Abstract
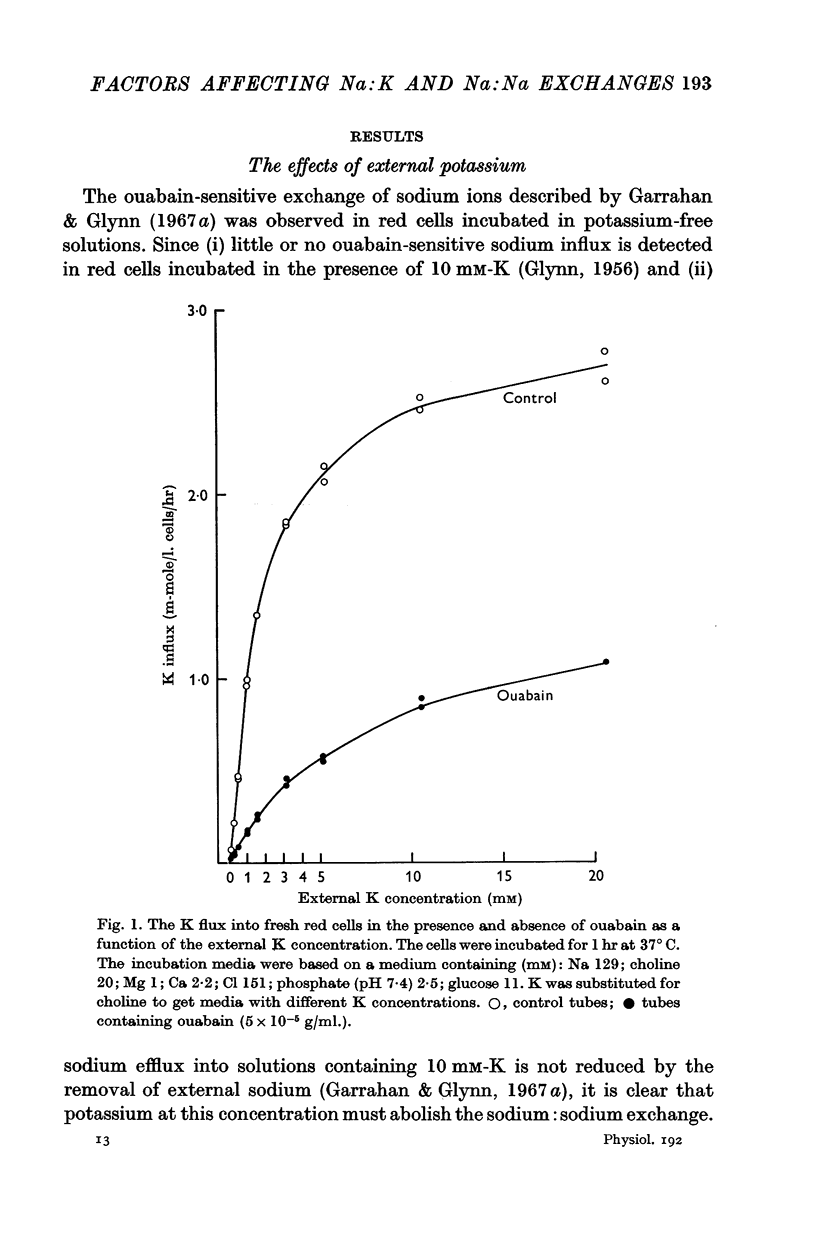
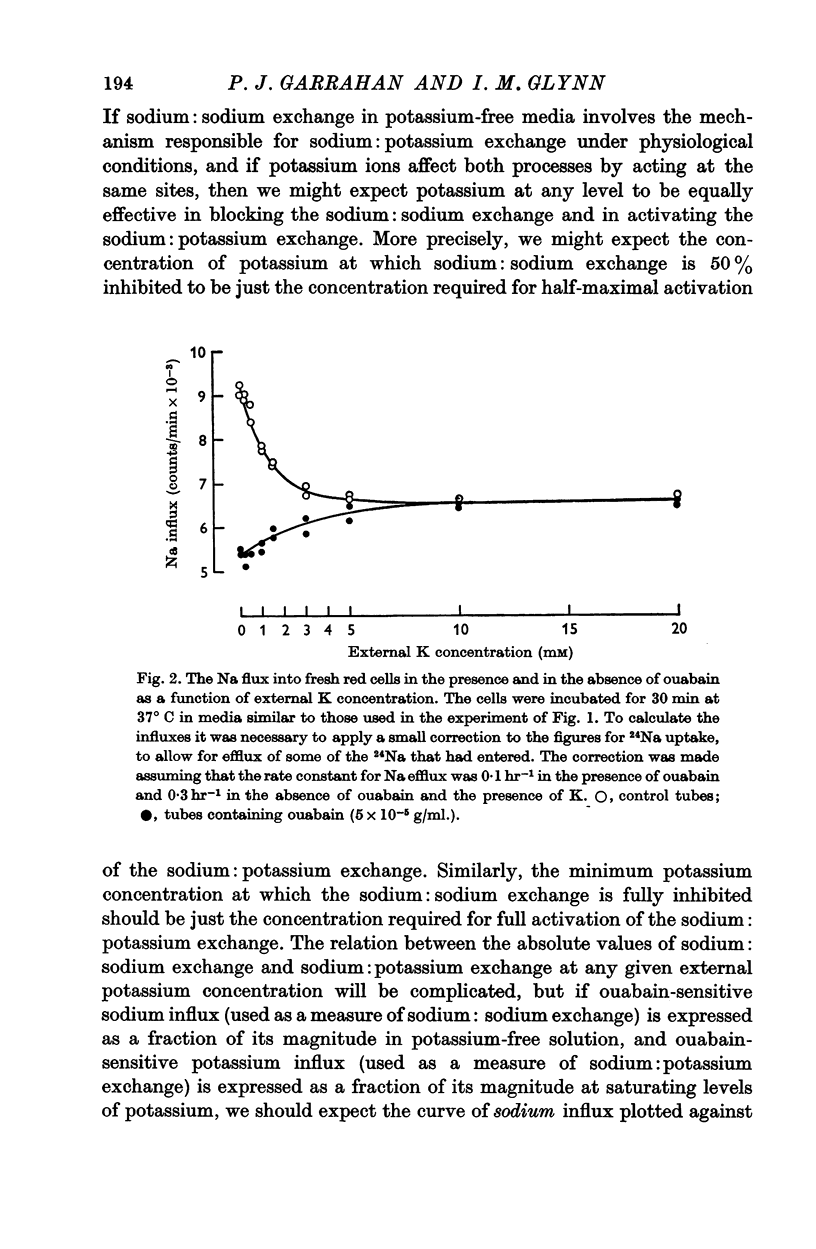
1. The effects of external potassium on sodium: potassium exchange and sodium: sodium exchange in human red cells have been estimated from measurements of ouabain-sensitive potassium influx and ouabain-sensitive sodium influx in media containing different concentrations of potassium.
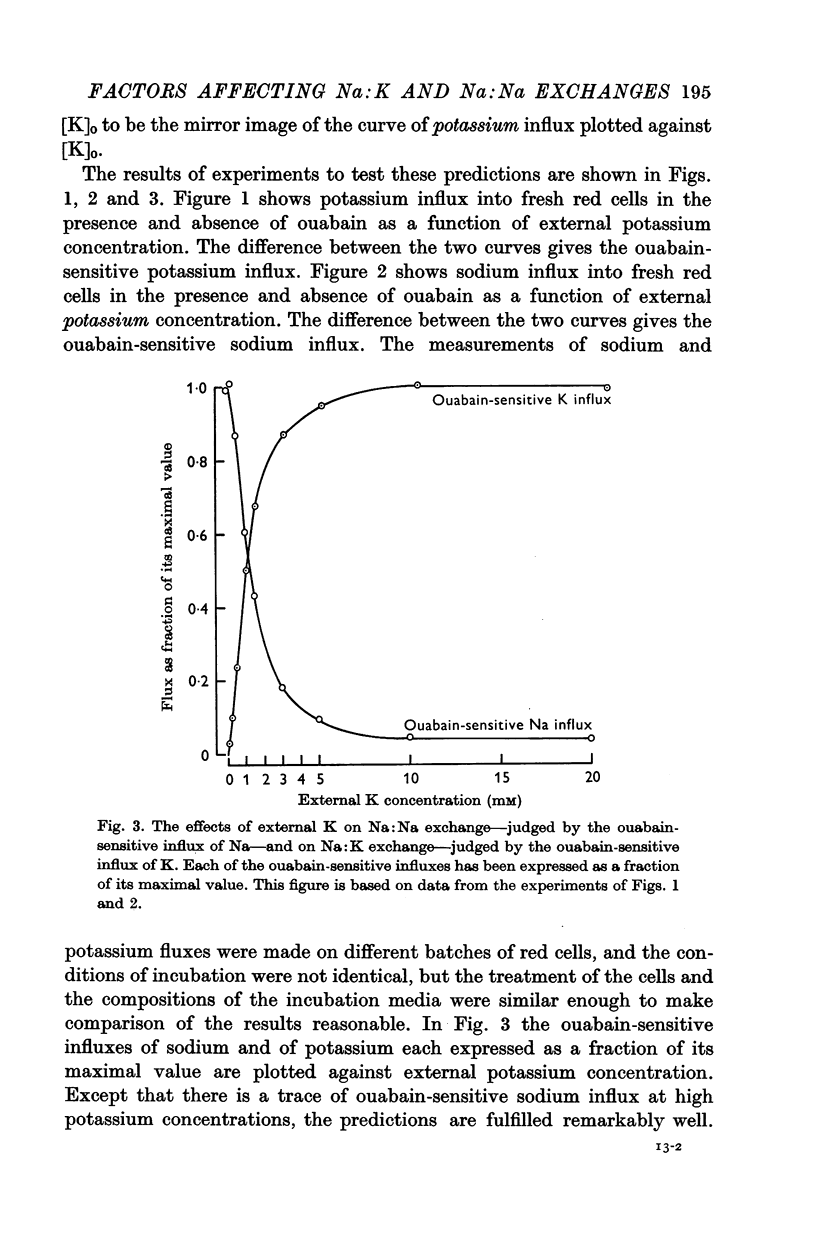
2. As the external potassium concentration is increased from zero to 5 mM, sodium:sodium exchange—as judged by ouabain-sensitive sodium influx—is progressively suppressed, and sodium:potassium exchange—as judged by ouabain-sensitive potassium influx—is progressively increased. Both exchanges are half-maximal between 1 and 2 mM-K, and at 5 mM-K sodium: sodium exchange becomes very small as sodium: potassium exchange approaches a maximum.
3. Experiments have been carried out, mainly on resealed ghosts, to determine what factors affect the magnitude of the sodium:sodium exchange in potassium-free solutions.
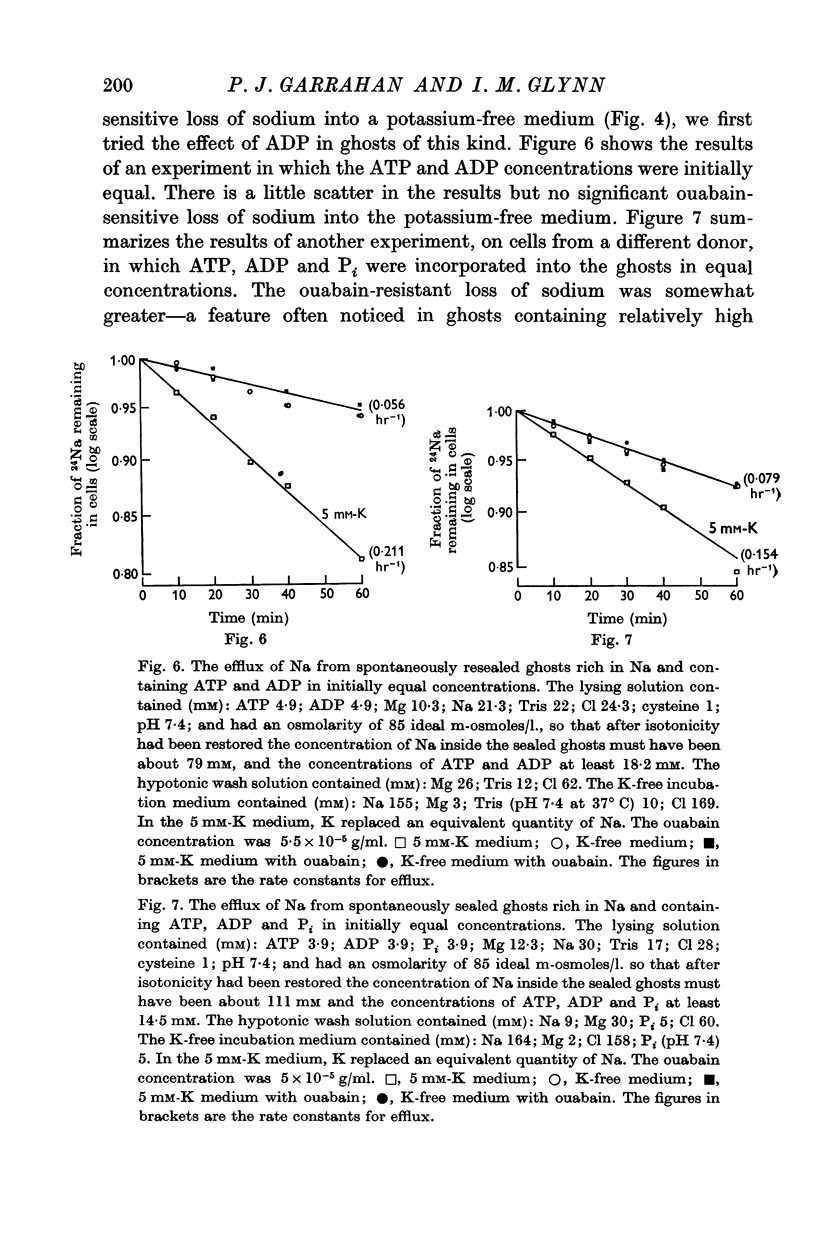
4. Sodium:sodium exchange does not occur in the absence of adenosine triphosphate (ATP).
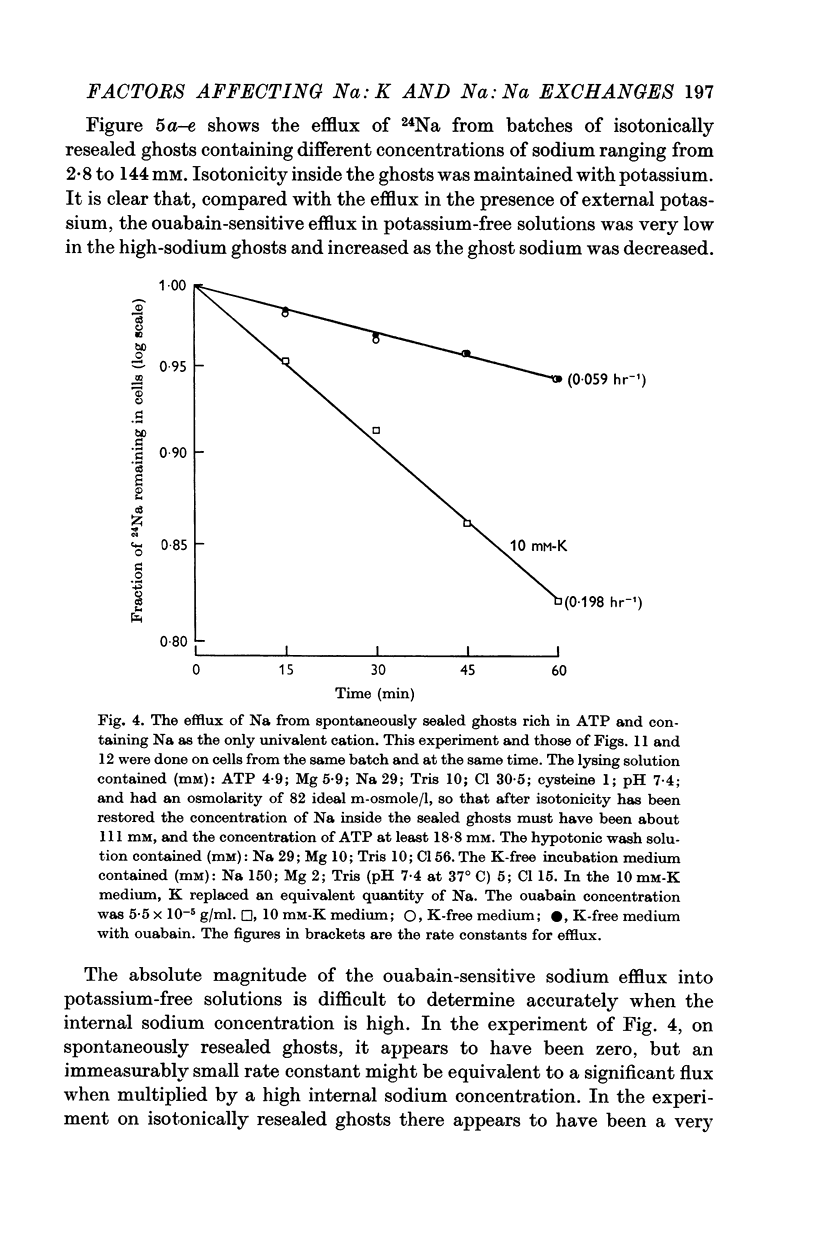
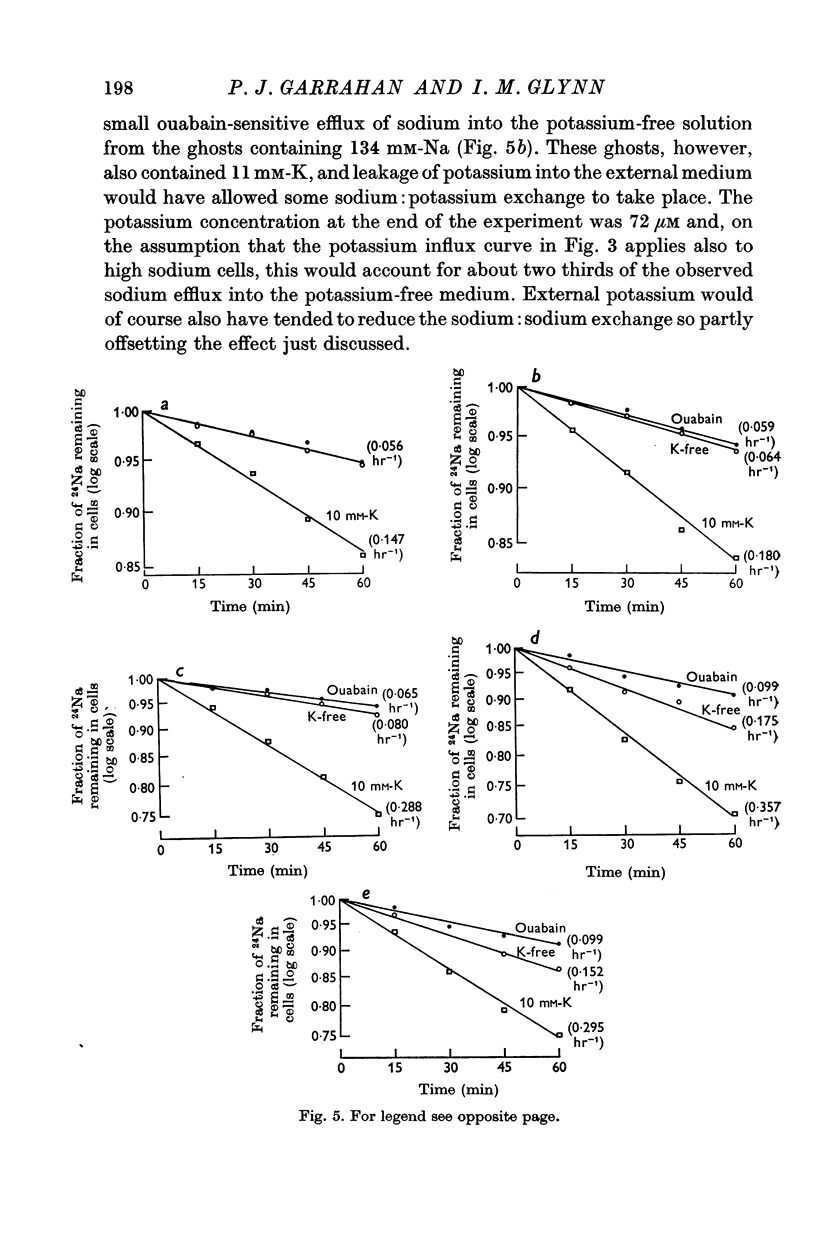
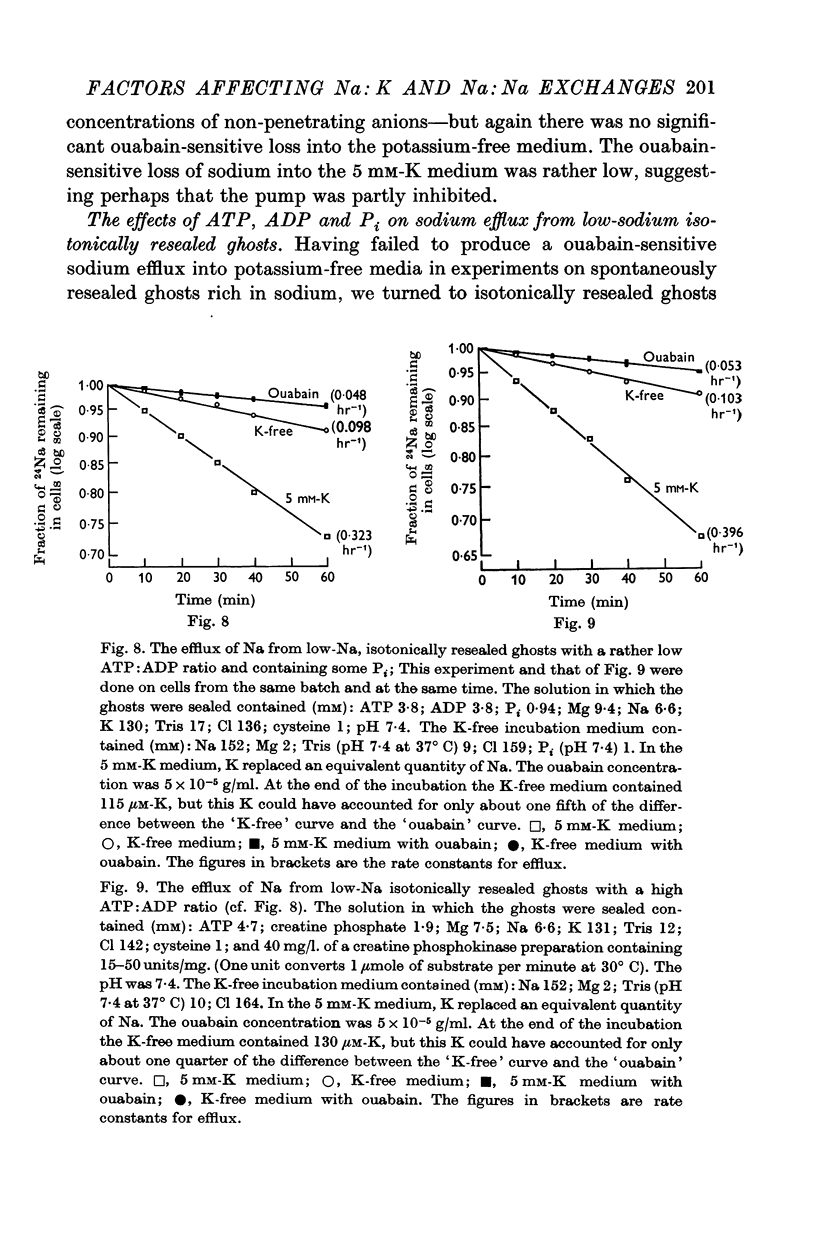
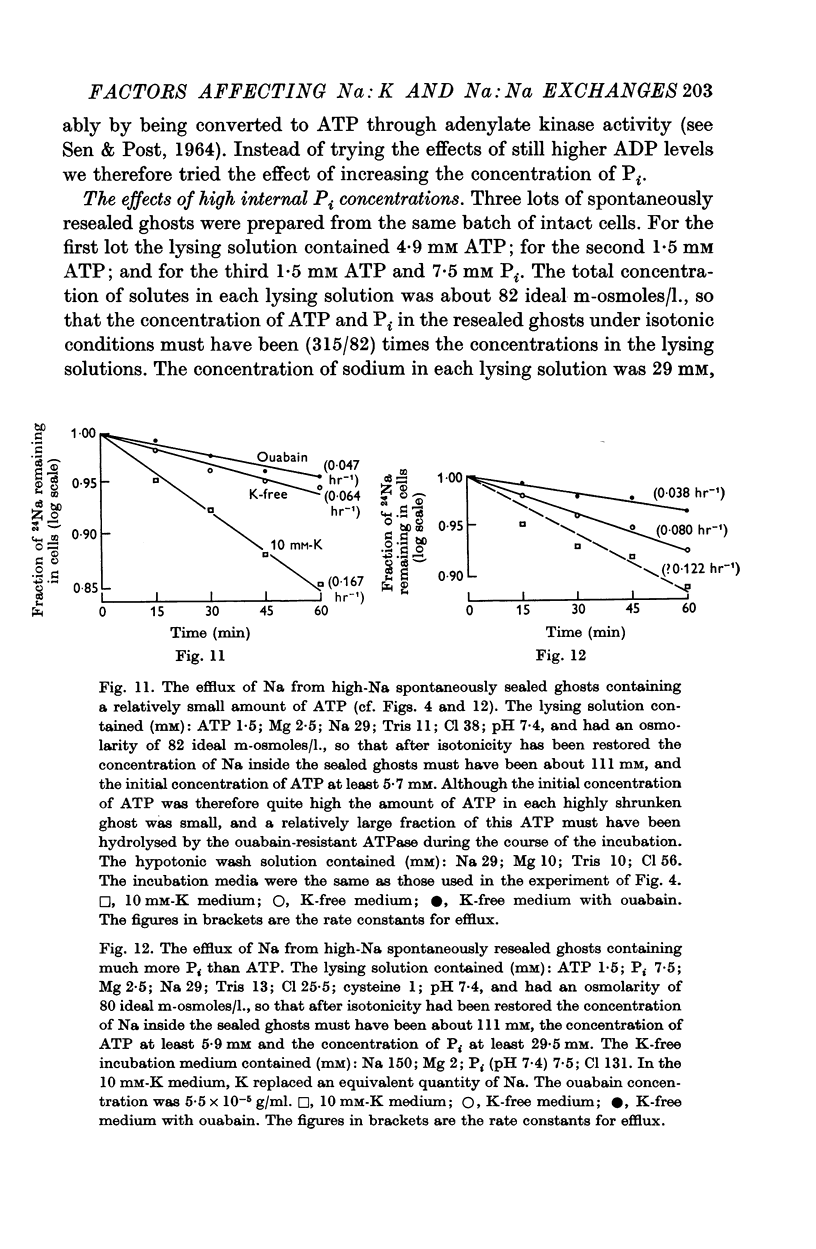
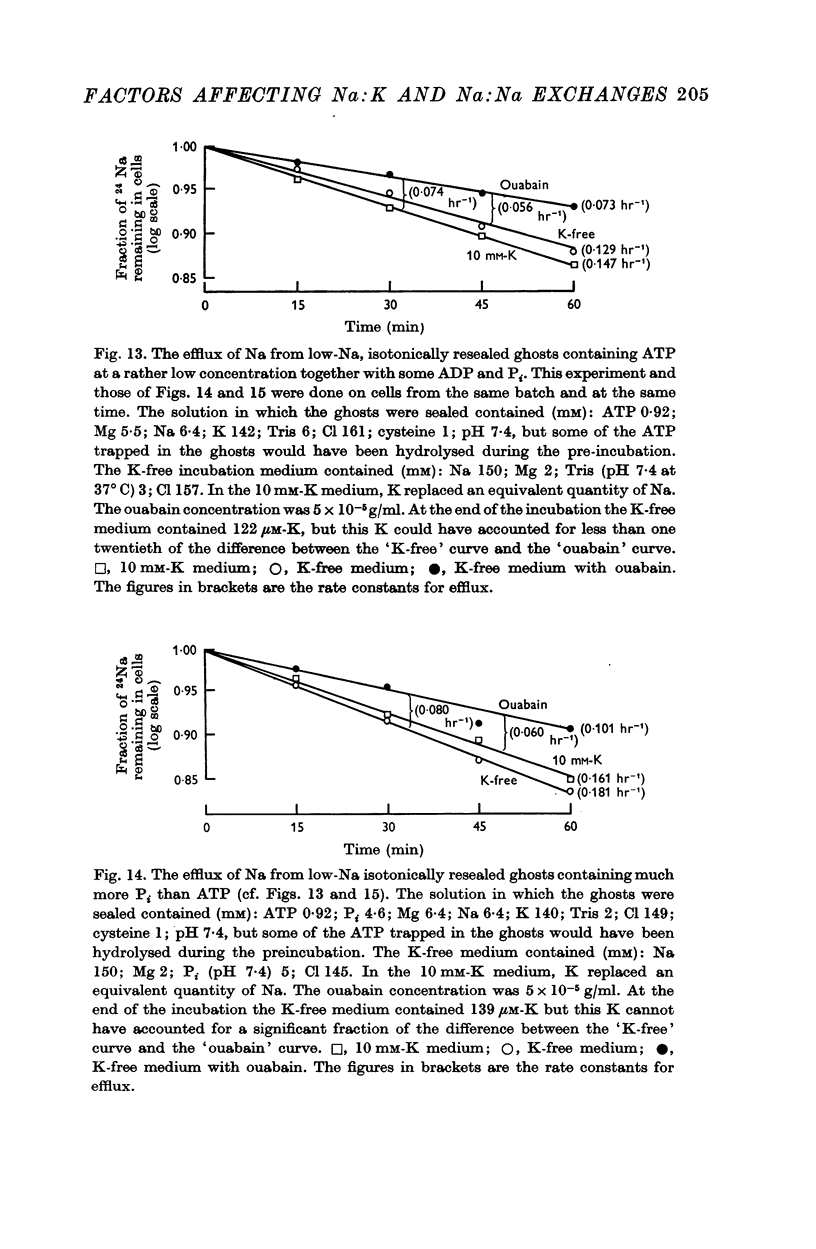
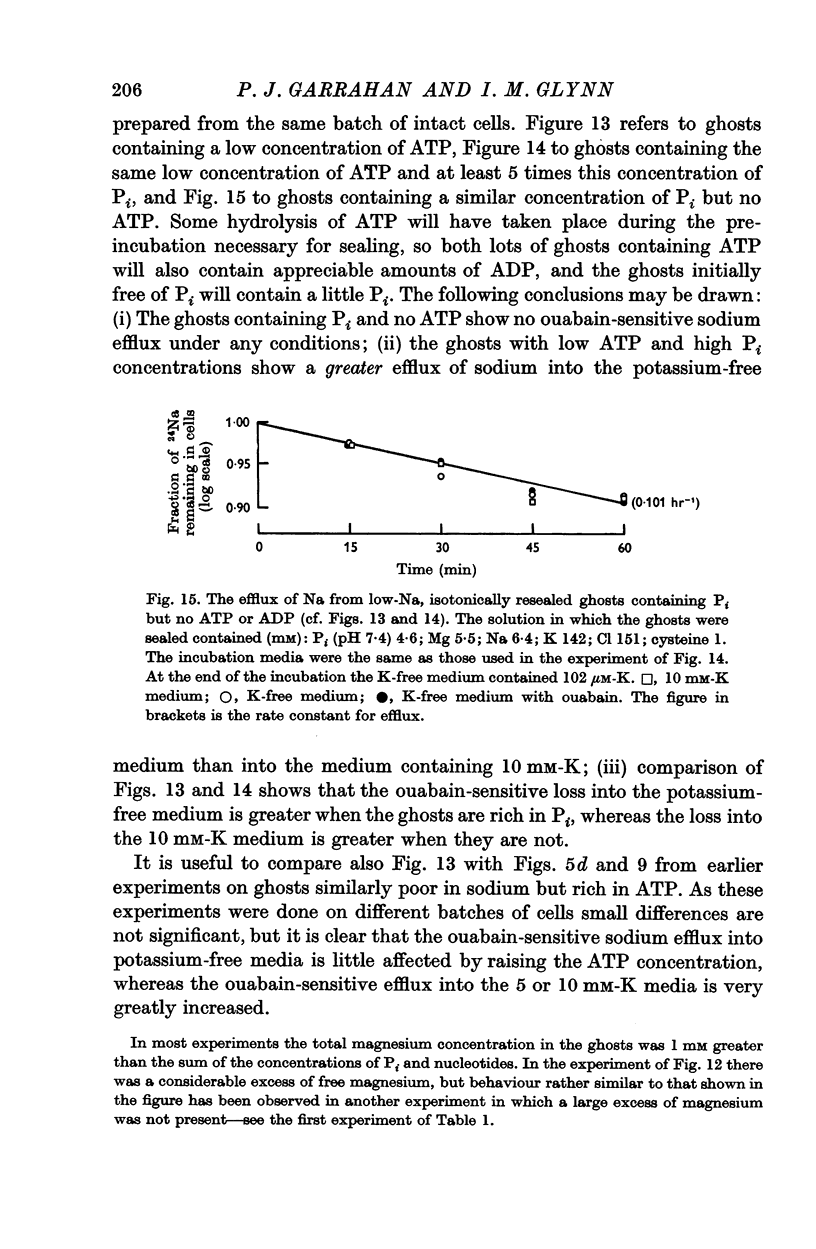
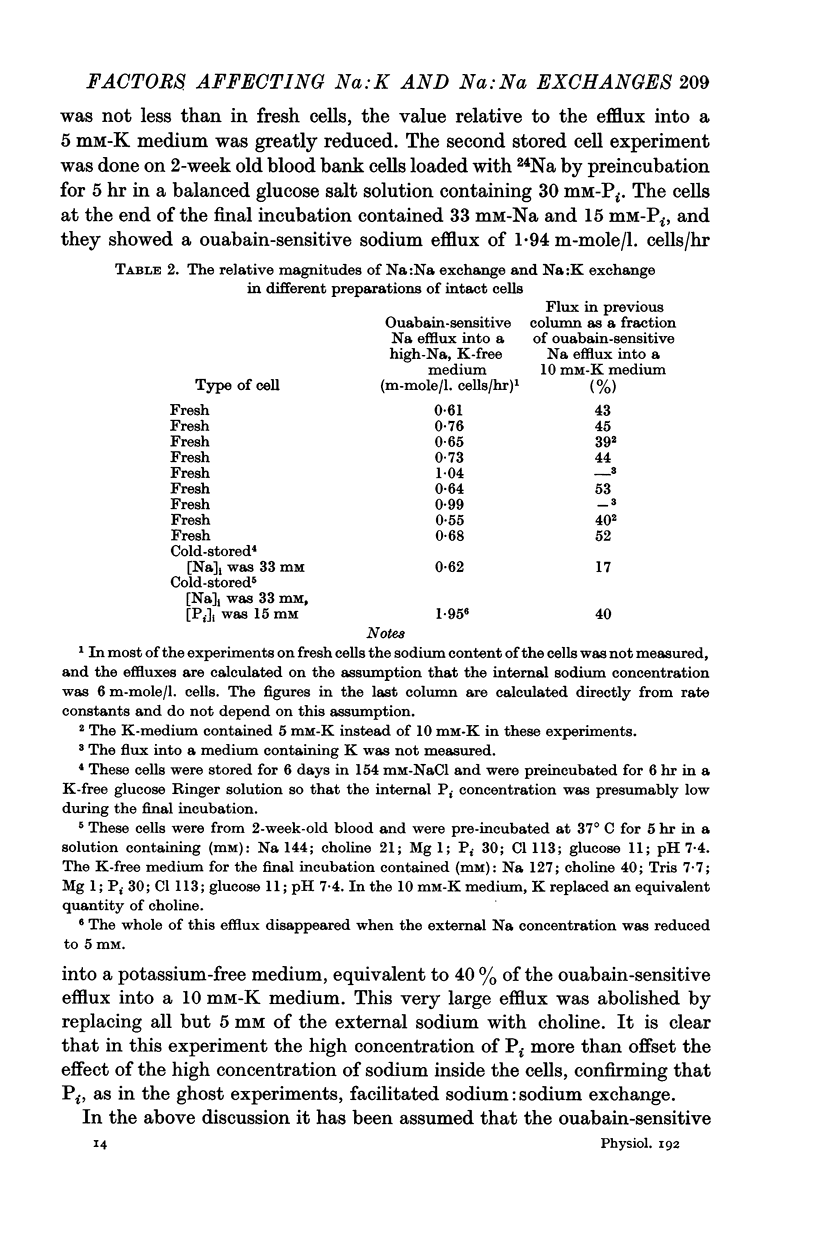
5. Ghosts containing high concentrations of sodium, no potassium and high concentrations of ATP show no ouabain-sensitive loss of sodium into potassium-free solutions. The ability to carry out sodium:sodium exchange can be restored by replacing most of the internal sodium with potassium or by preparing the cells so that they contain much more orthophosphate (Pi) than ATP.
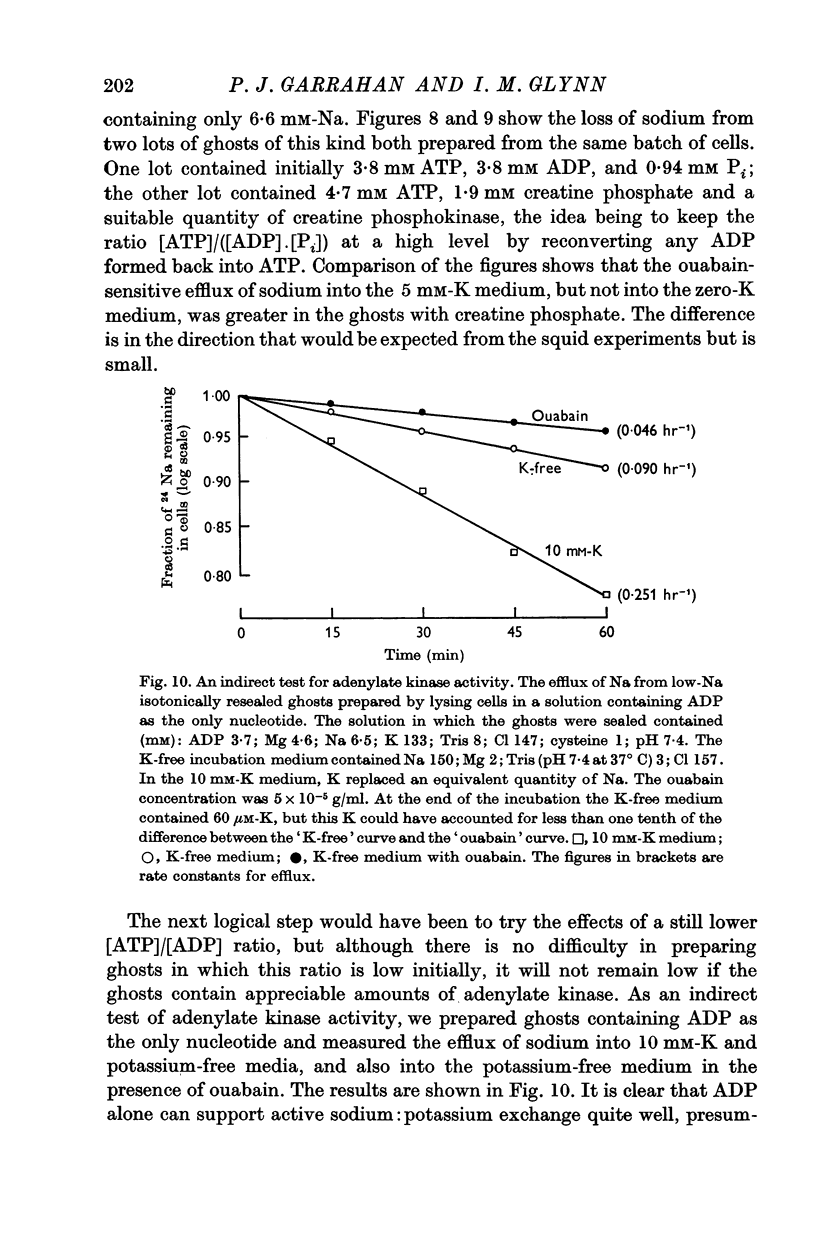
6. Ghosts containing sodium in low concentration, potassium in high concentration and with a low [ATP]/([ADP].[Pi]) ratio show a greater ouabain-sensitive loss of sodium into potassium-free media than into media containing potassium; i.e. external potassium reduces ouabain-sensitive sodium efflux.
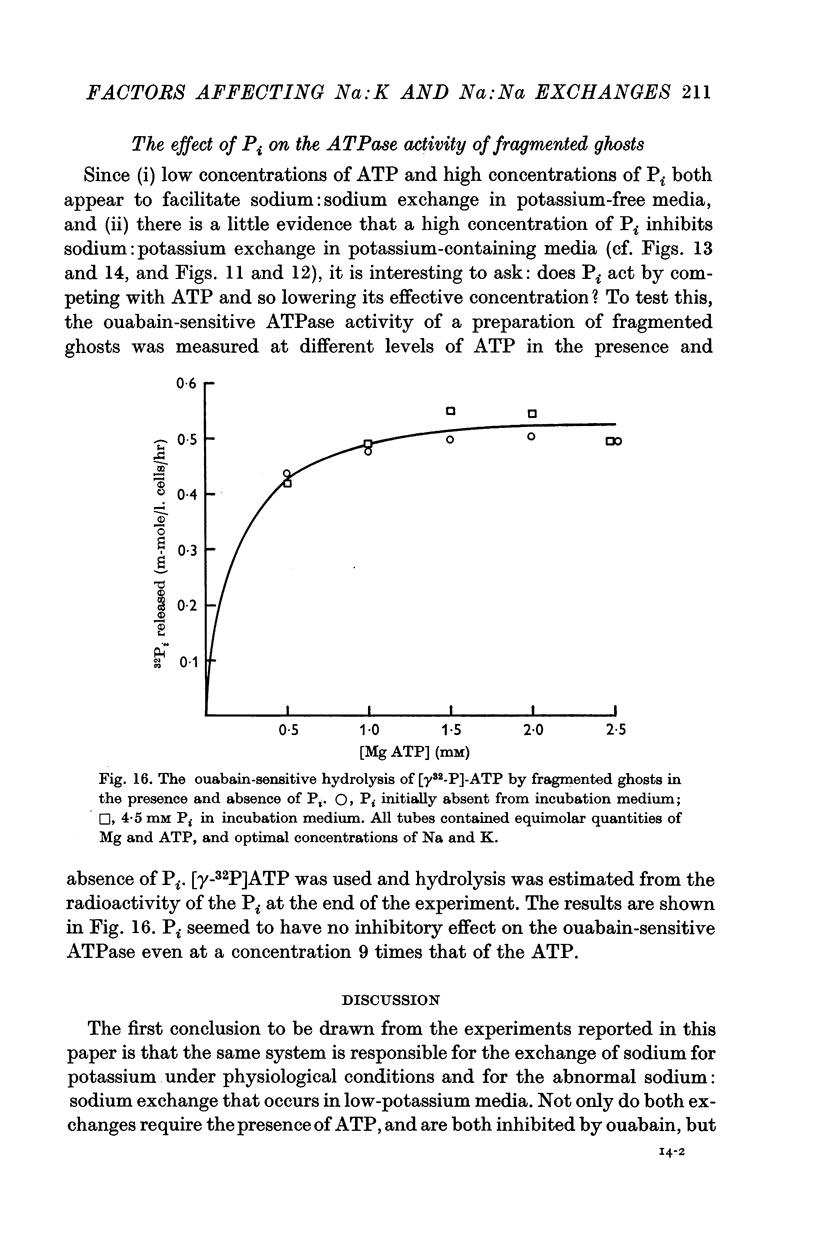
7. The effect of Pi is not the result of competitive inhibition of the transport ATPase since Pi at the concentrations used does not inhibit ATPase activity in fragmented ghosts.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. Partial inhibition of the active transport of cations in the giant axons of Loligo. J Physiol. 1960 Jul;152:591–600. doi: 10.1113/jphysiol.1960.sp006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. THE RATE OF FORMATION AND TURNOVER OF PHOSPHORUS COMPOUNDS IN SQUID GIANT AXONS. J Physiol. 1964 May;171:119–131. doi: 10.1113/jphysiol.1964.sp007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S., MULLINS L. J. POTASSIUM-FREE EFFECT IN SQUID AXONS. Nature. 1964 Dec 26;204:1312–1313. doi: 10.1038/2041312b0. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Uncoupling the sodium pump. Nature. 1965 Sep 4;207(5001):1098–1099. doi: 10.1038/2071098a0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lüthli U. Can the later stages of the 'transport ATPase' system be reversed independently of the earlier stages? J Physiol. 1967 Jul;191(2):104P–105P. [PubMed] [Google Scholar]
- HOFFMAN J. F., TOSTESON D. C., WHITTAM R. Retention of potassium by human erythrocyte ghosts. Nature. 1960 Jan 16;185:186–187. doi: 10.1038/185186a0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. Potassium movements and ATP in human red cells. J Physiol. 1958 Mar 11;140(3):479–497. [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Wiley J. S. Potassium transport and nucleoside metabolism in human red cells. J Physiol. 1967 Aug;191(3):633–652. doi: 10.1113/jphysiol.1967.sp008272. [DOI] [PMC free article] [PubMed] [Google Scholar]


