Abstract
Although several human immunodeficiency virus (HIV) vaccine approaches have elicited meaningful antigen-specific T-cell responses in animal models, no single vaccine candidate has engendered antibodies that broadly neutralize primary isolates of HIV type 1 (HIV-1). Thus, there remains a significant gap in the design of HIV vaccines. To address this issue, we exploited the existence of rare human monoclonal antibodies that have been isolated from HIV-infected individuals. Such antibodies neutralize a wide array of HIV-1 field isolates and have been shown to be effective in vivo. However, practical considerations preclude the use of antibody preparations as a prophylactic passive immunization strategy in large populations. Our concept calls for an antibody gene of choice to be transferred to muscle where the antibody molecule is synthesized and distributed to the circulatory system. In these experiments, we used a recombinant adeno-associated virus (rAAV) vector to deliver the gene for the human antibody IgG1b12 to mouse muscle. Significant levels of HIV-neutralizing activity were found in the sera of mice for over 6 months after a single intramuscular administration of the rAAV vector. This approach allows for predetermination of antibody affinity and specificity prior to “immunization” and avoids the need for an active humoral immune response against the HIV envelope protein.
Over the past several years, progress toward a safe and effective vaccine for human immunodeficiency virus (HIV) has been steady, and multiple approaches have shown promise in animal models and humans (1, 2, 4-6, 10). Many of these promising vaccine candidates have elicited measurable and significant antigen-specific T-cell responses. In contrast, there has been a notable lack of success in the induction of serum antibodies that broadly neutralize primary isolates of HIV type 1 (HIV-1) (30, 32, 34). Thus, if one considers such antibodies to be an important defense against HIV-1 infection and disease, there remains a significant gap in the design of current HIV-1 vaccine candidates.
There are several hypotheses put forth to explain this lack of neutralizing antibody induction after vaccination with envelope immunogens. First, most anti-envelope antibodies elicited do not recognize the mature oligomeric envelope complex but rather bind to unprocessed gp160 precursor or monomeric gp120 (35). This is due in part to the trimeric structure of the mature envelope spike, which yields a molecule of low inherent immunogenicity. Extensive glycosylation of surface-exposed domains renders a significant portion of the spike nonimmunogenic, giving rise to the so-called “silent face” of the molecule (37). Second, the compact structure of the trimeric moiety sterically interferes with antibody recognition of protein epitopes that are located within the core of the trimer. Importantly, these same epitopes are readily exposed on the unprocessed gp160 precursor or monomeric gp120 proteins and map to the nonneutralizing face of the protein. Consequently, it has been extremely difficult to isolate human monoclonal antibodies that neutralize primary viral isolates in a broad, cross-clade manner. In fact, only five such antibodies have currently been identified (b12, 2G12, 2F5, Z13, and 4E10) (43), despite efforts with a variety of techniques. The fact that such antibodies are rare in HIV-1-infected humans serves to underscore the ill-defined but substantial obstacles in eliciting broadly reactive antibodies by traditional methods of vaccination.
One potential solution to this problem might be to prophylactically administer antibody preparations (monoclonal or polyclonal) that possess the desired neutralizing activities. With regard to HIV-1, studies in nonhuman primates suggest that passively administered neutralizing antibodies can provide significant protection against “SIV/SHIV/HIV” infection (3, 16, 19, 25, 26, 38). This type of “passive immunization” scheme has been successfully applied on a large scale to a targeted population of infants at risk for serious respiratory syncytial virus infection (40, 41). However, such a strategy for HIV has significant drawbacks. It would be cost prohibitive and impractical to frequently administer antibody preparations to large numbers of people for an indefinite period of time.
Because of the significant obstacles that confront both active and passive immunization strategies, we have begun to explore an alternate strategy to generate serum antibodies that neutralize primary isolates of HIV-1. This novel approach exploits the existence of the aforementioned human monoclonal antibodies against gp160 and the unique gene delivery properties of recombinant adeno-associated virus (rAAV) vectors (28). rAAV vectors have been shown to transduce muscle with high efficiency and direct the long-term expression of a variety of transgenes (13, 22, 42). Because of the flexibility of this system, light- and heavy-chain antibody genes can be incorporated into a single rAAV vector, and the antibody-expressing vector can then be used to transduce muscle in vivo. This, in turn, leads to sustained expression of biologically active antibody molecules from transduced myofibers. We show here that the human monoclonal antibody IgG1b12 (9) can be expressed in exactly this fashion. Moreover, significant levels of HIV neutralizing activity are found in the sera of mice for over 6 months after a single intramuscular administration of vector. This approach allows for predetermination of antibody affinity and specificity prior to “immunization” and avoids the need for an active humoral immune response against the HIV envelope protein. Beyond the current application for HIV-1, this strategy might be useful for other situations where antibodies with predetermined specificities need to be delivered in vivo.
MATERIALS AND METHODS
Cells and virus.
HeLa cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. rAAV/IgG1b12 stable producer cells (CE71) were maintained in Dulbecco modified Eagle medium, 10% fetal bovine serum, penicillin-streptomycin, and G418 (700 μg/ml). Adenovirus type 5 (Ad5) was purified, and the titer was determined as previously described (14).
Animals.
All experiments involving animals were conducted in accordance with the Children's Hospital Institutional Animal Care and Use Committee. Six-week-old Rag-1 mice [C.129S7(B6)-Rag1tm1Mom] were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed in microisolator barrier housing. Mice were anesthetized with intraperitoneal injection of 2,2,2-tribromoethanol (Avertin; 240 mg/kg). A 5-mm skin incision was made over the distal femur and 50 μl of the viral suspension or phosphate-buffered saline (PBS) was injected in the quadriceps femoris muscle along the long axis of the muscle by using a 28-gauge needle. Blood samples were collected from the retroorbital-sinus under anesthesia. At the time of sacrifice, the entire quadriceps femoris muscles were removed and bisected along the transverse plane and half fixed in a non-cross-linking fixative (Histochoice; Amresco, Solon, Ohio) and paraffin embedded, and the other half quick-frozen in liquid nitrogen for subsequent DNA analysis. Tibialis anterior muscle was taken as control tissue.
Construction of a dual plasmid vector for rAAV/IgG1b12 production.
The coding sequences for the human monoclonal antibody, IgG1b12 were derived from plasmid pDR12. Isolation of IgG1b12 and plasmid pDR12 have been described elsewhere (8, 9). The dual promoter, rAAV cloning plasmid pAAV/IgG1b12 was constructed sequentially as follows. (i) Plasmid pCMV/β (Clontech) was digested with PstI and the 2.7-kb vector plasmid DNA fragment isolated and religated with itself to generate a ampicillin resistant vector (pB). (ii) PCR was used to amplify the hCMV promoter/enhancer and simian virus 40 (SV40) intron (808 bp) from plasmid pCMV/β. This fragment was cloned into the unique EcoRI site of plasmid pB to generate plasmid pCMV. These primers were designed with EcoRI sites flanking unique PacI and Srf I sites at the 5′ and 3′ ends. (iii) PCR was used to amplify the bovine growth hormone polyadenylation signal (190 bp) and the EF1-alpha promoter (770 bp) separately using plasmid pGT62lacZ as a template (InVivoGen). The resulting PCR fragments were subsequently XhoI digested and religated, followed by a second round of PCR performed with the BGH forward and EF1-alpha reverse primers to generate a single DNA fragment. These primers incorporated a 5′ SrfI site and 3′ AvrII, EcoRI, SwaI, and PstI sites, respectively. This 960-bp DNA fragment was directionally cloned into plasmid pCMV at the unique SrfI and PstI sites. The resulting plasmid (pCMV/EF1) now possessed the cytomegalovirus (CMV) promoter with a BGH polyadenyation site, followed by the EF1-alpha promoter. (iv) A 270-bp DNA fragment containing the SV40 polyadenylation signal (isolated from plasmid pGT62lacZ, Invivogen, Inc.) was directionally cloned into the EcoRI/SwaI sites of plasmid pCMV/EF1 to yield pCMV/EF1a. (v) The IgG1b12 heavy chain cDNA (1,463 bp) was isolated by transfecting CHO cells with plasmid pDR12 and isolating total RNA. The RNA was subjected to reverse transcription-PCR (RT-PCR) and cloned into the pZero vector (Invitrogen). These primers incorporated flanking SrfI sites that facilitated the cloning of the IgG1b12 heavy chain into the unique SrfI site in plasmid pCMV/EF1a, yielding plasmid pCMV/HC/EF1a. (vi) The IgG1b12 kappa light-chain gene was PCR amplified directly from plasmid pDR12, and the 720-bp product was cloned into plasmid pZero. The light-chain primers incorporated flanking AvrII restriction sites that were used to clone the kappa light-chain into the AvrII site of plasmid pCMV/HC/EF1a to generate plasmid pCMV/HC/EF1a/LC. (vii) Site-directed mutagenesis was used to introduce a unique MluI restriction site into the heavy-chain leader peptide sequence and a similar strategy was employed to introduce a BssHI site into the light chain leader peptide sequence. (viii) The dual expression cassette was isolated as a 4.5-kb PacI/SrfI DNA fragment and cloned between the AAV ITRs of plasmid pAAV/β-gal/rep-cap/neotk (14) to generate pAAV/IgG1b12/rep-cap/neotk. This tripartite plasmid contains the native rep-cap AAV helper sequences, as well as the neomycin resistance gene for stable cell line selection. Primer sequences used for the above construction were as follows: CMV forward, 5′-TCTAGAATTCTTTAATTAAGTCGTTACATAACTTACGG-3′; CMV reverse, 5′-TCTAGAATTCTGCCCGGGCTACAATTCCGCAGCTTTTAG-3′; BGH forward, 5′-TTAGTGTGCCCGGGCACTCGCTGATCAGC CTCGACT-3′; BGH reverse, 5′-TAGTGTCTCGAGAATCCTCCCCCTTGCTGTC-3′ EF1 forward, 5′-TTAGTGTCTCGAGAACTAACATACGCTCTCCA-3′; EF1 reverse, 5′-GTGTCTGCAGGTATTTAAATGTGGGAATTCGTCCTAGGCCCTCCTACCGGTGATCTC-3′; heavy chain cDNA forward,5′-TACTTCGCCCGGGCTAATTCGCCGCCACCATGGAA-3′; heavy chain cDNA reverse, 5′-TACTTCGCC CGGGCTTTATTCATTTACCCGGAGACAGGG-3′; light chain forward, 5′-CCTCACCTAGGCCACCATGGGTGTGCCACGCTGG-3′; and light chain reverse, 5′-CCTCACCTAGGATTAACACTCTCCCCTGTT-3′.
rAAV production.
rAAV/IgG1b12 was produced and purified by previously described methods (12, 14). Briefly, a producer cell line (CE71) was isolated after HeLa cell transfection with plasmid pAAV/IgG1b12/rep-cap/neotk and subsequent G418 (700 μg/ml) drug selection. Two hundred individual cell lines were screened following wild-type Ad5 infection (multiplicity of infection [MOI] = 20) and CE71 was identified as producing the highest number DNase-resistant particles (DRP) per cell (104 DRP/cell). For large-scale vector production, 1010 CE71 cells were expanded in a Corning Cell Cube adherent cell bioreactor and subsequently infected with wild-type Ad5 at an MOI of 20. After development of adenovirus CPE (72 h), rAAV/IgG1b12 was purified from the crude CE71 cell lysate by heparin chromatography as previously detailed (12). DRP titers were determined for purified rAAV/IgG1b12 by real-time PCR methodology utilizing a Prism 7700 Taqman Sequence Detector System (PE Applied Biosystems) as detailed by Clark et al. (12). The primer and fluorescent probe set used for rAAV/IgG1b12 quantitation were as follows: CMV forward primer, 5′-TGGAAATCCCCGTGAGTCAA-3′; CMV reverse primer, 5′-CATGGTGATGCGGTTTTGG-3′; and probe, 5′-FAM-CCGCTATCCACGCCCATTGATG-TAMRA-3′. An infectious rAAV/IgG1b12 titer was determined by using serial dilutions of the rAAV/IgG1b12 stock and infecting a rep-cap-expressing cell line (C12) in the presence of adenovirus. An endpoint titer determination was made based on quantitative PCR detection of replicating rAAV/IgG1b12 genomes in C12 cells, as previously described (15). The calculated DRP/IU ratio of rAAV/IgG1b12 used in these experiments was 28:1.
Immunohistochemical detection.
Muscle tissue for human IgG1 heavy-chain (Fc specific) detection was serial sectioned (6 μm) and deparaffinized in Americlear with successive ethanol baths, followed by a 1× PBS plus 0.2% Tween 20 wash. Tissue sections were initially processed by using the Antigen Retrieval Citra Solution (BioGenex) according to the manufacturer's instructions and then blocked for 10 min with Power Block reagent (BioGenex). A 1:100 dilution of a polyclonal rabbit anti-human IgG antiserum (Dako A0424) was incubated with the sections for 18 h at 4°C. After an extensive washing, a biotinylated anti-rabbit secondary antibody (1:100 dilution; Vector Laboratories) was added, followed by incubation for 30 min. Antigen was visualized by using an avidin-biotin-peroxidase conjugate according to the manufacturer's instructions (Vectastain Elite ABC-Peroxidase; Vector Laboratories). Color development was achieved by incubating the sections for 5 min in AEC Peroxidase Substrate (Dako). Images were captured by using a Jenoptik digital camera fitted to a Zeiss Axioskop light microscope by using the Prog/Res/3008 software bundle.
Enzyme immunoassays.
Human IgG1 levels were measured by using Bindazyme Human Immunoglobulin Subclass Enzyme Immunoassay Kit (The Binding Site) as directed by the manufacturer. The sensitivity of this assay is 2.9 ng of human IgG1/ml. Anti-HIV-1 gp120 antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) as follows: Immulon-4 immunoassay plates (Dynatech) were coated (100 ng/well) with recombinant HIV-1LAI gp120 produced in Chinese hamster ovary (CHO) cells (Quality Biological, Gaithersburg, Md.) diluted in carbonate buffer (BupH; Pierce, Rockford, Ill.) for 16 h at 4°C. The antigen was removed, and the wells were blocked with 1% normal goat serum in Blotto (5% skim dry milk in 1× PBS [pH 7.4]) for 1 h at 25°C. Mouse sera were diluted in 0.1% (vol/vol) Triton X-100 in PBS and incubated for 30 min and then washed five times by immersion in 0.1% (vol/vol) Triton X-100 in PBS. A goat anti-human IgG1 HRP-conjugated secondary antibody (1:5,000) was added for 1 h (Pierce, Rockford, Ill.). The colorimetric substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was added, and the reaction was stopped after 30 min by the addition of 1 N H2SO4. Both ELISA assays were read at an optical density of 450 nm (OD450) on a Perkin-Elmer HTS 7000 plate reader. Endpoint titers were derived by taking the reciprocal of the serum dilution that yielded OD450 values that were at least two times higher than the corresponding no-antigen control wells.
HIV-1 neutralization assays.
Antibody-mediated neutralization of HIV-1 IIIB was measured in an MT-2 cell-killing assay by using Finter's neutral red to quantify viable cells as described previously (29). Titers are reported as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing. This 50% endpoint corresponds to >90% reduction in p24 Gag antigen synthesis in this assay (7). Neutralization of SHIV-89.6 was measured in mitogen-stimulated human peripheral blood mononuclear cells by using a reduction in p27 Gag antigen synthesis (7). Virus stocks were generated in either H9 cells (HIV-1 IIIB) or human peripheral blood mononuclear cells (SHIV-89.6).
RESULTS
Construction of a dual-promoter rAAV vector for antibody expression.
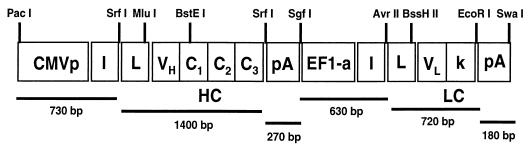
To achieve efficient antibody expression within target muscle cells, we hypothesized that a dual-promoter rAAV vector would result in optimal coexpression of heavy- and light-chain proteins within the same cell. To that end, we constructed a novel rAAV expression vector with the following features (Fig. 1). (i) This vector contains two constitutive promoters that are active in skeletal muscle in the context of a rAAV vector (hCMV promoter-enhancer and the human EF1-alpha promoter). (ii) Several unique 8-bp restriction enzyme sites were incorporated into the vector to allow for the rapid replacement of promoter elements or heavy and light chain coding sequences. (iii) Site-directed mutagenesis was performed on the heavy- and light-chain leader peptide sequences of IgG1b12 to introduce unique restriction sites (MluI for the heavy-chain leader and BssHII for the light-chain leader) that facilitate in-frame antibody gene cloning. (4) The IgG1b12 heavy-chain introns were removed by RT-PCR to reduce vector size and remain within the packaging limit of wild-type AAV. (5) A strong transcriptional termination site located 3′ to the first expression cassette was added to reduce possible promoter interference. Lastly, to enable high-titer rAAV/IgG1b12 vector production by using a stable producer cell line approach (14), the rAAV/IgG1b12 plasmid vector sequences were cloned into a larger tripartite plasmid (pAAV/IgG1b12/rep-cap/neotk) that also contains the AAV-2 rep-cap helper sequences and a neomycin resistance gene as previously described (14). The tripartite plasmid was then used to isolate an optimal HeLa based, rAAV/IgG1b12 producer cell line (designated CE71).
FIG. 1.
Dual-promoter rAAV antibody vector (pCMV/HC/EF1a/LC). Unique restriction sites are labeled at the top of the schematic. Vector components are labeled as follows: HC and LC, the heavy- and light-chain antibody genes, respectively; CMVp, human immediate-early promoter-enhancer; and I, SV40 small T-antigen intron. Antibody leader sequences are labeled “L,” and “pA” denotes the bovine growth hormone polyadenylation site. The second transcriptional unit contains the human elongation factor 1α (EF1-a) promoter and has been modified to enhance stability of DNA and RNA by using the R segment and part of the U5 sequence (R-U5′) of the HTLV-1 long terminal repeat. This promoter also contains the I117 intron, which is derived from plasmid pGT62LacZ (InVivoGen, Inc.). The light-chain polyadenylation site is from SV40.
The ability of the plasmid pAAV/IgG1b12/rep-cap/neotk and rAAV/IgG1b12 to produce human IgG1 antibody was initially confirmed in vitro by using several transformed cell lines (CHO-K1, HeLa, COS-7, and C2C12). After plasmid transfection or rAAV/IgG1b12 transduction, cell culture supernatant contained detectable levels of human IgG1 by using a commercial human IgG1 ELISA (data not shown).
Production of circulating human IgG1 in rAAV-transduced Rag1 mice.
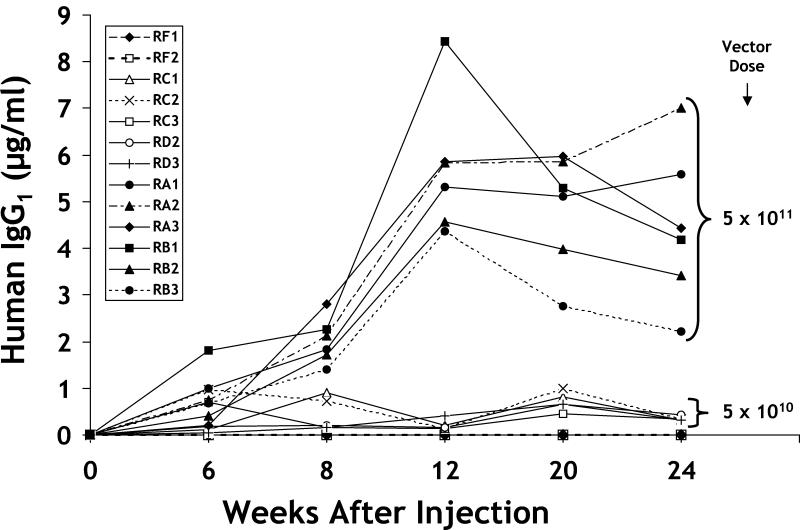
To determine whether antibody gene transfer into mouse muscle was a feasible strategy for systemic IgG production, immunodeficient Rag1 mice were inoculated with rAAV/IgG1b12 into both quadriceps muscles. Rag1 mice were used to avoid an anti-human IgG response. The study consisted of 16 animals: 6 received 5 × 1011 DRP of rAAV/IgG1b12, 6 received 5 × 1010 DRP, 2 received an irrelevant rAAV vector (rAAV/GUS, 4 × 1011 DRP), and 2 were given PBS diluent (used for vector DNA analysis only). No adverse effects attributable to the injection procedure were noted in any mice. Human IgG1 was detected at 6 weeks postinoculation in all 11 surviving rAAV/IgG1b12 mice (Fig. 2); one mouse died at 2 weeks from unrelated causes. On average, animals that received the higher dose of rAAV/IgG1b12 possessed 7 to 28 times more IgG1 than lower-dose animals. Maximal IgG1 concentrations were observed 12 weeks after injection and then plateaued over the next 3 months. The majority of high-dose animal sera consistently possessed between 4 and 5 μg of human IgG1 per ml, with the maximum level exceeding 8 μg/ml. In the low-dose group, antibody levels continued to increase 20 weeks after vector inoculation with circulating antibody levels in the 0.5- to 1.0-μg/ml range (Fig. 2). Mouse sera were also assayed for binding to gp120 by using an anti-HIV gp120 endpoint ELISA titer; the antigen used was recombinant HIV-1LAI gp120 produced in CHO cells. Sera taken at 16 weeks revealed endpoint titers in the range of 1:800 to 1:3,200 (Table 1). These data confirmed that IgG1b12 secreted from muscle retained gp120 binding specificity.
FIG. 2.
Human IgG1 concentrations in mouse serum after rAAV/IgG1b12 administration. The temporal profile of human IgG1 accumulation in mouse serum after rAAV/IgG1b12 administration is depicted. Data were obtained by using a commercial human IgG1 ELISA kit (Bindazyme) with a maximal sensitivity of 2.9 ng/ml. As shown to the right of the figure, two animal groups received either 5 × 1011 or 5 × 1010 DRP of rAAV/IgG1b12. Two animals (RF1 and RF2) received a control vector (rAAV/GUS, 4 × 1011 DRP), and their sera tested negative for human IgG1.
TABLE 1.
Serum anti-HIV-1 gp120 binding titers
| Vector | Mousea | Titer at (no. of wk postinjection)b:
|
|
|---|---|---|---|
| 0 | 16 | ||
| IgG1b12 | RA1 | <100 | 1:3,200 |
| RA2 | <100 | 1:1,600 | |
| RA3 | <100 | 1:1,600 | |
| RB1 | <100 | 1:800 | |
| GUS | RF1 | <100 | <100 |
| RF2 | <100 | <100 | |
RA1, RA2, RA3, and RB1 each received a single dose of 5 × 1011 DRP of rAAV/IgG1b12, whereas animals RF1 and RF2 received control rAAV/GUS (4 × 1011 DRP).
Titers are the reciprocal serum dilution at which the OD450 absorbance of antigen-coated wells was twofold greater than the minus-antigen control wells (duplicate wells were averaged).
Muscle-derived IgG1b12 neutralizes HIV-1.
While the IgG1 and gp120 ELISA data confirmed in vivo antibody expression, these assays did not address whether secreted IgG1b12 retained the ability to neutralize HIV-1. Therefore, 20-week serum samples from all six high-dose animals were analyzed for neutralization activity against TCLA strain HIV-1 IIIB. Sera from five of the six animals possessed detectable neutralization activity (Table 2). Significantly, the predicted IgG1b12 concentration in our mouse samples (extrapolated from the observed neutralization titer) by using this assay closely agreed with the levels of circulating human IgG determined by ELISA (Fig. 2). To extend these observations, the ability of mouse sera to neutralize a primary-like HIV-1 isolate (SHIV-89.6 containing the HIV 89.6 env) was also tested. Neutralization activity against SHIV-89.6 was observed in serum pools from 16, 20, and 24 weeks after injection (Table 3); sera were pooled because of limited volumes. Importantly, these same sera failed to neutralize the closely related viral isolate SHIV-89.6P, which is a known property of IgG1b12 that further confirms the integrity of the IgG1b12 produced in muscle (17). These data indicated that IgG1b12 originating from muscle retained the predicted ability to neutralize both TCLA and primary HIV-1 isolates.
TABLE 2.
Serum neutralization titers against HIV-1 IIIB
| Mousea | Titer at (no. of wk postinjection)b:
|
Predicted IgG1b12 concn (μg/ml)c | Observed human IgG1 concn (μg/ml)d | |
|---|---|---|---|---|
| 0 | 20 | |||
| RA1 | <20 | 93 | 6.7 | 5.1 |
| RA2 | <20 | 56 | 4.0 | 5.9 |
| RA3 | <20 | 55 | 4.0 | 6.0 |
| RB1 | <20 | 44 | 3.2 | 5.3 |
| RB2 | <20 | 47 | 3.4 | 4.0 |
| RB3 | <20 | <20 | 0.0 | 2.8 |
Each mouse received a single dose of 5 × 1011 DRP of rAAV/IgG1b12 in the quadriceps.
Antibody-mediated neutralization was measured in an MT-2 cell-killing assay. Titers are the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing, which corresponds to >90% reduction in p24 Gag antigen synthesis.
Concentration of purified IgG1b12 that is necessary to achieve the observed neutralization titer.
Actual concentration of human IgG1 in sera (taken from Fig. 2).
TABLE 3.
Serum neutralization titers against SHIV 89.6
| Test samplea | p27 concn (pg/ml) | % Reductionb |
|---|---|---|
| Diluent | 1,725 | 0 |
| IgG1b12 (4 μg/ml) | 89 | 95 |
| Pooled mouse sera at (no. of wk after injection): | ||
| 0 | 711 | 59 |
| 16 | 225 | 87 |
| 20 | 326 | 81 |
| 24 | 527 | 69 |
The preimmune (time zero) pool consisted of sera from mice RA1, RA2, RA3, RB1, RB2, and RB3. Sera from these same mice were pooled by collection date (16, 20, or 24 weeks after injection) as indicated. All pools were assayed at a 1:4 dilution.
The percent reduction in p27 antigen was calculated relative to the amount of p27 synthesized in the absence of serum.
rAAV vector persistence and protein production in muscle.
To show that muscle was the site of antibody production, we assayed for rAAV genome persistence and human IgG protein expression in muscle tissue harvested 24 weeks postinoculation. rAAV/IgG1b12 vector DNA persistence was analyzed by using real-time quantitative PCR. Approximately 50% of the left and right quadriceps from each animal were used for genomic DNA isolation and subjected to Taqman PCR by using a PCR primer-probe pair specific for the CMV promoter. As shown in Table 4, all inoculated muscle tissue possessed significant levels of vector DNA that ranged between 0.4 and 10 copies per nucleus. On average, muscle from animals that received the higher dose possessed five times more vector DNA per muscle nucleus than muscle from low-dose animals.
TABLE 4.
Persistence of vector DNA in mouse muscle
| Vector (dose)a | Mouse | Avg no. of genome copies/nucleusb |
|---|---|---|
| PBS | RF3 | 0.003 |
| RF4 | 0.012 | |
| rAAV/IgG1b12 (5 × 1010) | RC1 | 9.0 |
| RC2 | 5.5 | |
| RC3 | 0.5 | |
| RD2 | 1.2 | |
| RD3 | 4.5 | |
| rAAV/IgG1b12 (5 × 1011) | RA1 | 42.5 |
| RA2 | 3.2 | |
| RA3 | 20.0 | |
| RB1 | 27.1 | |
| RB2 | 0.4 | |
| RB3 | 19.3 |
Dose is measured as the DRP (see Materials and Methods).
Values represent the average number of rAAV genomes per nucleus observed in the quadriceps muscles after rAAV injection. A total of 60 ng of muscle DNA (10,000 nucleus equivalents) was analyzed by quantitative Taqman PCR by using the CMV primer-probe set (see Materials and Methods). All samples were harvested 24 weeks after injection.
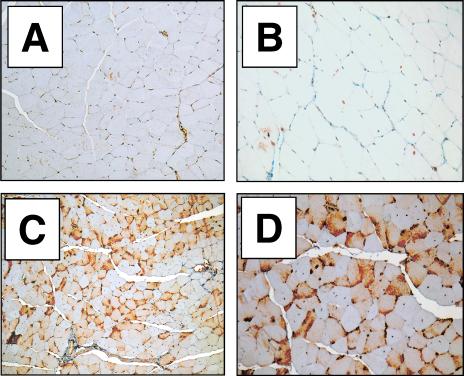
To demonstrate in situ antibody expression within the inoculated muscle, immunoperoxidase staining for human IgG1 was performed on paraffin-embedded muscle tissue. All higher-dose animals demonstrated appreciable, often punctate, immunostaining of specific myofibers consistent with endoplasmic reticulum-Golgi localization of the secreted protein (Fig. 3). Control rAAV/GUS quadriceps tissue was negative for the presence of human IgG1 (animals RF1 and RF2).
FIG. 3.
Immunohistochemical detection of human IgG1 in mouse quadriceps muscle. (A and B) Control animal RF1 (rAAV/GUS injected) quadriceps muscle cross section showing background immunostaining levels for human IgG1. (C and D) Animal RA3 (rAAV/IgG1b12 injected) right quadriceps cross section showing intense human IgG-specific immunoperoxidase straining throughout the tissue (brown color). No evidence of an overt inflammatory response was observed in any of the high-dose animals. Tissue slides were counterstained with hematoxylin. Magnifications: ×100 (A and C) and ×200 (B and D).
DISCUSSION
The goal of these studies was to demonstrate the feasibility of antibody gene transfer as a means of generating sustained HIV-1 neutralizing activity in serum. Our data show that, after injection of an rAAV vector, biologically active antibodies are synthesized in muscle and that these antibodies impart neutralizing activity to the sera of vector recipients. Moreover, after a single vector administration, neutralizing activity in serum was maintained for the 6-month duration of this study.
This new approach to serum antibody generation was made possible by the marriage of two distinct technologies: antibody gene cloning and rAAV vectors. In the first case, we exploited the ability to select and clone naturally occurring antibodies with predetermined specificity (20). This technology has been used to generate many human and nonhuman monoclonal antibodies with interesting and useful properties, and this is especially true for antibodies with activity against HIV-1 such as IgG1b12 (9). Although human monoclonal antibodies can be produced in large quantities, it is impractical to frequently administer such antibodies to large populations. Thus, it seemed reasonable to consider how one might use an antibody gene, rather than the protein itself, to allow for sustained serum activity. Although antibody gene transfer via ex vivo retroviral vector transduction of myoblasts has been previously reported (33), we were interested in a more direct approach, and rAAV vectors seemed like an ideal candidate to achieve this goal. Multiple studies have demonstrated long-term (>1.5 years) rAAV-mediated transgene expression in muscle (13, 22, 42). Moreover, because muscle is highly vascularized, rAAV transduction has resulted in the appearance of transgene products into the systemic circulation after intramuscular injection (21, 27, 31). Our data, as well as that of Noel et al. (33), confirm that skeletal myofibers possess the necessary cellular factors for correct antibody glycosylation, folding, and secretion.
As one looks forward to the practical application of these concepts, several issues remain to be addressed. One major concern is the antibody concentration in serum that can be achieved by this approach. In the present study, IgG1b12 peak antibody levels in serum were typically between 4 and 5 μg/ml at 5 months postinjection. Although it is uncertain what concentration of neutralizing antibodies would be necessary to provide benefit in humans, the results of passive transfer studies with the rhesus macaque model suggest that “sterilizing” protection against experimental SHIV challenge requires >99% neutralization in vitro (35, 36). This level of in vitro neutralization corresponds to neutralizing antibody titers in serum in excess of 1:100 (35, 36) for a single anti-HIV-1 monoclonal antibody and a concentration of ∼200 μg of IgG1b12/ml in rhesus macaque serum (36). Although we have not achieved these levels to date, the extant human monoclonal neutralizing antibodies (IgG1b12, 2F5, 2G12, Z13, and 4E10) have demonstrated synergy when combined together (23, 24, 43), achieving greater neutralization than simply the sum of their individual potencies. Triple or quadruple monoclonal antibody combinations increased the apparent neutralization titers ca. 10-fold over that predicted if the response were simply additive (43). Synergy is believed to be due in part to recognition of different envelope surface epitopes by the various antibodies (23, 24). Thus, the introduction of an rAAV cocktail encoding several monoclonal antibodies might be one strategy to reduce the total amount of antibody needed to observe a protective in vivo effect.
In addition to the cocktail-synergy approach, additional refinements should result in higher in vivo antibody activity. First, higher vector dosing can be explored, and it is noteworthy that intramuscular delivery of ∼1013 rAAV particles encoding the human α1 anti-trypsin gene resulted in hAAT concentrations in mouse serum of between 400 and 800 μg/ml (39). Second, the use of single-chain antibody (scFv) or Fab derivatives might allow for more efficient secretion from muscle. Also, it seems likely that additional human monoclonal antibodies reactive with HIV will be forthcoming. Third, alternative AAV serotypes (e.g., AAV-1 and AAV-5) appear to transduce skeletal myocytes more efficiently than AAV-2 vectors and represent another potential enhancement (11, 18). Finally, even if “sterilizing” levels of neutralizing MAb are never attained, “substerilizing” levels have lowered viral load setpoints and maintained CD4+ cell counts in infected rhesus macaques (25). Thus, even modest levels of preexisting, neutralizing antibodies might have a measurable and meaningful effect on the course of HIV infection. Ultimately, our goal is to combine this approach for generating neutralizing antibodies in serum with a vaccine that elicits a robust antigen-specific CD8+ T-cell response.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases.
We thank the CCRI Viral Vector Core Laboratory and Maera Flynn, Min Xia, David McNeely, and Florinda Jaynes for expert technical assistance. We also thank Dennis Burton and Paul Parren for the gift of IgG1b12.
REFERENCES
- 1.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger J.D. Altman, S. L. Lydy S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Baba,T.W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection at moderate serum levels. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 8.Burton, D. R., C. F. D. Barbas, M. A. Persson, S. Koenig, R. M. Chanock, and R. A. Lerner. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 88:10134-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, and P. L. Nara. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 10.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 11.Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. [DOI] [PubMed] [Google Scholar]
- 12.Clark, K. R., X. Liu, J. P. McGrath, and P. R. Johnson. 1999. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum. Gene Ther. 10:1031-1039. [DOI] [PubMed] [Google Scholar]
- 13.Clark, K. R., T. J. Sferra, and P. R. Johnson. 1997. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum. Gene Ther. 8:659-669. [DOI] [PubMed] [Google Scholar]
- 14.Clark, K. R., F. Voulgaropoulou, D. M. Fraley, and P. R. Johnson. 1995. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 6:1329-1341. [DOI] [PubMed] [Google Scholar]
- 15.Clark, K. R., F. Voulgaropoulou, P. R. Johnson. 1996. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 3:1124-1132. [PubMed] [Google Scholar]
- 16.Conley, A. J., J. A. Kessler, I. I., L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark III, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. H. I. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan, D., Z. Yan, Y. Yue, W. Ding, and J. F. Engelhardt. 2001. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. J. Virol. 75:7662-7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emini, E. A., W. A. Schleif, J. H. Nunberg, A. J. Conley, Y. Eda, S. Tokiyoshi, S. D. Putney, S. Matsushita, K. E. Cobb, C. M. Jett, et al. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728-730. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, A. D., and A. R. Duncan. 1998. Strategies for selection of antibodies by phage display. Curr. Opin. Biotechnol. 9:102-108. [DOI] [PubMed] [Google Scholar]
- 21.Herzog, R. W., J. N. Hagstrom, S. H. Kung, S. J. Tai, J. M. Wilson, K. J. Fisher, and K. A. High. 1997. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl. Acad. Sci. USA 94:5804-5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler, P. D., G. M. Podsakoff, X. Chen, S. A. McQuiston, P. C. Colosi, L. A. Matelis, G. J. Kurtzman, and B. J. Byrne. 1996. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 93:14082-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, A., T. W. Baba, J. Sodroski, S. Zolla-Pazner, M. K. Gorny, J. Robinson, M. R. Posner, H. Katinger, C. F. Barbas, D. R. Burton, T. C. Chou, and R. M. Ruprecht. 1997. Synergistic neutralization of a chimeric SIV/HIV type 1 virus with combinations of human anti-HIV type 1 envelope monoclonal antibodies or hyperimmune globulins. AIDS Res. Hum. Retrovir. 20:647-656. [DOI] [PubMed] [Google Scholar]
- 24.Li, A., H. Katinger, M. R. Posner, L. Cavacini, S. Zolla-Pazner, M. K. Gorny, J. Sodroski, T. C. Chou, T. W. Baba, and R. M. Ruprecht. 1998. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J. Virol. 72:3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S.Frankel, D. L. Birx, and M G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 27.Monahan, P. E., R. J. Samulski, J. Tazelaar, X. Xiao, T. C. Nichols, D. A. Bellinger, M. S. Read, and C. E. Walsh. 1998. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 5:40-49. [DOI] [PubMed] [Google Scholar]
- 28.Monahan, P. E., and R. J. Samulski. 2000. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol. Med. Today 6:433-440. [DOI] [PubMed] [Google Scholar]
- 29.Montefiori, D. C., W. E. Robinson, Jr., S. S. Schuffman, and W. M. Mitchell. 1988. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J. Clin. Microbiol. 26:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore, J. P., P. W. Parren, and D. R. J. Burton. 2001. Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J. Virol. 75:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, J. E., S. Zhou, K. Giese, L. T. Willaims, J. A. Escobedo, and V. J. Dwarki. 1997. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc. Natl. Acad. Sci. USA 94:13921-13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathanson, N., and B. J. Mathieson. 2000. Biological considerations in the development of a human immunodeficiency virus vaccine. J. Infect. Dis. 182:579-589. [DOI] [PubMed] [Google Scholar]
- 33.Noel, D., M. Pelegrin, M. Marin, M. Biard-Piechaczyk, J. C. Ourlin, J. C. Mani, and M. Piechaczyk. 1997. In vitro and in vivo secretion of cloned antibodies by genetically modified myogenic cells. Hum. Gene Ther. 8:1219-1229. [DOI] [PubMed] [Google Scholar]
- 34.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed] [Google Scholar]
- 35.Parren, P. W., M. C. Gauduin, R. A. Koup, P. Poignard, P. Fisicaro, D. R. Burton, and Q. J. Sattentau. 1997. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol. Lett. 57:105-112. [DOI] [PubMed] [Google Scholar]
- 36.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poignard, P., E. O. Saphire, P. W. H. Parren, and D. R. Burton. 2001. GP120: biologic aspects of structural features. Annu. Rev. Immunol. 19:253-274. [DOI] [PubMed] [Google Scholar]
- 38.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 39.Song, S., M. Morgan, T. Ellis, A. Poirier, K. Chesnut, J. Wang, M. Brantly, N. Muzyczka, B. J. Byrne, M. Atkinson, and T. R. Flotte. 1998. Sustained secretion of human α1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA 95:14384-14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian, K. N., L. E. Weisman, T. Rhodes, R. Ariagno, P. J. Sanchez, J. Steichen, L. B. Givner, T. L. Jennings, F. H. Top, Jr., D. Carlin, and E. Connor. 1998. Safety, tolerance, and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr. Infect. Dis. J. 17:110-115. [DOI] [PubMed] [Google Scholar]
- 41.Vujovic, O., and J. Mills. 2001. Preventive and therapeutic strategies for respiratory syncytial virus infection. J. Curr. Opin. Pharmacol. 1:497-503. [DOI] [PubMed] [Google Scholar]
- 42.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]