOI] [PubMed] [Google Scholar]
- 169.Lee, K. H., H. J. Choi, M. G. Lee, and J. B. Lee. 2000. Virulent Treponema pallidum 47 kDa antigen regulates the expression of cell adhesion molecules and binding of T-lymphocytes to cultured human dermal microvascular endothelial cells. Yonsei Med. J. 41:623-633. [DOI] [PubMed] [Google Scholar]
- 170.Lee, R. V., G. F. Thornton, and H. O. Conn. 1971. Liver disease associated with secondary syphilis. N. Engl. J. Med. 284:1423-1425. [DOI] [PubMed] [Google Scholar]
- 171.Lee, Y. H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6:628-633. [DOI] [PubMed] [Google Scholar]
- 172.Lerner, V., Y. Finkelstein, and E. Witztum. 2004. The enigma of Lenin's (1870-1924) malady. Eur. J. Neurol. 11:371-376. [DOI] [PubMed] [Google Scholar]
- 173.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 174.Limberger, R. J., L. L. Slivienski, M. C. El-Afandi, and L. A. Dantuono. 1996. Organization, transcription, and expression of the 5′ region of the fla operon of Treponema phagedenis and Treponema pallidum. J. Bacteriol. 178:4628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lombard, M., D. Touati, M. Fontecave, and V. Niviere. 2000. Superoxide reductase as a unique defense system against superoxide stress in the microaerophile Treponema pallidum. J. Biol. Chem. 275:27021-27026. [DOI] [PubMed] [Google Scholar]
- 176.Luger, A. 1993. The origin of syphilis. Clinical and epidemiologic considerations on the Columbian theory. Sex. Transm. Dis. 20:110-117. [PubMed] [Google Scholar]
- 177.Lukehart, S. A. 1992. Immunology and pathogenesis of syphilis. .In T. C. Quinn (ed.), Sexually transmitted diseases. Raven Press, Ltd., New York, N.Y.
- 178.Lukehart, S. A., S. A. Baker-Zander, and E. R. Gubish, Jr. 1982. Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J. Immunol. 129:833-838. [PubMed] [Google Scholar]
- 179.Lukehart, S. A., S. A. Baker-Zander, R. M. Lloyd, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J. Immunol. 124:461-467. [PubMed] [Google Scholar]
- 180.Lukehart, S. A., S. A. Baker-Zander, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 124:454-460. [PubMed] [Google Scholar]
- 181.Lukehart, S. A., S. A. Baker-Zander, and S. Sell. 1986. Characterization of the humoral immune response of the rabbit to antigens of Treponema pallidum after experimental infection and therapy. Sex. Transm. Dis. 13:9-15. [DOI] [PubMed] [Google Scholar]
- 182.Lukehart, S. A., C. Godornes, B. J. Molini, P. Sonnett, S. Hopkins, F. Mulcahy, J. Engelman, S. J. Mitchell, A. M. Rompalo, C. M. Marra, and J. D. Klausner. 2004. Macrolide resistance in Treponema pallidum in the United States and Ireland. N. Engl. J. Med. 351:154-158. [DOI] [PubMed] [Google Scholar]
- 183.Lukehart, S. A., E. W. Hook III, S. A. Baker-Zander, A. C. Collier, C. W. Critchlow, and H. H. Handsfield. 1988. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann. Intern. Med. 109:855-862. [DOI] [PubMed] [Google Scholar]
- 184.Lukehart, S. A., and J. N. Miller. 1978. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J. Immunol. 121:2014-2024. [PubMed] [Google Scholar]
- 185.Lukehart, S. A., J. M. Shaffer, and S. A. Baker-Zander. 1992. A subpopulation of Treponema pallidum is resistant to phagocytosis: possible mechanism of persistence. J. Infect. Dis. 166:1449-1453. [DOI] [PubMed] [Google Scholar]
- 186.Lukehart, S. A., M. R. Tam, J. Hom, S. A. Baker-Zander, K. K. Holmes, and R. C. Nowinski. 1985. Characterization of monoclonal antibodies to Treponema pallidum. J. Immunol. 134:585-592. [PubMed] [Google Scholar]
- 187.Lyons, J. B. 2000. James Joyce: steps towards a diagnosis. J. Hist. Neurosci. 9:294-306. [DOI] [PubMed] [Google Scholar]
- 188.Magnuson, H. J., E. W. Thomas, S. Olansky, B. I. Kaplan, L. DeMello, and J. C. Cutler. 1956. Inoculation syphilis in human volunteers. Medicine 35:33-82. [DOI] [PubMed] [Google Scholar]
- 189.Magnuson, H. J., H. Eagle, and R. Fleischmann. 1948. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain), and a consideration of its rate of multiplication in vivo. Am. J. Syph. Gonorrhea Vener. Dis. 32:1-18. [PubMed] [Google Scholar]
- 190.Mahoney, J. F., K. K. Bryant. 1934. The time element in the penetration of the genital mucosa of the rabbit by the Treponema pallidum. Vener. Dis. Inf. 15:1-5. [Google Scholar]
- 191.Marangoni, A., R. Aldini, V. Sambri, L. Giacani, K. Di Leo, and R. Cevenini. 2004. Production of tumor necrosis factor alpha by Treponema pallidum, Borrelia burgdorferi s.l., and Leptospira interrogans in isolated rat Kupffer cells. FEMS Immunol. Med. Microbiol. 40:187-191. [DOI] [PubMed] [Google Scholar]
- 192.Marra, C., S. A. Baker-Zander, E. W. Hook III, and S. A. Lukehart. 1991. An experimental model of early central nervous system syphilis. J. Infect. Dis. 163:825-829. [DOI] [PubMed] [Google Scholar]
- 193.Marra, C. M., C. L. Maxwell, S. L. Smith, S. A. Lukehart, A. M. Rompalo, M. Eaton, B. P. Stoner, M. Augenbraun, D. E. Barker, J. J. Corbett, M. Zajackowski, C. Raines, J. Nerad, R. Kee, and S. H. Barnett. 2004. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J. Infect. Dis. 189:369-376. [DOI] [PubMed] [Google Scholar]
- 194.Marra, C. M., C. L. Maxwell, L. Tantalo, M. Eaton, A. M. Rompalo, C. Raines, B. P. Stoner, J. J. Corbett, M. Augenbraun, M. Zajackowski, R. Kee, and S. A. Lukehart. 2004. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin. Infect. Dis. 38:1001-1006. [DOI] [PubMed] [Google Scholar]
- 195.Marra, C. M., L. C. Tantalo, C. L. Maxwell, K. Dougherty, and B. Wood. 2004. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology 63:85-88. [DOI] [PubMed] [Google Scholar]
- 196.Mascola, L., R. Pelosi, J. H. Blount, C. E. Alexander, and W. Cates, Jr. 1985. Congenital syphilis revisited. Am. J. Dis. Child. 139:575-580. [DOI] [PubMed] [Google Scholar]
- 197.McArthur, J., E. Medina, A. Mueller, J. Chin, B. J. Currie, K. S. Sriprakash, S. R. Talay, G. S. Chhatwal, and M. J. Walker. 2004. Intranasal vaccination with streptococcal fibronectin binding protein Sfb1 fails to prevent growth and dissemination of Streptococcus pyogenes in a murine skin infection model. Infect. Immun. 72:7342-7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McBroom, R. L., A. R. Styles, M. J. Chiu, C. Clegg, C. J. Cockerell, and J. D. Radolf. 1999. Secondary syphilis in persons infected with and not infected with HIV-1: a comparative immunohistologic study. Am. J. Dermatopathol. 21:432-441. [DOI] [PubMed] [Google Scholar]
- 199.McGrew, B. E., M. J. DuCros, G. W. Stout, and V. H. Falcone. 1968. Automation of a flocculation test for syphilis. Am. J. Clin. Pathol. 50:52-59. [DOI] [PubMed] [Google Scholar]
- 200.McKevitt, M., K. Patel, D. Smajs, M. Marsh, M. McLoughlin, S. J. Norris, G. M. Weinstock, and T. Palzkill. 2003. Systematic cloning of Treponema pallidum open reading frames for protein expression and antigen discovery. Genome Res. 13:1665-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Merritt, H. H., R. D. Adams, and H. C. Solomon. 1946. Neurosyphilis. Oxford University Press, New York, N.Y.
- 202.Metzger, M., P. H. Hardy, Jr., and E. E. Nell. 1961. Influence of lysozyme upon the treponeme immobilization reaction. Am. J. Hyg. 73:236-244. [DOI] [PubMed] [Google Scholar]
- 203.Miller, J. N. 1973. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J. Immunol. 110:1206-1215. [PubMed] [Google Scholar]
- 204.Mindel, A., S. J. Tovey, D. J. Timmins, and P. Williams. 1989. Primary and secondary syphilis, 20 years' experience. 2. Clinical features. Genitourin. Med. 65:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moore, M. B., Jr., E. V. Price, J. M. Knox, and L. W. Elgin. 1963. Epidemiologic treatment of contacts to infectious syphilis. Public Health Rep. 78:966-970. [PMC free article] [PubMed] [Google Scholar]
- 206.Morgan, C. A., S. A. Lukehart, and W. C. Van Voorhis. 2002. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect. Immun. 70:6811-6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Morgan, C. A., S. A. Lukehart, and W. C. Van Voorhis. 2003. Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect. Immun. 71:5605-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morgan, C. A., B. J. Molini, S. A. Lukehart, and W. C. Van Voorhis. 2002. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J. Immunol. 169:952-957. [DOI] [PubMed] [Google Scholar]
- 209.Morton, H. E., G. Rake, and N. R. Rose. 1951. Electron microscope studies of treponemes. III. Flagella. Am. J. Syph. Gonorrhea Vener. Dis. 35:503-516. [PubMed] [Google Scholar]
- 210.Morton, R. S. 1991. Did Catherine the Great of Russia have syphilis? Genitourin. Med. 67:498-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Muller, F., and S. Oelerich. 1981. Treponema-specific and antilipoidal 19S (IgM) antibodies in penicillin-treated and untreated rabbits after infection with Treponema pallidum. Br. J. Vener. Dis. 57:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Muller, N., H. G. Heine, and W. Boos. 1985. Characterization of the Salmonella typhimurium mgl operon and its gene products. J. Bacteriol. 163:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mullick, C. J., A. P. Liappis, D. A. Benator, A. D. Roberts, D. M. Parenti, and G. L. Simon. 2004. Syphilitic hepatitis in HIV-infected patients: a report of 7 cases and review of the literature. Clin. Infect. Dis. 39:100-105. [DOI] [PubMed] [Google Scholar]
- 214.Musher, D. M., M. Hague-Park, F. Gyorkey, D. C. Anderson, and R. E. Baughn. 1983. The interaction between Treponema pallidum and human polymorphonuclear leukocytes. J. Infect. Dis. 147:77-86. [DOI] [PubMed] [Google Scholar]
- 215.Myint, M., H. Bashiri, R. D. Harrington, and C. M. Marra. 2004. Relapse of secondary syphilis after benzathine penicillin G: molecular analysis. Sex. Transm. Dis. 31:196-199. [DOI] [PubMed] [Google Scholar]
- 216.Nelson, R. A., and M. M. Mayer. 1949. Immobilization of Treponema pallidum in vitro by antibody produced in syphilis infection. J. Exp. Med. 89:369-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nichols, J. C., and J. B. Baseman. 1975. Carbon sources utilized by virulent Treponema pallidum. Infect. Immun. 12:1044-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Norgard, M. V., L. L. Arndt, D. R. Akins, L. L. Curetty, D. A. Harrich, and J. D. Radolf. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect. Immun. 64:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Norgard, M. V., B. S. Riley, J. A. Richardson, and J. D. Radolf. 1995. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect. Immun. 63:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Norris, S. J. 1982. In vitro cultivation of Treponema pallidum: independent confirmation. Infect. Immun. 36:437-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Norris, S. J., and D. G. Edmondson. 1986. Factors affecting the multiplication and subculture of Treponema pallidum subsp. pallidum in a tissue culture system. Infect. Immun. 53:534-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Norris, S. J., and S. Sell. 1984. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J. Immunol. 133:2686-2692. [PubMed] [Google Scholar]
- 223.O'Regan, A. W., C. Castro, S. A. Lukehart, J. M. Kasznica, P. A. Rice, and M. F. Joyce-Brady. 2002. Barking up the wrong tree? Use of polymerase chain reaction to diagnose syphilitic aortitis. Thorax 57:917-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.O'Regan, S., J. S. Fong, J. P. de Chadarevian, J. R. Rishikof, and K. N. Drummond. 1976. Treponemal antigens in congenital and acquired syphilitic nephritis: demonstration by immunofluorescence studies. Ann. Intern. Med. 85:325-327. [DOI] [PubMed] [Google Scholar]
- 225.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 226.Pallesen, L., and P. Hindersson. 1989. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect. Immun. 57:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 228.Penn, C. W. 1981. Avoidance of host defences by Treponema pallidum in situ and on extraction from infected rabbit testes. J. Gen. Microbiol. 126:69-75. [DOI] [PubMed] [Google Scholar]
- 229.Penn, C. W., M. J. Bailey, and A. Cockayne. 1985. The axial filament antigen of Treponema pallidum. Immunology 54:635-641. [PMC free article] [PubMed] [Google Scholar]
- 230.Penn, C. W., A. Cockayne, and M. J. Bailey. 1985. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J. Gen. Microbiol. 131:2349-2357. [DOI] [PubMed] [Google Scholar]
- 231.Penn, C. W., and J. G. Rhodes. 1982. Surface-associated antigens of Treponema pallidum concealed by an inert outer layer. Immunology 46:9-16. [PMC free article] [PubMed] [Google Scholar]
- 232.Perine, P. L., R. S. Weiser, and S. J. Klebanoff. 1973. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect. Immun. 8:787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Peterson, K. M., J. B. Baseman, and J. F. Alderete. 1986. Isolation of a Treponema pallidum gene encoding immunodominant outer envelope protein P6, which reacts with sera from patients at different stages of syphilis. J. Exp. Med. 164:1160-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peterson, K. M., J. B. Baseman, and J. F. Alderete. 1983. Treponema pallidum receptor binding proteins interact with fibronectin. J. Exp. Med. 157:1958-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pope, V., M. B. Fears, W. E. Morrill, A. Castro, and S. E. Kikkert. 2000. Comparison of the Serodia Treponema pallidum particle agglutination, Captia Syphilis-G, and SpiroTek Reagin II tests with standard test techniques for diagnosis of syphilis. J. Clin. Microbiol. 38:2543-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Porcella, S. F., T. G. Popova, K. E. Hagman, C. W. Penn, J. D. Radolf, and M. V. Norgard. 1996. A mgl-like operon in Treponema pallidum, the syphilis spirochete. Gene 177:115-121. [DOI] [PubMed] [Google Scholar]
- 237.Portnoy, J., W. Carson, and C. A. Smith. 1957. Rapid plasma reagin card test for syphilis. Public Health Rep. 72:761-766. [PMC free article] [PubMed] [Google Scholar]
- 238.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Puavilai, S., S. Charuwichitratana, N. Polnikorn, A. Sakuntabhai, and P. Timpatanapong. 1993. Clinical and histopathological features of secondary syphilis. J. Med. Assoc. Thai. 76:85-92. [PubMed] [Google Scholar]
- 240.Purcell, B. K., N. R. Chamberlain, M. S. Goldberg, L. P. Andrews, E. J. Robinson, M. V. Norgard, and J. D. Radolf. 1989. Molecular cloning and characterization of the 15-kilodalton major immunogen of Treponema pallidum. Infect. Immun. 57:3708-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Quist, E. E., L. A. Repesh, R. Zeleznikar, and T. J. Fitzgerald. 1983. Interaction of Treponema pallidum with isolated rabbit capillary tissues. Br. J. Vener. Dis. 59:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 243.Radolf, J. D., D. R. Blanco, J. N. Miller, and M. A. Lovett. 1986. Antigenic interrelationship between endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum (Nichols): molecular characterization of endoflagellar proteins. Infect. Immun. 54:626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Radolf, J. D., N. R. Chamberlain, A. Clausell, and M. V. Norgard. 1988. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent Triton X-114. Infect. Immun. 56:490-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Radolf, J. D., E. B. Lernhardt, T. E. Fehniger, and M. A. Lovett. 1986. Serodiagnosis of syphilis by enzyme-linked immunosorbent assay with purified recombinant Treponema pallidum antigen 4D. J. Infect. Dis. 153:1023-1027. [DOI] [PubMed] [Google Scholar]
- 246.Radolf, J. D., C. Moomaw, C. A. Slaughter, and M. V. Norgard. 1989. Penicillin-binding proteins and peptidoglycan of Treponema pallidum subsp. pallidum. Infect. Immun. 57:1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Radolf, J. D., and M. V. Norgard. 1988. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect. Immun. 56:1825-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Radolf, J. D., M. V. Norgard, M. E. Brandt, R. D. Isaacs, P. A. Thompson, and B. Beutler. 1991. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J. Immunol. 147:1968-1974. [PubMed] [Google Scholar]
- 249.Radolf, J. D., M. V. Norgard, and W. W. Schulz. 1989. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc. Natl. Acad. Sci. USA 86:2051-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Radolf, J. D., E. J. Robinson, K. W. Bourell, D. R. Akins, S. F. Porcella, L. M. Weigel, J. D. Jones, and M. V. Norgard. 1995. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect. Immun. 63:4244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Reis e Sousa, C., A. Sher, and P. Kaye. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392-399. [DOI] [PubMed] [Google Scholar]
- 252.Rekart, M. L., D. M. Patrick, B. Chakraborty, J. J. Maginley, H. D. Jones, C. D. Bajdik, B. Pourbohloul, and R. C. Brunham. 2003. Targeted mass treatment for syphilis with oral azithromycin. Lancet 361:313-314. [DOI] [PubMed] [Google Scholar]
- 253.Ricord, P. 1838. A practical treatise on venereal diseases. Rouvier et le Bouvier, Paris, France. (In French.)
- 254.Rietschel, E. T., M. Rietschel, and B. Beutler. 2004. How the mighty have fallen: fatal infectious diseases of divine composers. Infect. Dis. Clin. N. Am. 18:311-339. [DOI] [PubMed] [Google Scholar]
- 255.Riley, B. S., N. Oppenheimer-Marks, E. J. Hansen, J. D. Radolf, and M. V. Norgard. 1992. Virulent Treponema pallidum activates human vascular endothelial cells. J. Infect. Dis. 165:484-493. [DOI] [PubMed] [Google Scholar]
- 256.Riley, B. S., N. Oppenheimer-Marks, J. D. Radolf, and M. V. Norgard. 1994. Virulent Treponema pallidum promotes adhesion of leukocytes to human vascular endothelial cells. Infect. Immun. 62:4622-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Riviere, G. R., D. D. Thomas, and C. M. Cobb. 1989. In vitro model of Treponema pallidum invasiveness. Infect. Immun. 57:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Robins, A. H., and S. L. Sellars. 2000. Oscar Wilde's terminal illness: reappraisal after a century. Lancet 356:1841-1843. [DOI] [PubMed] [Google Scholar]
- 259.Rompalo, A. M., M. R. Joesoef, J. A. O'Donnell, M. Augenbraun, W. Brady, J. D. Radolf, R. Johnson, and R. T. Rolfs. 2001. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex. Transm. Dis. 28:158-165. [DOI] [PubMed] [Google Scholar]
- 260.Rosahn, P. D. 1947. Autopsy studies in syphilis. J. Vener. Dis. Inf. 649:1-67. [Google Scholar]
- 261.Rosahn, P. D., B. Gueft, and C. Rowe. 1948. Experimental mouse syphilis. I. Organ distribution of the infectious agent. Am. J. Syph. Gonorrhea Vener. Dis. 32:327. [PubMed] [Google Scholar]
- 262.Ross, J. J. 2005. Shakespeare's chancre: did the bard have syphilis? Clin. Infect. Dis. 40:399-404. [DOI] [PubMed] [Google Scholar]
- 263.Rudolph, A. H. 1976. The microhemagglutination assay for Treponema pallidum antibodies (MHA-TP), a new treponemal test for syphilis: where does it fit? J. Am. Vener. Dis. Assoc. 3:3-8. [PubMed] [Google Scholar]
- 264.Salakhov, E., L. Tikhonova, K. Southwick, A. Shakarishvili, C. Ryan, and S. Hillis. 2004. Congenital syphilis in Russia: the value of counting epidemiologic cases and clinical cases. Sex. Transm. Dis. 31:127-132. [DOI] [PubMed] [Google Scholar]
- 265.Salazar, J. C., K. R. Hazlett, and J. D. Radolf. 2002. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 4:1133-1140. [DOI] [PubMed] [Google Scholar]
- 266.Sambri, V., A. Marangoni, L. Giacani, R. Gennaro, R. Murgia, R. Cevenini, and M. Cinco. 2002. Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J. Antimicrob. Chemother. 50:895-902. [DOI] [PubMed] [Google Scholar]
- 267.Sambri, V., A. Marangoni, M. A. Simone, A. D'Antuono, M. Negosanti, and R. Cevenini. 2001. Evaluation of recomWell Treponema, a novel recombinant antigen-based enzyme-linked immunosorbent assay for the diagnosis of syphilis. Clin. Microbiol. Infect. 7:200-205. [DOI] [PubMed] [Google Scholar]
- 268.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Schaudinn, F. N., and E. Hoffman. 1905. Vorlaufiger bericht uber das vorkommen von spirochaeten in syphilitischen krankheitsprodukten und bei papillomen. Arb. K. Gesund. 22:527-534. [PubMed] [Google Scholar]
- 270.Schiller, N. L., and C. D. Cox. 1977. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect. Immun. 16:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schober, P. C., G. Gabriel, P. White, W. F. Felton, and R. N. Thin. 1983. How infectious is syphilis? Br. J. Vener. Dis. 59:217-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schouls, L. M., R. Mout, J. Dekker, and J. D. van Embden. 1989. Characterization of lipid-modified immunogenic proteins of Treponema pallidum expressed in Escherichia coli. Microb. Pathog. 7:175-188. [DOI] [PubMed] [Google Scholar]
- 273.Schouls, L. M., H. G. van der Heide, and J. D. van Embden. 1991. Characterization of the 35-kilodalton Treponema pallidum subsp. pallidum recombinant lipoprotein TmpC and antibody response to lipidated and nonlipidated T. pallidum antigens. Infect. Immun. 59:3536-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schroeter, A. L., R. H. Turner, J. B. Lucas, and W. J. Brown. 1971. Therapy for incubating syphilis. Effectiveness of gonorrhea treatment. JAMA 218:711-713. [PubMed] [Google Scholar]
- 275.Sell, S., S. Baker-Zander, and H. C. Powell. 1982. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab. Investig. 46:355-364. [PubMed] [Google Scholar]
- 276.Sell, S., D. Gamboa, S. A. Baker-Zander, S. A. Lukehart, and J. N. Miller. 1980. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J. Investig. Dermatol. 75:470-475. [DOI] [PubMed] [Google Scholar]
- 277.Sell, S., J. Salman, and S. J. Norris. 1985. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am. J. Pathol. 118:248-255. [PMC free article] [PubMed] [Google Scholar]
- 278.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049-2056. [PubMed] [Google Scholar]
- 279.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 280.Sellati, T. J., S. L. Waldrop, J. C. Salazar, P. R. Bergstresser, L. J. Picker, and J. D. Radolf. 2001. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J. Immunol. 166:4131-4140. [DOI] [PubMed] [Google Scholar]
- 281.Sellati, T. J., D. A. Wilkinson, J. S. Sheffield, R. A. Koup, J. D. Radolf, and M. V. Norgard. 2000. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J. Infect. Dis. 181:283-293. [DOI] [PubMed] [Google Scholar]
- 282.Sepetjian, M., D. Salussola, and J. Thivolet. 1973. Attempt to protect rabbits against experimental syphilis by passive immunization. Br. J. Vener. Dis. 49:335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shaffer, J. M., S. A. Baker-Zander, and S. A. Lukehart. 1993. Opsonization of Treponema pallidum is mediated by immunoglobulin G antibodies induced only by pathogenic treponemes. Infect. Immun. 61:781-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sheffield, J. S., P. J. Sanchez, G. Morris, M. Maberry, F. Zeray, D. D. McIntire, and G. D. Wendel, Jr.2002. Congenital syphilis after maternal treatment for syphilis during pregnancy. Am. J. Obstet. Gynecol. 186:569-573. [DOI] [PubMed] [Google Scholar]
- 285.Shevchenko, D. V., T. J. Sellati, D. L. Cox, O. V. Shevchenko, E. J. Robinson, and J. D. Radolf. 1999. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect. Immun. 67:2266-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shin, J. L., K. Y. Chung, J. M. Kang, T. H. Lee, and M. G. Lee. 2004. The effects of Treponema pallidum on human dendritic cells. Yonsei Med. J. 45:515-522. [DOI] [PubMed] [Google Scholar]
- 287.Sieling, P. A., W. Chung, B. T. Duong, P. J. Godowski, and R. L. Modlin. 2003. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J. Immunol. 170:194-200. [DOI] [PubMed] [Google Scholar]
- 288.Simms, I., K. A. Fenton, M. Ashton, K. M. Turner, E. E. Crawley-Boevey, R. Gorton, D. R. Thomas, A. Lynch, A. Winter, M. J. Fisher, L. Lighton, H. C. Maguire, and M. Solomou. 2005. The re-emergence of syphilis in the United Kingdom: the new epidemic phases. Sex. Transm. Dis. 32:220-226. [DOI] [PubMed] [Google Scholar]
- 289.Simon, R. P. 1985. Neurosyphilis. Arch. Neurol. 42:606-613. [DOI] [PubMed] [Google Scholar]
- 290.Singh, A. E., and B. Romanowski. 1999. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin. Microbiol. Rev. 12:187-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sohaskey, C. D., C. Arnold, and A. G. Barbour. 1997. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J. Bacteriol. 179:6837-6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Staggs, T. M., M. K. Greer, J. B. Baseman, S. C. Holt, and V. V. Tryon. 1994. Identification of lactoferrin-binding proteins from Treponema pallidum subspecies pallidum and Treponema denticola. Mol. Microbiol. 12:613-619. [DOI] [PubMed] [Google Scholar]
- 293.Stamm, L. V., and P. J. Bassford, Jr. 1985. Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect. Immun. 47:799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Stamm, L. V., and H. L. Bergen. 2000. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob. Agents Chemother. 44:806-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Stamm, L. V., and H. L. Bergen. 2000. The sequence-variable, single-copy tprK gene of Treponema pallidum Nichols strain UNC and Street strain 14 encodes heterogeneous TprK proteins. Infect. Immun. 68:6482-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Stamm, L. V., F. C. Gherardini, E. A. Parrish, and C. R. Moomaw. 1991. Heat shock response of spirochetes. Infect. Immun. 59:1572-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Stamm, L. V., S. R. Greene, H. L. Bergen, J. M. Hardham, and N. Y. Barnes. 1998. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol. Lett. 169:155-163. [DOI] [PubMed] [Google Scholar]
- 298.Stamm, L. V., R. L. Hodinka, P. B. Wyrick, and P. J. Bassford, Jr. 1987. Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect. Immun. 55:2255-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stamm, L. V., N. R. Young, J. G. Frye, and J. M. Hardham. 1996. Identification and sequences of the Treponema pallidum mglA and mglC genes. DNA Seq. 6:293-298. [DOI] [PubMed] [Google Scholar]
- 300.Stamm, W. E., H. H. Handsfield, A. M. Rompalo, R. L. Ashley, P. L. Roberts, and L. Corey. 1988. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA 260:1429-1433. [PubMed] [Google Scholar]
- 301.Stokes, J. H., H. Beerman, and N. R. Ingraham. 1944. Modern clinical syphilology. The W.B. Saunders Co., Philadelphia, Pa.
- 302.Sun, E. S., B. J. Molini, L. K. Barrett, A. Centurion-Lara, S. A. Lukehart, and W. C. Van Voorhis. 2004. Subfamily I Treponema pallidum repeat protein family: sequence variation and immunity. Microbes Infect. 6:725-737. [DOI] [PubMed] [Google Scholar]
- 303.Swain, R. H. 1955. Electron microscopic studies of the morphology of pathogenic spirochaetes. J. Pathol. Bacteriol. 69:117-128. [DOI] [PubMed] [Google Scholar]
- 304.Swancutt, M. A., B. S. Riley, J. D. Radolf, and M. V. Norgard. 1989. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect. Immun. 57:3314-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sykes, J. A., and J. N. Miller. 1973. Ultrastructural studies of treponemes: location of axial filaments and some dimensions of Treponema pallidum (Nichols strain), Treponema denticola, and Treponema reiteri. Infect. Immun. 7:100-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tantalo, L. C., S. A. Lukehart, and C. M. Marra. 2005. Treponema pallidum strain-specific differences in neuroinvasion and clinical phenotype in a rabbit model. J. Infect. Dis. 191:75-80. [DOI] [PubMed] [Google Scholar]
- 307.Theus, S. A., D. A. Harrich, R. Gaynor, J. D. Radolf, and M. V. Norgard. 1998. Treponema pallidum, lipoproteins, and synthetic lipoprotein analogues induce human immunodeficiency virus type 1 gene expression in monocytes via NF-kappaB activation. J. Infect. Dis. 177:941-950. [DOI] [PubMed] [Google Scholar]
- 308.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1986. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect. Immun. 52:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J. Exp. Med. 161:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Putative Treponema pallidum cytadhesins share a common functional domain. Infect. Immun. 49:833-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tight, R. R., and R. L. Perkins. 1976. Treponema pallidum infection in subcutaneous polyethylene chambers in rabbits. Infect. Immun. 13:1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Titus, R. G., and R. S. Weiser. 1979. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J. Infect. Dis. 140:904-913. [DOI] [PubMed] [Google Scholar]
- 314.Tosca, A., J. Lehou, M. Hatjivasiliou, A. Varelzidis, and J. D. Stratigos. 1988. Infiltrate of syphilitic lesions before and after treatment. Genitourin. Med. 64:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tourville, D. R., L. H. Byrd, D. U. Kim, D. Zajd, I. Lee, L. B. Reichman, and S. Baskin. 1976. Treponemal antigen in immunopathogenesis of syphilitic glomerulonephritis. Am. J. Pathol. 82:479-492. [PMC free article] [PubMed] [Google Scholar]
- 316.Turner, T. B. 1939. Protective antibodies in the serum of syphilitic rabbits. J. Exp. Med. 69:867-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Turner, T. B., and D. H. Hollander. 1957. Biology of the treponematoses. World Health Organization, Geneva, Switzerland.
- 318.Turner, T. B., P. H. Hardy, Jr., B. Newman, and E. E. Nell. 1973. Effects of passive immunization on experimental syphilis in the rabbit. Johns Hopkins Med. J. 133:241-251. [PubMed] [Google Scholar]
- 319.Umemoto, T., and I. Namikawa. 1994. Binding of host-associated treponeme proteins to collagens and laminin: a possible mechanism of spirochetal adherence to host tissues. Microbiol. Immunol. 38:655-663. [DOI] [PubMed] [Google Scholar]
- 320.U.S. Department of Health, Education, and Welfare. 1968. Syphilis—a synopsis. Center for Disease Control, Atlanta, Ga.
- 321.van der Bij, A. K., I. G. Stolte, R. A. Coutinho, and N. H. Dukers. 2005. Increase of sexually transmitted infections, but not HIV, among young homosexual men in Amsterdam: are STIs still reliable markers for HIV transmission? Sex. Transm. Infect. 81:34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.van der Sluis, J. J., J. A. Koehorst, and A. M. Boer. 1987. Factors that inhibit adherence of Treponema pallidum (Nichols strain) to a human fibroblastic cell line: development in serum of patients with syphilis. Genitourin. Med. 63:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Van Voorhis, W. C., L. K. Barrett, D. M. Koelle, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 324.Van Voorhis, W. C., L. K. Barrett, S. A. Lukehart, B. Schmidt, M. Schriefer, and C. E. Cameron. 2003. Serodiagnosis of syphilis: antibodies to recombinant Tp0453, Tp92, and Gpd proteins are sensitive and specific indicators of infection by Treponema pallidum. J. Clin. Microbiol. 41:3668-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Van Voorhis, W. C., L. K. Barrett, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect. Immun. 64:1048-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wagner von Jauregg, J. 1918. Uber die Einwirkung der Malaria auf die progressive Paralyse. Psychiatr. Neurol. Wochenschr. 20:132. [Google Scholar]
- 327.Walker, E. M., J. K. Arnett, J. D. Heath, and S. J. Norris. 1991. Treponema pallidum subsp. pallidum has a single, circular chromosome with a size of approximately 900 kilobase pairs. Infect. Immun. 59:2476-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Walker, E. M., J. K. Howell, Y. You, A. R. Hoffmaster, J. D. Heath, G. M. Weinstock, and S. J. Norris. 1995. Physical map of the genome of Treponema pallidum subsp. pallidum (Nichols). J. Bacteriol. 177:1797-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Walker, E. M., G. A. Zampighi, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1989. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J. Bacteriol. 171:5005-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Watson-Jones, D., J. Changalucha, B. Gumodoka, H. Weiss, M. Rusizoka, L. Ndeki, A. Whitehouse, R. Balira, J. Todd, D. Ngeleja, D. Ross, A. Buve, R. Hayes, and D. Mabey. 2002. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J. Infect. Dis. 186:940-947. [DOI] [PubMed] [Google Scholar]
- 331.Weigel, L. M., J. D. Radolf, and M. V. Norgard. 1994. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc. Natl. Acad. Sci. USA 91:11611-11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weiser, R. S., D. Erickson, P. L. Perine, and N. N. Pearsall. 1976. Immunity to syphilis: passive transfer in rabbits using serial doses of immune serum. Infect. Immun. 13:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wenhai, L., Z. Jianzhong, and Y. Cao. 2004. Detection of Treponema pallidum in skin lesions of secondary syphilis and characterization of the inflammatory infiltrate. Dermatology 208:94-97. [DOI] [PubMed] [Google Scholar]
- 334.Wicher, K., L. M. Schouls, V. Wicher, J. D. Van Embden, and S. S. Nakeeb. 1991. Immunization of guinea pigs with recombinant TmpB antigen induces protection against challenge infection with Treponema pallidum Nichols. Infect. Immun. 59:4343-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]
- 336.Zakoucka, H., V. Polanecky, and V. Kastankova. 2004. Syphilis and gonorrhoea in the Czech Republic. Euro Surveill. 9(12):18-20. [PubMed] [Google Scholar]
- 337.Zhang, Z., J. N. Feige, A. B. Chang, I. J. Anderson, V. M. Brodianski, A. G. Vitreschak, M. S. Gelfand, and M. H. Saier, Jr. 2003. A transporter of Escherichia coli specific for L- and D-methionine is the prototype for a new family within the ABC superfamily. Arch. Microbiol. 180:88-100. [DOI] [PubMed] [Google Scholar]
- 338.Zoechling, N., E. M. Schluepen, H. P. Soyer, H. Kerl, and M. Volkenandt. 1997. Molecular detection of Treponema pallidum in secondary and tertiary syphilis. Br. J. Dermatol. 136:683-686. [PubMed] [Google Scholar]