Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. The latency-associated nuclear antigen (LANA) is a multifunctional protein that is consistently expressed in all KSHV-associated malignancies. LANA interacts with a variety of cellular proteins, including the transcriptional cosuppressor complex mSin3 and the tumor suppressors p53 and Rb, thereby regulating viral and cellular gene expression. In addition, LANA is required for maintenance of the episomal viral DNA during latency in dividing cells. Colocalization studies suggest that LANA tethers the viral genome to chromosomes during mitosis. In support of this model, a specific LANA- binding site has recently been identified within the terminal repeat unit, and a chromatin interaction domain was mapped to a short amino acid stretch within the N-terminal domain of LANA. Epstein-Barr virus nuclear antigen 1 (EBNA-1), a functional homologue of LANA, is also required for genome segregation; in addition, EBNA-1 also supports efficient DNA replication of oriP-containing plasmids. By performing short-term replication assays, we demonstrate here for the first time that de novo synthesis of terminal-repeat (TR)-containing plasmids is highly dependent on the presence of LANA. We map the required cis-acting sequences within the TR to a 79-bp region and demonstrate that the DNA-binding domain of LANA is required for this DNA replication activity. Surprisingly, the 233-amino-acid C domain of LANA by itself partially supports replication. Our data show that LANA is a sequence-specific DNA-binding protein that, like EBNA-1, plays an important role in DNA replication and genome segregation. In addition, we show that all necessary cis elements for the origin of replication (ori) function are located within a single TR, suggesting that the putative ori of KSHV is different from those of other gammaherpesviruses, which all contain ori sequences within the unique long sequence outside of their TR. This notion is further strengthened by the unique modular structure of the KSHV TR element.
Kaposi's sarcoma-associated herpesvirus (KSHV), also called human herpesvirus 8, is associated with Kaposi's sarcoma (KS) and two lymphoproliferative diseases: primary effusion lymphomas (PEL) and a plasmablastic variant of multicentric Castleman's disease (for a review, see reference 16). Most cells in these tumors are latently infected with KSHV and express only a subset of viral genes (6, 54, 61). One of these gene products, the latency-associated nuclear antigen (LANA) is shown to be highly expressed in all tumor tissues by in situ hybridization techniques and immunohistochemical analysis (13, 29, 30, 46). LANA, encoded by ORF73, is expressed from a bicistronic mRNA located in the major latency-associated region of KSHV. LANA plays a pivotal role during latency in KSHV-infected cells. First, LANA is required for long-term maintenance of viral episomal DNA in dividing cells (3, 4). Second, LANA has been shown to modulate the cellular transcription program in KSHV-infected cells by physically interacting with a variety of host cellular proteins, including transcriptional cosupressors such as mSin3 and the tumor suppressors pRb and p53. p53 and pRb interactions have functional consequences in downregulating p53-dependent transcription and upregulating E2F-dependent transcription, respectively (15, 32, 45). Furthermore, a variety of cellular and viral promoters have been shown to be responsive to the presence of LANA by either transient-transfection assays or by gene array analysis (27, 28, 47).
Based on the primary amino acid sequence, LANA can be subdivided in three major protein domains. The N-terminal proline-rich region contains a nuclear localization signal (NLS) and a sequence motif that tethers LANA to chromosomes during mitosis (44). The central domain contains three different highly repetitive blocks of acidic amino acids. The core acidic block varies considerably in length from isolate to isolate, giving rise to LANA proteins of less than 1,000 to 1,163 amino acids (aa) (17). The acidic region close to the C-terminal domain contains a leucine zipper motif that has been demonstrated to be necessary for LANA's interaction with cellular proteins (p53, pRb, and CREB/ATF) (15, 39, 45). Recently, we demonstrated that the C terminus of LANA (aa 770 to 1003) encodes a sequence-specific DNA-binding domain that binds to a 18-bp imperfect palindrome within the terminal repeat (TR) of KSHV (19). Based on electrophoretic mobility shift analysis (EMSA), this binding site has been identified by several laboratories by using different protein sources and supershift controls to confirm the presence of LANA in specific complexes (3, 19). Additional binding sites outside of the TR have recently been reported; however, these LANA complexes varied in size and were not further confirmed (8). In support of a palindromic LANA-binding site, Schwam et al. previously demonstrated that LANA forms dimers in solution and that the C domain alone is sufficient for efficient dimerization (51). In addition to LANA's ability to modulate transcription by protein-protein interactions, LANA has also been shown to suppress transcription when bound to DNA. When fused to a Gal4 DNA-binding domain, LANA can suppress transcription of a Gal4-binding site-containing promoter (32, 51). We have recently shown that LANA inhibits transcription when bound to its native binding site within TR (19).
In summary, all of these observations suggest that LANA is a functional homologue to other DNA tumor virus replication or transcription proteins, such as Epstein-Barr virus nuclear antigen 1 (EBNA-1) or the E1/E2 proteins of papillomaviruses. Members of this protein family, including the simian virus 40 (SV40) large T antigen are multifunctional polypeptides that affect transcription of cellular and viral genes as well as replication and segregation of viral genomes in infected cells (14, 36, 55). Thus far, many studies have addressed LANA's ability to modulate transcription. Ballestas et al. demonstrated that LANA is necessary and sufficient to support long-term episomal maintenance of TR-containing plasmids in dividing cells. These data, together with studies from Piollot et al., suggest a model by which LANA ensures faithful segregation by tethering viral episomes to chromosomes during mitosis. However, replication of viral DNA during latency can be divided into two steps: (i) DNA synthesis and (ii) segregation of newly synthesized viral genomes (for a review, see reference 36). By utilizing short-term replication assays in epithelial and endothelial cell lines, we demonstrate that LANA is required for viral DNA synthesis and provide a detailed analysis of the trans and cis requirements for LANA-dependent DNA replication.
MATERIALS AND METHODS
Plasmids.
pCRII/TR containing one copy of the TR element has previously been described (19). pCRII/2TR contains two copies of the TR element in the same orientation, inserted into pCRII into the NotI site. Probes TR1, TR2, and TR3 were gifts from Mike Lagunoff and have been described earlier, as were the LANA expression plasmids pcDNA3/ORF73 and the deletion mutants A, AB, AC, BC, and C (19, 47). pJH1 (termed pTRΔ1) contains TR nucleotides (nt) 1 to 581 (nucleotide numbers are as defined in reference 35) and was constructed by digestion of pCRII/TR with SrfI and XhoI, followed by Klenow filling in and self-ligation. pJH2, termed pTRΔ3 (nt 268 to 801), and pJH4, termed pTR2 (nt 268 to 581), were derived from plasmids pCRII/TR and pJH1, respectively, by deletion of the TR3 fragment with AscI and EcoRV, Klenow filling in, and self-ligation. pJH5 (pTRΔLBS [nt 1 to 559]) was created by Bsu36I and XhoI double digestion of pJH1. The resultant 4.5-kb fragment was filled in by Klenow and then ligated. pJH8, termed pTRΔ3/DraIII (nt 391 to 801), was constructed by partial digestion of pJH2 with DraIII, followed by complete digestion with KpnI, and then blunt ended with T4 DNA polymerase and self-ligated. pJH9, termed pTRΔ3/SanD III (nt 509 to 801), was constructed by double digestion of pJH2 with SanDI and EcoRI, treated with mung bean nuclease (NEB), and self-ligated. Plasmid pZ6Δ contains a BamHI fragment from the cosmid Z6 from the PEL cell line BC-1 (Russo). pJH6, derived from pZ6Δ by SrfI digestion and religation, contains one copy of terminal repeat and a flanking unique KSHV sequence (600 bp on one side and 2.6 kbp, including the K1 gene, respectively). pJH13 (pUL0.6) contains the 600-bp fragment and was recovered from pZ6Δ by NotI and inserted into SuperCos vector (Invitrogen); pJH14 (pUL1.9) containing 1.9-kbp unique long sequence from the left end of the KSHV genome was generated by inserting a BsrGI/NotI fragment into SuperCos (Invitrogen).
Cell lines.
293 cells, human embryonic kidney cells, and SLK cells, a KS-derived KSHV-negative human endothelial cell line (25), were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and antibiotics at 37°C under a 5% CO2 atmosphere.
Short-term replication assays.
293 cells were plated at a density of 3 × 106 cells per 10-cm dish. Plasmids were introduced into cells by the calcium phosphate transfection system (Gibco-BRL) according to the manufacturer's instructions. The transfection efficiency was monitored by a parallel transfection of pcDNA3/LacZ. To exclude nontransfected DNA from the analysis, cells were treated with trypsin, washed three times 16 h posttransfection, and seeded into two plates.
Transfected cells were harvested 72 h posttransfection. Cells were lysed in 700 μl of lysis buffer (10 mM Tris, 10 mM EDTA, 0.6% sodium dodecyl sulfate). Chromosomal DNA was precipitated at 4°C overnight by adding 5 M NaCl to a final concentration of 0.85 M. Cell lysates were centrifuged at 4°C at 14,000 rpm for 30 min. The supernatant containing extrachromosomal DNA was subjected to phenol-chloroform extraction. The extrachromosomal DNA was precipitated by ethanol precipitation and dissolved in 20 μl of H2O, including RNase A. Then, 10% of the DNA was digested with HindIII or KpnI to linearize the plasmid DNA to measure the input DNA by Southern blot analysis. Next, 90% of the DNA was subjected to HindIII (or KpnI) and DpnI digestion in a final volume of 100 μl. A total of 180 U of DpnI was used for digestion at 37°C for 48 h. After digestion, DNA was ethanol precipitated and redissolved in 20 μl of Tris-EDTA buffer. The single-digested and double-digested plasmids were electrophoretically separated in 0.8% agarose gels, transferred to nylon membranes (Amersham), and assayed by Southern blot analysis. Then, 20 ng of an 800-bp fragment of TR or 45 ng of the entire plasmid containing TR fragment were labeled with the random prime labeling system (Amersham Pharmacia Biotech, Ltd.) and purified with QuickSpin columns (Roche). The blots were hybridized in Church buffer at 65°C, washed, exposed to the Molecular Dynamics Phosphor Screen overnight, and analyzed with ImageQuant.
Expression of recombinant mutant LANA proteins by using the MVA/T7 vaccinia virus expression system.
Mutant LANA proteins were all produced by using the vaccinia virus T7 expression system (MVA-T7) (57) to infect CV-1 cells, essentially as described previously (9, 42, 57). Briefly, a confluent 10-cm plate of CV-1 cells was split 1 to 2.5 h prior to infection with MVA-T7 virus at an approximate multiplicity of infection of 10. Cells were transfected at 1 h postinfection with 1 μg of each respective plasmid by using Effectine (Qiagen) according to the manufacturer's instructions. Nuclear protein extractions were performed 24 to 36 h posttransfection as previously described (2, 19).
Western blot analysis.
Protein extracts were electrophoretically separated on sodium dodecyl sulfate-8% polyacrylamide gels. Proteins were transferred to nylon membranes (Amersham). Membranes were blocked for 2 h in Tween-Tris-buffered saline (TTBS) containing 5% dry milk. Primary antibodies were diluted in TTBS, and membranes were incubated for 1 h at room temperature. After being washed the membranes were incubated with secondary antibody for 1 h. The primary antibody was a monoclonal mouse antibody against the V5 epitope tag (Invitrogen, Carlsbad, Calif.). Peroxidase-conjugated secondary antibodies were goat anti-mouse and were diluted 1 to 7,500 prior to incubation. After final washes, Western blots were developed with the Pierce Super Signal detection system and exposed to Kodak film.
EMSA.
Probes were labeled with Klenow fragment by using [α-32P]dCTP (3,000 Ci/mmol; Amersham) according to the manufacturer's instructions (Promega). To purify the probe from nonincorporated nucleotides, we used Sephadex 50 spin columns (Boehringer). TR5 was made as previously described and contained TR sequences from nt 551 to 675. In each reaction, labeled fragment was combined with 0.5 to 1 μg of MVA/T7-infected CV-1 nuclear extract. The protein extracts were incubated at room temperature for 25 min in a total volume of 20 μl of binding buffer which contained 10 mM HEPES (pH 7.9), 50 mM KCl, 1 mM EDTA, 0.05 μg of poly(dI-dC)/μl, 0.5 μg of bovine serum albumin/μl, 10 mM dithiothreitol, and 10% glycerol. The samples were then separated by electrophoresis through a native 4% polyacrylamide gel (55 mA in a room kept at 4°C). Gels were dried and either exposed to Kodak film or analyzed by using a Storm PhosphorImager.
RESULTS
LANA is required for efficient DNA synthesis of TR-containing plasmids in 293 and in SLK cells.
To determine whether LANA plays a role in viral DNA synthesis, we performed transient replication assays, also known as DpnI assays, that represent the standard method for monitoring de novo DNA synthesis in many viral systems (5, 23). Plasmid DNA containing a putative origin of replication (ori) is transfected into cells, and newly synthesized DNA is detected by Southern blot analysis after DpnI digestion 48 to 72 h posttransfection; DpnI cleaves only DNA methylated during replication in a dam+ Escherichia coli strain. Therefore, transfected DNA can be digested, whereas DNA newly synthesized in eukaryotic cells is resistant to DpnI.
We have previously shown that LANA specifically binds to an 18-bp imperfect palindrome within the TR (19). Ballestas and Kaye recently demonstrated that a plasmid containing two TR units is efficiently maintained in long-term replication assays, whereas a plasmid containing a single copy of TR does so with less efficiency (3).
We first prepared a plasmid containing two copies of TR by head-to-tail multimerization of the NotI unit length fragment into pCRII (p2TR) and cotransfected the resulting construct into 293 cells either with filler DNA or with pcDNA3/ORF73. To eliminate extracellular DNA from the analysis, cells were detached from the plates 16 h posttransfection, washed twice with PBS, and seeded into new dishes. Cells were harvested 48 h later, and episomal DNA was extracted by the Hirt protocol (26). DNA was analyzed by digesting 10% of the extracted DNA with a single cutting restriction enzyme to linearize plasmid DNA. The remaining 90% of DNA was digested for 12 h with DpnI in conjunction with a single cutter. After electrophoretic separation and transfer to nylon membranes, DNA was detected by using a radiolabeled probe containing the TR sequence. As can be seen in Fig. 1A, linear p2TR is detectable at nearly similar amounts in the input lanes independent of the presence of LANA (lanes 4 and 5). However, DpnI-resistant DNA is only detectable in the presence of LANA, clearly indicating that LANA supports DNA replication of p2TR in 293 cells (compare lanes 7 and 8). By comparing the amounts of newly synthesized DNA and intracellular plasmid DNA by densitometric analysis in several experiments, we determined that about 3 to 5% of the DNA taken up by the cells replicates in the presence of LANA in 293 cells.
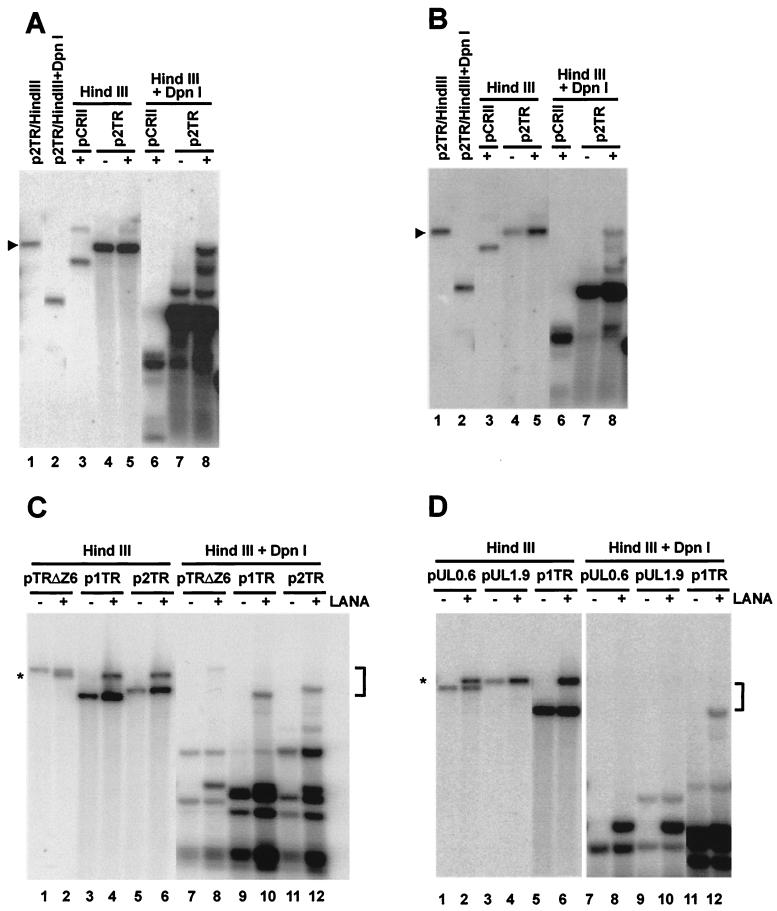
FIG. 1.
LANA supports replication of TR-containing plasmid in 293 and SLK cells in short-term replication assays. A total of 10 μg of p2TR or empty vector pCRII was cotransfected with 10 μg of pcDNA3/ORF73 or carrier DNA into 293 cells (A) or SLK cells (B). At 72 h after transfection, extrachromosomal DNA was extracted as described in Materials and Methods. Then, 10% of the episomal DNA was digested with 20 U of HindIII, whereas 90% was double digested with 20 U of HindIII and 20 U of DpnI for 16 h. After electrophoresis, DNA was immobilized on nylon membranes and hybridized with a radiolabeled TR probe. (A) Replication in 293 cells. Lane 1 contains 150 pg of p2TR digested with HindIII to indicate the position of linear plasmids. Lane 2 contains 150 pg of p2TR digested with HindIII and DpnI. Lanes 3, 4, and 5 show the input of indicated plasmids in the absence or presence of LANA, respectively. pCRII, a plasmid not containing TR sequences, was used as a negative control (lane 6). p2TR can only replicate in the presence of LANA (lane 8) but not without LANA (lane 7). (B) Replication in SLK cells. The gel was loaded in the same order as described for panel A. (C) All cis requirements are located in TR, and p1TR replicates as efficiently as p2TR. A total of 10% of the extracted episomal DNA was digested with 20 U of HindIII (lanes 1 to 6). We observed some partial digestion in panels A and B (lane 8), and therefore Hirt-extracted DNA for all following assays was digested with 100 U of HindIII and 180 U of DpnI for 48 h (lanes 7 to 12). pTRΔZ6, p1TR, and p2TR only replicate in the presence of LANA (lanes 8, 10, and 12, respectively). In contrast, none of them can replicate without LANA (lanes 7, 9, and 11). (D) Unique long sequences adjacent to TR do not contribute to ori activity. pUL0.6 and pUL1.9 do not replicate in the presence of LANA (lanes 8 and 10). The brackets in panels C and D indicate the size range of the replicated plasmids. This blot was hybridized to a probe containing the entire pCRII/TR, which also detects the backbone of pcDNA3/ORF73, marked by asterisks. A triangle (▸) indicates the linear position of p2TR.
KS spindle cells are believed to be of endothelial origin and several reports have shown that upon cultivation ex vivo, tumor-derived cells rapidly lose the KSHV genome (24, 25). This phenomenon could be due to defects in either DNA synthesis or genome segregation in explanted endothelial cells. To test whether cells derived from KS lesions are able to support LANA-dependent DNA synthesis, we cotransfected p2TR alone or in combination with pcDNA3/ORF73 into SLK cells as described above. The SLK cell line, which is negative for KSHV, has been established from a KS lesion and expresses surface markers indicating an endothelial lineage (25). At 72 h posttransfection DpnI-resistant episomal DNA could only be detected in cells cotransfected with LANA (Fig. 1B, lanes 8). Hence, LANA-dependent synthesis of TR-containing plasmids seems to be efficient in epithelial and endothelial cell lines and therefore does not explain why tumor cells lose viral genomes upon ex vivo cultivation. These data show for the first time that LANA, in addition to its role in segregation, plays an active role in supporting DNA replication of TR-containing episomes.
Ballestas et al. had originally reported that a short sequence outside of the TR within cosmid clone Z6 would be necessary for efficient long-term replication (4). In addition, LANA-binding sites have been proposed outside of the TR (8). Based on these observations, we constructed a deletion clone which contains one full copy of the TR plus the adjacent sequences from cosmid clone Z6 (kindly provided by Yuan Chang, Columbia University). In addition to one TR unit, this clone contains a 600-bp unique long sequence on one side and a 2.6-kbp sequence, including one partial TR and the regulatory and coding sequences for K1 (35, 49). This clone, pTRΔZ6, was analyzed in parallel with p2TR in the presence or absence of LANA in 293 cells. We also tested p1TR, a construct containing a single TR unit, to determine whether there is a difference in replication efficiency for one copy of the TR versus two. As shown in Fig. 1C, all three plasmids only replicate in the presence of LANA (lanes 8, 10, and 12). It is important to note that we measured DNA replication within the sensitivity range of Southern blot-based analysis. Therefore, we cannot rule out that a small fraction of TR-containing episomes replicate in the absence of LANA, as was shown by sensitive competitive PCR methods for EBV oriP-containing plasmids (1). The comparison of input and replicated DNA for each construct by densitometry revealed that p1TR replicates at the same efficiency as a plasmid containing two TR units and as pTRΔZ6 containing one copy of TR and sequences outside of the TR unit (the reduced input of this plasmid is due to its much larger size and decreased transfection efficiency). To further rule out that unique long sequences adjacent to the TR units contain redundant ori activity, we constructed two plasmids which contained either a 600-bp (pUL0.6) or a 1.9-kbp (pUL1.9) long fragment from the previously described cosmid Z6. Both of these plasmids did not replicate in the presence of LANA (Fig. 1D, lanes 8 and 10). In summary, these data clearly demonstrate that a single copy of the TR contains all of the elements necessary for efficient viral DNA replication in the presence of LANA and also suggest that the KSHV ori is located within the TR. This finding is remarkably different from what is found with all other gammaherpesviruses. EBV and herpesvirus saimiri also contain TR sequences, but both viruses harbor ori elements within the unique long region of their respective genomes (33, 58, 60). The observation that plasmids containing one or two TR units replicate with the same efficiency in short-term replication assays (Fig. 1C) but have been reported to show differences with respect to long-term maintenance (3) indicates that both processes have different cis requirements.
Mapping of the minimal cis-regulatory sequences within TR that confer LANA-dependent DNA replication.
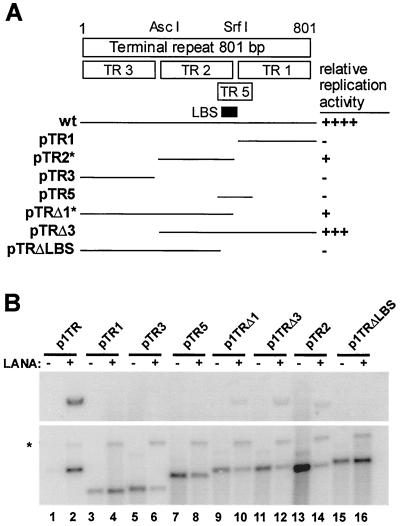
After we established that sequences in the TR are sufficient for LANA-dependent viral DNA replication, we wanted to map the minimal cis-regulatory elements required for ori function. We therefore created a series of deletion constructs and compared their replication efficiency with wild-type (wt) p1TR. The first series of deletion mutants is described in Fig. 2A. The TR unit length is 801 bp, and its GC content is 89%. The previously described plasmids pTR1, pTR2, pTR3, and pTR5 have been instrumental in identifying the LANA-binding site (LBS) that maps within TR5 to nt 571 to 587, partially overlapping TR2 and TR3. As shown in Fig. 2B, pTR1 and pTR3 do not replicate in the presence of LANA to levels detectable by Southern blot analysis. Also, pTR5 containing the LBS and additional sequences on either site does not replicate (Fig. 2B, lanes 4, 6, and 8). In contrast, pTR2, which contains 70% of the LANA binding site sufficient for LANA-binding in EMSA (A. C. Garber and R. Renne, unpublished data), replicates at ca. 20% of wt efficiency (Fig. 2B, lane 14). A construct where only TR3 is deleted replicates with nearly 75% of wt activity (Fig. 2B, lane 12); conversely, a construct with TR1 and the LBS deleted does not replicate (Fig. 2B, lane 16). These data show that the binding of LANA is absolutely required for replication and that, in addition, sequences outside the LANA-binding site are required for oriP function; moreover, these sequences are not located within TR3, which is dispensable for oriP function. The mapping experiments from Fig. 2B, which were repeated with identical results, are qualitatively summarized in Fig. 2A. Interestingly, Cotter et al. have recently suggested an additional LANA-binding site located within TR3 (8). However, this site, which was not confirmed by antibody shift experiments and does not show significant sequence homology to the LBS in TR5, does not contribute to LANA-dependent replication.
FIG. 2.
Mapping of cis requirements for LANA-dependent DNA replication. (A). Illustration of TR unit indicating constructs TR1, -2, -3, and -5, which have been used to identify the LBS within the TR. Below this, the solid lines represent TR-derived fragments that were inserted into either pCRII or pBS and were tested in short-term replication assays (pTR1, nt 1 to 268; pTR2, nt 268 to 581; pTR3, nt 581 to 801; pTR5, nt 551 to 675; pTRΔ1, nt 1 to 581; pTRΔ3, nt 268 to 801; and pTRΔLBS, nt 1 to 559). Also indicated are the relative replication efficiencies of these plasmids compared to wt p1TR, which were derived by comparing the ratios of input to replicated DNA after densitometric measurement. The asterisks at pTR2 and pTRΔ1 indicate that the LBS is not fully contained in these constructs. (B) Mapping data from short-term replication assays in 293 cells. All assays were performed as described in Fig. 1. Odd-numbered lanes indicate plasmids in the absence of LANA; even-numbered lanes indicate plasmids in the presence of LANA. Only wt p1TR, pTRΔ1, pTRΔ3, and pTR2 replicate, as shown in the upper blot in lanes 2, 10, 12, and 14; the lower panel shows linearized plasmids as a loading control. The asterisk shows the linearized pcDNA3/ORF73, since the blots were hybridized to a probe containing the entire pCRII/TR plasmid.
On the basis of these data, we further examined the TR primary sequence flanking the LBS for elements commonly found in eukaryotic origins of replication. Although only a few eukaryotic ori's are characterized on the molecular level, a hallmark of all of them is the presence of an AT-rich stretch close to the DNA initiation site. This feature is conserved from yeast to Drosophila to human and also includes the viral ori sequences of herpesvirus saimiri and EBV (22, 33, 34, 53). As mentioned above, the GC content of the KSHV TR is 89%. However, based on our observation that TR alone has ori activity in the presence of LANA, we scanned the TR for AT-rich stretches. Figure 3A shows the entire TR sequence and illustrates that AT residues are not statistically distributed over the length of this sequence. Instead, we identified a region, ranging from nt 380 to 481, that has a GC content of only 53% compared to a GC content of >90% for the remaining 700 bp (Fig. 3A, underlined). Furthermore, this region is located only 89 bp away from the LBS, which is required for replication. We therefore speculated that the 47% AT-rich region located within TR2 and the 89 bp upstream of the LBS are required for DNA replication and might contain an initiator element and sequences necessary for binding of cellular origin recognition complex (ORC) proteins required for DNA replication. To test this hypothesis, we created two additional deletion mutants. The first pTRΔ3/DraIII deleted TR2 up to the beginning of the AT-rich region, and the second pTRΔ3/SanDI deleted sequences up to nt 509 containing the entire AT-rich region plus an additional 27 bp from the GC-rich element (see Fig. 3B). As shown in Fig. 3C, both plasmids replicate at levels identical to that of wt p1TR (lanes 5, 6, and 8). Although this experiment could not prove the importance of the AT-rich region within the TR, these data, together with mapping data presented in Fig. 2, identify a 79-bp fragment within TR that can function as ori in the presence of LANA (nt 509 to 587). Within this sequence, two cis-acting elements are absolutely required for ori function: (i) the LBS, which confirms that LANA-binding is necessary for initiation of DNA replication, and (ii) an adjacent GC-rich region that presumably contributes to ori function by binding of ORC proteins. Very recently, a GC-rich sequence that binds ORC proteins has been identified near the DS element in oriP of EBV (7, 12, 50). The fact that pTR5 is inactive while pTRΔ3/SanDI replicates at wt efficiency (Fig. 2B, lane 8, and 3C, lane 6) suggests that important cis-acting sequences are located between nt 509 and 550. Our observation that the AT-rich region is not required within the background of a bacterial vector backbone that is ca. 50% AT-rich does not rule out the possibility that this element might contain an initiator element important in the context of the viral episome. To further address the role of the AT-rich module, we are currently conducting experiments to map the initiation site of replication within p1TR. It is thus important to note that analysis of TR sequences of EBV and HVS, both of which are gammaherpesviruses in which the ori is located within the unique long region, revealed an equal distribution of AT residues throughout the length of their respective GC-rich TR units. The modular structure of the KSHV TR unit, which is conserved in TR sequences from different KSHV isolates (35, 43, 49), represents a unique ori remarkably different from those of other gammaherpesviruses. ori activity within the TR also suggests that each KSHV genome contains 30 to 40 functional copies of this ori element.
FIG. 3.
Mapping of a second sequence element required for ori function. (A) The 801-bp KSHV TR sequence, with sequence elements identified by the mapping analysis highlighted. The LBS (nt 570 to 587) is in red; the AT-rich region (47% versus 9% in the remainingsequence) is underlined (nt 380 to 481). Between the AT-rich region and the LBS is an 89-bp GC-rich region required for replication. Restriction sites for DraIII (nt 384) and SanDI (nt 506) have been boxed. A second low-affinity LBS located 5 bp from LBS 1 is also underlined (nt 592 to 609) (18). (B) Representation of constructs used for the identification of ori elements within the TR. Lines represent fragments inserted into pCRII. The wt pTR1 and construct pTR5 have been described in Fig. 2 (the asterisk indicates replication data for TR5 shown in Fig. 2B, lane 8). Construct pTRΔ3/DraIII contains sequences from nt 391 to 801, whereas pTRΔ3/SanDI contains sequences from nt 509 to 801. Also shown are relative replication efficiencies from the plasmids derived from panel C. (C) Short-term replication assay as described in Fig. 1. Lanes 1 to 4 contain 10% of linearized plasmid DNA as input control and indicate nearly identical loading. Lanes 5 to 7 show replicated DNA, indicating that all three plasmids replicate with similar efficiency. Lanes 3 and 7, controls showing LANA-dependent replication.
The DNA-binding domain of LANA is necessary for its DNA replication activity and alone has partial activity.
Based on its primary structure, LANA can be divided into three main domains: (i) a proline-rich N-terminal domain containing an NLS and (ii) a central acidic and highly repetitive domain that contains a leucine zipper at its border with (iii) the C terminus, which is enriched for basic residues, and contains a second NLS. In agreement with this modular structure of LANA, several distinct activities and/or molecular interactions have recently been mapped to individual domains. The N-terminal region is required for the interaction of LANA with chromatin, and a short 18-aa sequence has been determined that can direct this phenotype to reporter proteins (44, 51). In addition, LANA's interaction with mSin3 has been mapped to this domain. Most LANA-protein interactions (pRb, ATF/CREB, and CBP) have been mapped to the central region containing the leucine zipper motif (32, 38, 39, 45). By utilizing a panel of mutant vaccinia virus-expressed LANA proteins, we have previously shown that the C-terminal 233 aa of LANA are sufficient for sequence-specific DNA binding to the TR (19).
Accordingly, we assayed a panel of LANA mutants to determine which domains of LANA are necessary for DNA replication in 293 cells and compared their ability to support replication of p2TR with pcDNA3/ORF73 expressing wt LANA. The expression plasmids encoding the N terminus (aa 1 to 332), termed A; the central domain (aa 332 to 770), termed B; the C terminus (aa 770 to 1003), termed C; and combinations thereof have been described elsewhere (19).
To measure the activity of each construct in comparison to that of wt LANA, we first determined the amount of input DNA in each sample and then normalized for these amounts. As can be seen in Fig. 4, all proteins containing the C domain (C, AC, and BC) support DNA replication (Fig. 4, lanes 14, 16, and 17), whereas mutant proteins lacking the DNA-binding domain were completely inactive (Fig. 4, lanes 13 and 15). Both C and AC replicate p2TR with ca. 20% of wt LANA efficiency. In contrast, replication of p2TR in BC-transfected cells is barely detectable; however, this can be explained by the fact that this protein is extremely unstable (19). These data show that the C-terminal 233 aa of LANA alone partially support DNA replication. During the assay period, cells go through about three or four divisions; therefore, we do not know whether the observed differences in efficiency between LANA-C and the wt are based on a decreased DNA replication rate or on the fact that these proteins are deficient in segregation, which is dependent on the N terminus of LANA to tether the episome to chromatin during mitosis (4, 44, 51). These data also further confirm the mapping of the KSHV ori sequences and show that only plasmids containing an LBS are replicated in the presence of LANA (see Fig. 2 and 3).
FIG. 4.
C domain of LANA partially supports DNA replication of TR-containing plasmids. p2TR was cotransfected with either expression vectors encoding wt LANA or LANA mutant A, C, AB, AC, or BC as described in detail in Materials and Methods. Transient replication assays were performed as described in Fig. 1, and the blot was hybridized to a probe containing a radiolabeled TR fragment. Lanes 1 and 2 indicate the positions of DpnI-digested and linearized p2TR (▸). Lanes 3 to 9 show input DNA as loading control, whereas lanes 11 to 17 show DpnI double-digested DNA. Newly synthesized DNA can be detected in lanes 12, 14, 16, and 17, in which cells were transfected with wt LANA, C, AC, and BC, respectively. As an additional control for DNA digestion, 2 ng of p2TR was mixed into a mock Hirt extract prior to DpnI digestion (lane 10). The replication efficiency of each LANA mutant was determined by densitometric comparison of replicated DNA to input DNA. LANA mutants C and AC replicate p2TR at ca. 20% efficiency compared to the wt. Mutants lacking the C domain do not support replication (lanes 13 and 15).
Testing mutant LANA proteins for dominant-negative activity.
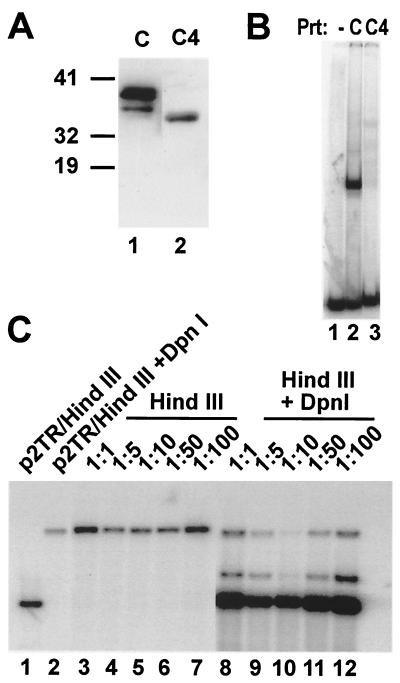
The presented mapping data of cis and trans requirements strongly point out the requirement of specific LANA-DNA interaction for latent DNA replication. Based on these data, we hypothesized that a LANA protein that lost its ability to efficiently bind to LBS might have dominant-negative activity by forming heterodimers with wt LANA. In our previous EMSA analysis, we had mapped the DNA-binding domain of LANA to residues 770 to 1003 (19). Further deletions of 15 aa from the N terminus of LANA-C yielded proteins deficient in binding, similar to what occurred after deletion of more than 15 aa from the C terminus. Using the MVA/T7 vaccinia virus expression system as previously described, we expressed LANA-C4, a construct that contains LANA residues 785 to 1003. As can be seen in Fig. 5A, C4 mutant protein is expressed at levels comparable to that of LANA-C. Using equal amounts of protein for EMSA showed that LANA-C4 lost >95% of its binding activity compared to LANA-C (Fig. 5B). Before we tested whether LANA-C4 interferes with wt LANA in DNA replication assays, we tested the minimum amount of LANA expression vector necessary for efficient replication of p2TR. Surprisingly, transfection of 100 ng of pCDNA3/ORF73 was sufficient for efficient replication (data not shown). We then transfected increasing amounts of pcDNA3.1V5-His/LANA-C4 ranging from 1- to 100-fold, together with 100 ng of pcDNA3/ORF73, into 293 cells and sought to determine whether LANA-C4 acts in a dominant-negative manner. However, LANA-C4 did not act as dominant negative even when present in a ratio of 100 to 1 compared to wt LANA (Fig. 5, lanes 14 to 18). The fact that even at high levels C4 did not further stimulate replication is in agreement with its poor binding activity in vitro. These results could be explained by a failure of C4 to interact with wt protein. For instance, it has been shown for EBNA-1 that mutations affecting dimerization also lead to a loss of DNA-binding activity. However, other explanations are also possible, and we are currently testing a larger panel of LANA mutants for their ability to dimerize and possibly act as dominant-negative proteins.
FIG. 5.
LANA-C4 does not possess dominant-negative activity. (A) Western blot analysis of LANA-C (aa 770 to 1003) and LANA-C4 (aa 785 to 1003) expressed in MVA/T7 vaccinia virus-infected CV1 cells. (B) DNA binding of LANA-C and LANA-C4 to TR5. Equal amounts of recombinant proteins were incubated with radiolabeled TR5 under conditions described in Materials and Methods. Lane 1 contains probe alone, whereas lanes 2 and 3 contain binding reactions with LANA mutants C and C4; C shows strong complex formation, whereas C4 shows extremely weak binding. (C) Titration of increasing amount of pcDNA3.1V5His/LANA-C4 with a constant amount of pcDNA3/ORF73. Short-term replication assays were performed as described for Fig. 1. Then, 100 ng of wt LANA expression construct was cotransfected with a vector expressing LANA-C4 ranging from 100 ng (1:1) to 10 μg (1:100) into 293 cells. Lanes 1 and 2 indicate the positions of DpnI-digested and linearized p2TR. Lanes 3 to 7 contain input DNA, whereas lanes 8 to 12 show newly synthesized DNA. The comparison of input and replicated DNA at each ratio shows that LANA-C4 does not inhibit LANA wt function even when present at a ratio of 100 to 1 (lane 12).
DISCUSSION
LANA supports DNA replication of TR-containing plasmids.
Using short-term replication assays, we demonstrate for the first time that LANA plays a role in supporting the DNA replication of plasmids containing a single copy of the viral TR and that LANA binding to sequences within the TR is absolutely required for this activity. This observation places LANA in a diverse family of DNA tumor virus replication and/or segregation proteins such as SV40 large T, EBNA-1, and the human papillomavirus E1/E2 protein complex. Since it was known that LANA was necessary and sufficient for long-term maintenance of TR-containing plasmids, the fact that LANA also has replication activity was anticipated given that EBNA-1 of EBV, the closest human relative of KSHV, also has segregation and replication activities (4). However, it is important to note that there are no amino acid sequence homologies between LANA and EBNA-1 or between the cis-acting sequences bound by both proteins. A major difference between these different viral ori binding proteins is whether they possess intrinsic helicase activity (SV40 large T) or interact upon DNA binding with either additional viral (papillomavirus E1) or cellular proteins (EBV EBNA-1 ORC interactions) that directly act on DNA structure (21). Computer analysis of LANA did not reveal any motifs suggesting the presence of a prototype helicase domain. By mutational analysis of LANA, we showed that the C-terminal 233 aa by itself support the replication of TR plasmids in 293 cells at levels ca. 20% of that of the wt at 72 h posttransfection (Fig. 4). Although we do not know at this point whether this reduction is due to a failure in segregation or to a decrease in de novo synthesis of DNA, it is astonishing that this activity is partially preserved in a protein in which 770 of 1,003 aa had been deleted. These C-terminal 233 aa also closely correspond to the DNA-binding domain of LANA. We have previously shown that LANA-C binds with high affinity to its target sequence within TR, whereas further deletions lead to a loss of binding activity (Fig. 5) (19). These data are in agreement with EBNA-1 and E2, both of which have been shown to support replication only when DNA-binding activity is conserved (41, 52, 56). Furthermore, EBNA-1 and E2 bind to DNA as dimers, and EBNA-1 mutations that prevent dimer formation also lead to loss of DNA binding (36, 40). By differential tagging of recombinant LANA proteins, it was recently shown that the C terminus of LANA exists predominantly as a dimer in solution (51). An N-terminal truncation of 15 aa from LANA-C results in a protein (LANA-C4) that has nearly completely lost its ability to bind to DNA in EMSA and failed to support DNA replication. In addition, LANA-C4 does not inhibit wt LANA in replication assays (Fig. 5C), suggesting that this mutant might be defective in dimer formation. In order to develop dominant-negative LANA proteins, we are currently testing LANA-C4 and additional mutants for their ability to dimerize. However, at this point we cannot rule out other explanations for the failure of LANA-C4 to inhibit wt LANA function. For EBNA-1 it was shown that only proteins retaining their DNA-binding activity function as dominant negative, and it was suggested that this inhibition is dependent on complex interactions within oriP, in addition to heterodimer formation (31).
In summary, our data lead to the hypothesis that all functions necessary to support DNA replication: sequence-specific DNA binding, presumably interactions with cellular ORC proteins, and dimer formation are intrinsic to the C-terminal domain of LANA. Many of the protein-protein interactions causing transcriptional regulation of viral and cellular genes have been mapped to the leucine zipper within the central domain of LANA (38, 39, 45). These molecular interactions might represent independent functions not directly related to LANA's replication or segregation activity, suggesting that KSHV has evolved a single polypeptide containing a plethora of activities that in EBV are distributed over the entire EBNA protein family (37).
All sequences necessary for LANA-dependent DNA replication are located within the TR of KSHV.
By direct comparison of a plasmid containing TR sequences or, in addition, sequences derived from both sides of the unique long region, we showed that only sequences within the TR itself confer LANA-dependent replication and that sequences adjacent to TR do not contribute to this (Fig. 1C and D). By performing a detailed deletion analysis of the 801-bp 89% GC-rich TR unit, we mapped the cis-regulatory elements necessary for replication to a 101-bp sequence (nt 509 to 609). This sequence contains two elements absolutely required for LANA-dependent DNA replication: (i) the LANA-binding site and (ii) a 62-bp upstream element. The importance of this element is supported by the observation that mutant TR5 containing a LANA-binding site but only 20 of the 62 bp upstream is completely inactive, whereas mutant pTRΔ3/SanDI containing an additional 42 bp replicates like wt p1TR (compare lane 8 of Fig. 2B with lane 6 of Fig. 3C). This sequence contains many CpG residues often found within eukaryotic ori's (10, 11). Very recently, it was shown that EBNA-1 directly interacts with ORC-2 and thereby facilitates ORC-2 binding to a site adjacent to the DS element of oriP (7, 11, 50). Although there is no extended sequence homology between sequences within oriP and the TR, this site also contains CpG nucleotides. By using EMSA in combination with immunoprecipitation assays, we are currently testing whether ORC complex proteins bind to this region and whether LANA plays a role in this process.
TR sequences of gammaherpesviruses are very GC-rich and are comprised of highly repetitive elements even within the TR unit. Common to these viruses (i.e., EBV and HVS) is the fact that their latent ori's of replication have been mapped within the unique long region of their genomes (22, 33, 59, 60). Based on the observation that the TR element of KSHV was sufficient to confer LANA-dependent DNA replication, we examined the TR sequence for signatures different from those of TRs of other gammaherpesviruses and identified a 100-bp region which is only 53% GC-rich, as illustrated in Fig. 3A. In contrast, AT residues are randomly distributed within the TR sequences of EBV, HVS, and MHVγ-68 (GenBank accession numbers A9625578, NC_001987, and NC_001826). This suggests that the TR element of KSHV contains three sequence elements that contribute to efficient DNA replication: in addition to LBS, there is a sequence that allows binding of cellular factors and also a 47% AT-rich putative initiator region embedded in sequences that show a >90% GC content. Although deletion of 40 bp of this 62-bp GC-rich element between LBS and the AT-rich stretch leads to complete loss of replication (see Fig. 2, lane 8), deleting the AT-rich region did not reduce its ability to replicate (Fig. 3B and C). However, deletion of nt 1 to 508, including the 91% GC-rich region upstream of the AT-rich element, positioned the LBS and putative ORC-binding region in pTRΔ3/SanDI in close proximity to bacterial sequences that are ca. 50% AT-rich and thereby might facilitate initiation. This result might prevent us from supporting the importance of this element in the context of small plasmids. Nevertheless, to our best knowledge the TR of KSHV is the first to contain a functional ori element and the only one in which we identified an AT-rich element in close proximity to sequences required for ori function; this suggests that this element plays a role in the context of the viral genome. To further address this question, it will be necessary to molecularly analyze replication initiation start points within latently infected PEL cells (20, 48).
In summary, we have provided strong data suggesting an ori structure that contains at least two elements which are absolutely required for ori function: a binding site for LANA and a 62-bp GC-rich region directly adjacent to it. Even though the presence of a functional ori element within each TR and, as a consequence, its high copy number in the viral episome is unique to KSHV, there are also important similarities between KSHV and the oriP element of EBV. OriP is comprised of two major elements. The first is the family of repeats that contain 20 copies of a palindromic EBNA-1 binding site that are crucial for long-term maintenance. The second is located 1 kbp away from the DS element that is required for the initiation of DNA replication. The DS element contains four EBNA-1 binding sites—two high-affinity sites and two low-affinity sites—that are bound in a cooperative fashion. Adjacent to the DS element lies a sequence that can be bound by ORC proteins; this binding is facilitated by direct protein-protein interactions of EBNA-1 and ORC proteins, leading to the formation of a preinitiation complex and subsequently to the initiation of DNA synthesis in close proximity to this site (7, 12, 50). We have recently identified a second LANA-binding site 5 bp downstream of the originally reported sequence (see Fig. 3A). As in the DS element of EBV, this site is bound by LANA in a cooperative fashion and is necessary for efficient DNA replication (18). These data suggest that both LANA-binding sites are functionally equivalent to half a DS element and, together with the data presented here, show that the putative ori of KSHV, although remarkably different in its location within the TR, contains conserved structural elements comparable to oriP of EBV. The high copy number of the ori sequence might provide a functional element, similar to the FR within oriP, that is required in the context of genome segregation. This analogy is supported by our finding that plasmids containing one or two copies of the TR sequence replicate with identical efficiencies, whereas these plasmids show strong differences in their efficiency to establish long-term maintenance (3).
Our data provide the first analysis of the putative ori structure of KSHV and greatly add to our understanding of latent DNA replication of KSHV. A better understanding of molecular mechanisms involved in viral latent replication might provide the basis for molecular targeted antiviral treatment during latency, the phase associated with human malignancies.
Acknowledgments
R.R. is a Mount Sinai Healthcare Foundation scholar. This work was supported by a grant from the National Institutes of Health (CA 88763-2) to R.R. and the CWRU Center for AIDS Research. A.C.G. is a recipient of the Research Oncology Training grant (T32HL07147).
REFERENCES
- 1.Aiyar, A., C. Tyree, and B. Sugden. 1998. The plasmid replicon of EBV consists of multiple cis-acting elements that facilitate DNA synthesis by the cell and a viral maintenance element. EMBO J. 17:6394-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballestas, M., and K. M. Kaye. 2001. Kaposi's sarcoma associated herpesvirus latency associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein, J. A., J. M. Porter, and M. D. Challberg. 1986. Template requirements for in vivo replication of adenovirus DNA. Mol. Cell. Biol. 6:2115-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, B., H. Xu, I. Todorov, A. Dutta, and J. L. Yates. 2001. Human DNA replication initiation factors, ORC and MCM, associate with oriP of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 98:10085-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, M. A., II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 9.Deak, J. C., J. V. Cross, M. Lewis, Y. Qian, L. A. Parrott, C. W. Distelhorst, and D. J. Templeton. 1998. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc. Natl. Acad. Sci. USA 95:5595-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado, S., M. Gomez, A. Bird, and F. Antequera. 1998. Initiation of DNA replication at CpG islands in mammalian chromosomes. EMBO J. 17:2426-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar, S. K., L. Delmolino, and A. Dutta. 2001. Architecture of the human origin recognition complex. J. Biol. Chem. 276:29067-29071. [DOI] [PubMed] [Google Scholar]
- 12.Dhar, S. K., K. Yoshida, Y. Machida, P. Khaira, B. Chaudhuri, J. A. Wohlschlegel, M. Leffak, J. Yates, and A. Dutta. 2001. Replication from oriP of Epstein-Barr virus requires human ORC and is inhibited by geminin. Cell 106:287-296. [DOI] [PubMed] [Google Scholar]
- 13.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanning, E., and R. Knippers. 1992. Structure and function of simian virus 40 large tumor antigen. Annu. Rev. Biochem. 61:55-85. [DOI] [PubMed] [Google Scholar]
- 15.Friborg, J., W. Kong, M. O. Hottiger, and G. J. Nabel. 2000. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 16.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91:157-160. [DOI] [PubMed] [Google Scholar]
- 17.Gao, S. J., Y. J. Zhang, J. H. Deng, C. S. Rabkin, O. Flore, and H. B. Jenson. 1999. Molecular polymorphism of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 180:1466-1476. [DOI] [PubMed] [Google Scholar]
- 18.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 19.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerbi, S. A., and A. K. Bielinsky. 1997. Replication initiation point mapping. Methods 13:271-280. [DOI] [PubMed] [Google Scholar]
- 21.Grossman, S. R., and L. A. Laimins. 1996. EBNA1 and E2: a new paradigm for origin-binding proteins? Trends Microbiol. 4:87-89. [DOI] [PubMed] [Google Scholar]
- 22.Harrison, S., K. Fisenne, and J. Hearing. 1994. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J. Virol. 68:1913-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay, R. T. 1985. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 4:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herndier, B., and D. Ganem. 2001. The biology of Kaposi's sarcoma. Cancer Treat. Res. 104:89-126. [DOI] [PubMed] [Google Scholar]
- 25.Herndier, B. G., A. Werner, P. Arnstein, N. W. Abbey, F. Demartis, R. L. Cohen, M. A. Shuman, and J. A. Levy. 1994. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS 8:575-581. [DOI] [PubMed] [Google Scholar]
- 26.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 27.Hyun, T. S., C. Subramanian, M. A. Cotter II, R. A. Thomas, and E. S. Robertson. 2001. Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 75:8761-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (ORF74) and LANA (ORF73) promoters. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellam, P., C. Boshoff, D. Whitby, S. Matthews, R. A. Weiss, and S. J. Talbot. 1997. Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1:19-29. [PubMed] [Google Scholar]
- 31.Kirchmaier, A. L., and B. Sugden. 1997. Dominant-negative inhibitors of EBNA-1 of Epstein-Barr virus. J. Virol. 71:1766-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kung, S. H., and P. G. Medveczky. 1996. Identification of a herpesvirus Saimiri cis-acting DNA fragment that permits stable replication of episomes in transformed T cells. J. Virol. 70:1738-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladenburger, E. M., C. Keller, and R. Knippers. 2002. Identification of a binding region for human origin recognition complex proteins 1 and 2 that coincides with an origin of DNA replication. Mol. Cell. Biol. 22:1036-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 36.Leight, E. R., and B. Sugden. 2000. EBNA-1: a protein pivotal to latent infection by Epstein-Barr virus. Rev. Med. Virol. 10:83-100. [DOI] [PubMed] [Google Scholar]
- 37.Liebowitz, D., and E. Kieff. 1993. Epstein-Barr virus, p. 107-172. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, Inc., New York, N.Y.
- 38.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016-31022. [DOI] [PubMed] [Google Scholar]
- 39.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81(Pt. 11):2645-2652. [DOI] [PubMed] [Google Scholar]
- 40.McBride, A. A., J. C. Byrne, and P. M. Howley. 1989. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domain: transactivation is mediated by the conserved amino-terminal domain. Proc. Natl. Acad. Sci. USA 86:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohr, I. J., R. Clark, S. Sun, E. J. Androphy, P. MacPherson, and M. R. Botchan. 1990. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science 250:1694-1699. [DOI] [PubMed] [Google Scholar]
- 42.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. Product review: new mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 43.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 46.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by ORF73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. Brown, and D. Ganem. 2001. Modulation of cellular and viral transcription by the latency-associated nuclear antigen (LANA/ORF73) of KSHV. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 49.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schepers, A., M. Ritzi, K. Bousset, E. Kremmer, J. L. Yates, J. Harwood, J. F. Diffley, and W. Hammerschmidt. 2001. Human origin recognition complex binds to the region of the latent origin of DNA replication of Epstein-Barr virus. EMBO J. 20:4588-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo, Y. S., F. Muller, M. Lusky, E. Gibbs, H. Y. Kim, B. Phillips, and J. Hurwitz. 1993. Bovine papilloma virus (BPV)-encoded E2 protein enhances binding of E1 protein to the BPV replication origin. Proc. Natl. Acad. Sci. USA 90:2865-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirakata, M., and K. Hirai. 1998. Identification of minimal oriP of Epstein-Barr virus required for DNA replication. J. Biochem. 123:175-181. [DOI] [PubMed] [Google Scholar]
- 54.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubenrauch, F., and L. A. Laimins. 1999. Human papillomavirus life cycle: active and latent phases. Semin. Cancer Biol. 9:379-386. [DOI] [PubMed] [Google Scholar]
- 56.Wu, H., P. Kapoor, and L. Frappier. 2002. Separation of the DNA replication, segregation, and transcriptional activation functions of Epstein-Barr nuclear antigen 1. J. Virol. 76:2480-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyatt, L. S., B. Moss, and S. Rozenblatt. 1995. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202-205. [DOI] [PubMed] [Google Scholar]
- 58.Yates, J., N. Warren, D. Reisman, and B. Sugden. 1984. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. USA 81:3806-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yates, J. L., S. M. Camiolo, and J. M. Bashaw. 2000. The minimal replicator of Epstein-Barr virus oriP. J. Virol. 74:4512-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]
- 61.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]