Abstract
Y-family DNA polymerases can replicate past a variety of damaged bases in vitro but, with the exception of DNA polymerase η (polη), which is defective in xeroderma pigmentosum variants, there is little information on the functions of these polymerases in vivo. Here, we show that DNA polymerase ι (polι), like polη, associates with the replication machinery and accumulates at stalled replication forks following DNA-damaging treatment. We show that polη and polι foci form with identical kinetics and spatial distributions, suggesting that localization of these two polymerases is tightly co-ordinated within the nucleus. Furthermore, localization of polι in replication foci is largely dependent on the presence of polη. Using several different approaches, we demonstrate that polη and polι interact with each other physically and that the C-terminal 224 amino acids of polι are sufficient for both the interaction with polη and accumulation in replication foci. Our results provide strong evidence that polη targets polι to the replication machinery, where it may play a general role in maintaining genome integrity as well as participating in translesion DNA synthesis.
Keywords: DNA polymerase/replication foci/UV light/xeroderma pigmentosum variants
Introduction
DNA damage occurs ubiquitously in all cells. In order to maintain the stability of the genome, cells have evolved mechanisms not only to repair all types of DNA damage, but also to replicate DNA from which the damage has not been removed (post-replication repair). In the case of human cells, a major mechanism for carrying out post-replication repair involves translesion synthesis (TLS) past damaged sites. TLS is deficient in the variant form of the sun-sensitive cancer-prone disorder xeroderma pigmentosum (XP). The gene defective in these XP variants (XP-V) encodes a DNA polymerase, polη (Johnson et al., 1999; Masutani et al., 1999), which is able to replicate undamaged templates or those containing cyclobutane pyrimidine dimers (CPDs, the major UV photoproduct) with equal efficiencies (Masutani et al., 1999). TLS by polη is the principal mechanism for bypassing CPDs in human cells. Although the lack of polη in XP-V cells does not confer substantial hypersensitivity to killing by UV light, UV hypermutability is increased to levels approaching those in classical XP cells, which are deficient in nucleotide excision repair (Maher et al., 1976).
Polη is a member of the recently discovered Y-family of DNA polymerases (Ohmori et al., 2001), which have been best characterized for their lesion-bypassing properties (reviewed in Goodman, 2002). There are, however, few studies to date to indicate how these polymerases function inside cells. In previous work, we showed that in S-phase cells, polη localizes in replication foci. On exposure to DNA-damaging treatments, we observed an accumulation of polη-containing foci. These appear to represent replication factories in which replication forks are stalled at lesions (Kannouche et al., 2001). The C-terminal 70 amino acids of polη are required to localize it in the nucleus, and a further 50 are needed for the relocalization into replication foci (Kannouche et al., 2001).
In addition to polη, human cells possess three other Y-family polymerases (polι, polκ and Rev1) (Woodgate, 1999; Friedberg et al., 2002; Goodman, 2002). Polι, like polη, is phylogenetically related to DNA polymerases in the Saccharomyces cerevisiae Rad30 branch of the Y-family (McDonald et al., 1999; Ohmori et al., 2001). It is extremely error prone on undamaged templates and, most strikingly, polι incorporates dGMP opposite T 3–10 times more frequently than the correct nucleotide dAMP (Johnson et al., 2000b; Tissier et al., 2000b; Zhang et al., 2000c). Although polι can also insert bases opposite several DNA lesions, such as an abasic site or the 3′ T of a 6–4 T–T photoproduct (Tissier et al., 2000a; Zhang et al., 2001), extension from the inserted base is often limited. It has been suggested that in such cases, the polι-dependent (mis)insertion might be extended by another polymerase, such as polζ (Johnson et al., 2000b; Tissier et al., 2000a). Using a model system containing human polι in conjunction with S.cerevisiae polζ, the combined action of the two polymerases leads to the complete bypass of abasic sites and 6–4 T–T photoproducts in vitro (Johnson et al., 2000b; Guo et al., 2001). Despite a wealth of data on the enzymatic properties of polι in vitro (McDonald et al., 2001; Vaisman and Woodgate, 2001; Vaisman et al., 2001; Zhang et al., 2001), there are no data on the role of polι in vivo or on its functional relationship to other Y-family DNA polymerases.
Here, we show that the localization of polι following DNA damage is identical to that of polη, and that recruitment of polι into replication foci is dependent on polη. We also show by several criteria that polη and polι physically interact. Taken together, our results suggest that an interaction between pols η and ι helps target polι to the replication machinery, where it can participate in genome maintenance and TLS.
Results
Pol ι does not correct the UV sensitivity of XP-variant cells
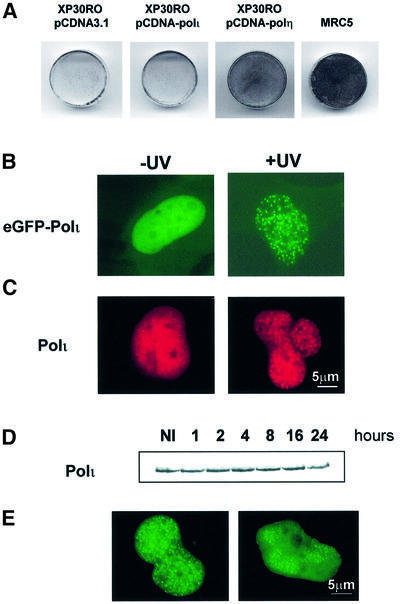
Since polη and polι are paralogues and contain similar catalytic domains, we investigated whether overexpression of polι was able to correct the defect in XP-V cells. Although XP-V cells are only marginally hypersensitive to killing by UV, incubating them in caffeine following UV treatment confers a substantial sensitization to UV-induced cell killing, which is not seen in normal cells (Arlett et al., 1975). pCDNA-polι was stably transfected into XP30RO cells and transfectant clones were analysed for resistance to UV + caffeine. As shown in Figure 1A, XP30RO cells overexpressing polι displayed the same UV + caffeine sensitivity as cells transfected with vector alone. In contrast, XP-V cells overexpressing polη were resistant to UV + caffeine, with survival similar to that of the normal cell line MRC5. Thus, unlike polη, polι is not able to correct the defect in XP-V cells.
Fig. 1. Polι does not correct the UV + caffeine sensitivity of XP-V cells but localizes into nuclear foci after UV irradiation. (A) XP30RO cells were transfected with the plasmids pCDNA3.1 vector, pCDNA-polι or pCDNA-polη as indicated. Stable clones were UV irradiated (7 J/m2) and then incubated in the presence of 75 µg/ml caffeine. Right panel: MRC5 cells treated with the same doses of UV + caffeine. After 4 days, the cells were stained with methylene blue. (B and C) MRC5 cells were transfected with plasmids encoding either eGFP–polι (B) or pCDNA-polι (C). At 20 h post-transfection, cells were irradiated with 7 J/m2 (right panels). After 12 h, the distribution of eGFP–polι was examined following paraformaldehyde fixation. For cells transfected with pCDNA-polι (C), polι distribution was revealed with anti-polι antibody and tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary antibody. (D) Western blots of extracts of MRC5 cells irradiated with 7 J/m2 and incubated for the indicated times. Blots were probed with anti-polι. In (E), MRC5 cells were transfected with eGFP–polι and irradiated either uniformly (left) or through a Millipore filter (right).
Polι localizes into nuclear foci after UVC irradiation
In order to improve our understanding of the biological function of polι, we have analysed its cellular localization. The cDNA encoding enhanced green fluorescent protein (eGFP) was fused in-frame to the N-terminus of polι (eGFP–polι), and this construct was transfected into MRC5 normal fibroblasts. Despite the absence of any obvious nuclear localization signal (NLS) consensus motif in its amino acid sequence, eGFP–polι predominantly localized within the nucleus (Figure 1B, left) and appeared homogeneously distributed. In 10–15% of transfected cells, eGFP–polι was localized in many intranuclear foci (not shown). This localization pattern was strik ingly similar to our previous observations with polη (Kannouche et al., 2001). Consequently, we asked whether the distribution of polι changes after DNA damage. We irradiated the transfected cells with 7 J/m2 UVC and analysed the pattern of eGFP–polι 12 h later (Figure 1B, right). As with polη, we found that in >60% of transfected cells, eGFP–polι was concentrated into foci throughout the nucleoplasm. We verified that the localization of eGFP–polι was not due to tagging artefacts by examining the distribution of untagged polι using a pCDNA-polι construct and anti-polι antibodies. Similar distributions were observed (Figure 1C). Furthermore, accumulation of nuclear foci was also observed after methyl methanesulfonate treatment but not after γ-irradiation, indicating that polι focus formation is specific to certain classes of DNA lesions (data not shown). Using western blotting, we were able to show that there was no increase in the endogenous level of polι following UV irradiation of MRC5 cells (Figure 1D). This result suggests that the accumulation of polι in foci is not associated with induction of the protein.
Polι is recruited at sites of UV-induced DNA damage
By irradiating cells through a membrane filter containing holes of 8 µm in diameter (Volker et al., 2001), it is possible to induce local UV damage in the nucleus in cells transfected with peGFP-polι. After incubation for a further 12 h, cells were fixed and polι distribution was detected by autofluorescence of eGFP. In parallel, we irradiated the transfected cells without the membrane to compare the localization after total irradiation. As shown in Figure 1E (left), eGFP–polι is concentrated into foci throughout the nucleoplasm after total irradiation. In contrast, after local irradiation, polι foci accumulated almost exclusively in the areas of the nucleus that had been exposed to UV (Figure 1E, right). This result supports the idea that polι foci accumulate at the sites of UV-induced DNA damage.
Polι co-localizes with polη in replication foci
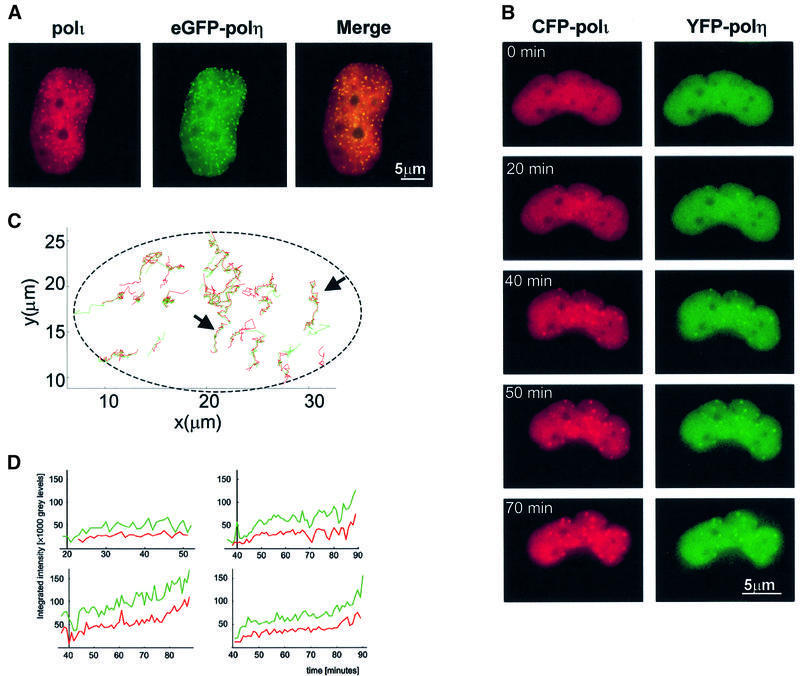
The localization patterns of polι are strikingly similar to those of polη (Kannouche et al., 2001). We therefore checked by immunofluorescence if these proteins co-localize after UV irradiation. MRC5 cells were co-transfected with pCDNA-polι and peGFP-polη plasmids and irradiated with 7 J/m2. At 12 h post-irradiation, polι was visualized using anti-polι antibodies (red staining, Figure 2A, left) and polη was detected by autofluorescence of eGFP (green staining, Figure 2A, middle). Interestingly, in the nucleus of cells displaying polι foci, we detected co-localization with eGFP–polη dots. The overlapping of polη with polι is visualized as yellow foci (Figure 2A, right) and indicates that polι and polη are localized in the same regions after DNA damage. We obtained similarly striking co-localization using confocal microscopy (data not shown). These results strongly suggest that polι, like polη, accumulates at replication forks stalled at sites of UV damage.
Fig. 2. Co-localization of polη and polι. (A) MRC5 cells co-transfected with eGFP–polη and pCDNA-polι were irradiated (7 J/m2), fixed 12 h later and stained with anti-polι antibody and TRITC-conjugated secondary antibody. The staining pattern of polι (red) (left) and the autofluorescent signal of GFP–polη (green) (middle) in the same cell are shown. Co-localization of polι and eGFP–polη is indicated by a yellow pattern (right). (B–D) MRC5 cells were co-injected with peYFP-polη and peCFP-polι, and UV irradiated (10 J/m2) 8 h later. The cells were examined by time-lapse microscopy using appropriate filters. (B) The fluorescent signals for the two tagged proteins at various times after irradiation. In (C), the co-ordinates of each focus in the cell were determined at different times. Each track represents the movement of a specific focus of polη (green) and polι (red). The dashed line represents the approximate position of the nuclear envelope. In (D), each of the four panels represents the intensity of the yellow and blue signals of an individual focus as a function of time. Green, polη; red, polι.
Co-ordination of polη and polι foci in living cells
In order to investigate the co-localization of polη and polι in more detail, we used dual labelling of polη and polι and time-lapse microscopy. The cDNAs encoding enhanced cyan fluorescent protein (eCFP) and enhanced yellow fluorescent protein (eYFP) were fused in-frame to the N-termini of polι (peCFP-polι) and polη (peYFP-polη), respectively. Both plasmids (peCFP-polι and peYFP-polη) were microinjected together into MRC5 cells and 8 h later the fibroblasts were UV irradiated (10 J/m2). Foci formation of polη and polι was analysed in living cells using time-lapse microscopy. A time-lapse series following foci in a single nucleus is shown in Figure 2B. At each time after irradiation, the foci of polη and polι appeared co-incidentally.
Co-ordination in time and in space. The positions of each focus of polη and polι within a single nucleus are plotted as a function of time in Figure 2C. Red and green lines correspond to the tracks of eCFP–polι and eYFP–polη, respectively, in the cell. There was a striking overlap of most of the red and green lines over the whole period of time, indicating that polη and polι foci are tightly associated after UV irradiation and not just for a short time. The trajectories in Figure 2C are in absolute space. Visual examination of the relative positions of the individual foci shows that these trajectories reflect a combination of movement of the whole nucleus and that of the foci relative to the nuclear envelope.
Co-ordination in intensity and in time. In order to determine whether the two polymerases accumulate at the same time on unrepaired damage, we have plotted the appearance and intensity of the fluorescence due to polη and polι in four individual foci as a function of time (Figure 2D). These plots show that both the time of appearance of the focus and the increase in intensity of the fluorescent signals were very similar for polι and polη. This indicates that, inside the nucleus, these two polymerases are closely co-ordinated in both space and time after UV irradiation. We observed a similar co-localization with the fluorophores reversed, i.e. using eYFP–polι- and eCFP–polη-expressing plasmids, thus ruling out the possibility of artefacts due to cross-talk (data not shown).
Polι relocalization is dependent on polη
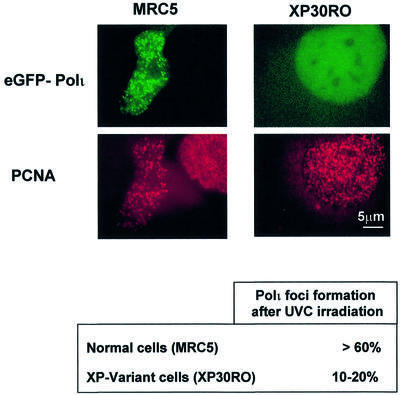
In view of this co-ordinated response of polη and polι after UV irradiation, we asked whether polι could relocalize at sites of replication forks blocked by UV damage in the absence of polη. We transfected XP-V (XP30RO) cells with peGFP-polι, irradiated the cells with 7 J/m2 and analysed polι distribution 12 h after irradiation. Strikingly, the number of transfected cells containing polι foci was never more than 20%, in contrast to MRC5 cells in which >60% of cells contained polι foci after irradiation. In both cases, we also analysed the localization of proliferating cell nuclear antigen (PCNA; red staining). In MRC5 cells, eGFP–polι relocalized in foci which co-localized with PCNA dots (Figure 3, left), whereas in XP30RO fibroblasts eGFP–polι displayed diffuse staining even in cells in which PCNA formed dots (Figure 3, right). The experiments were repeated several times and in each experiment we observed only 10–20% of irradiated XP30RO cells with eGFP–polι foci. We conclude that the accumulation of polι in UV-induced replication foci requires the presence of polη. In contrast, we observed that polι does remain in the nucleus in XP30RO cells, demonstrating that it is only the foci formation and not nuclear localization that is dependent on polη.
Fig. 3. Dependence of polι foci on polη. MRC5 (left) or XP30RO cells (right) transfected with eGFP–polι were UV irradiated, fixed 12 h later and stained with anti-PCNA monoclonal and TRITC-conjugated secondary antibody. The staining patterns of the autofluorescent signal of GFP (green staining in upper panels) and PCNA (red staining in lower panels) in the same cell are shown.
Polι interacts with polη
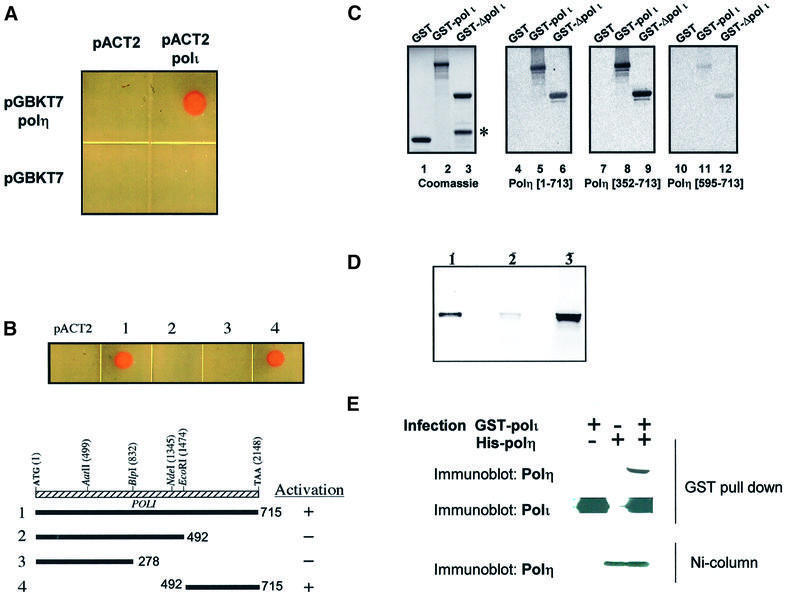
Our cellular localization data provide strong evidence that polη and polι are closely linked in the nucleus. We therefore carried out several tests to determine if the two polymerases might interact directly. In the first series of experiments, we used the yeast two-hybrid system. polη cDNA was cloned downstream of the GAL4 DNA-binding domain and polι cDNA downstream of the GAL4 activation domain. The plasmids were co-transfected into a yeast reporter strain, AH109. Interaction resulted in the ability of the yeast cells to grow in selective medium in the absence of tryptophan, leucine, histidine and adenosine. Figure 4A shows that only cells containing both polη- and polι-expressing plasmids were able to grow in selective medium, whereas if either of the plasmids contained no insert, there was no growth. To determine which domain in polι was responsible for the interaction with polη, we used three different polι deletion constructs. As indicated in Figure 4B, the C-terminal 224 amino acids of polι (residues 492–715) were sufficient to interact with polη, whereas no interaction was observed with the N-terminal 492 amino acids containing the whole of the catalytic domain.
Fig. 4. Interaction between pol η and polι. (A and B) In vivo interaction of polη and polι using the yeast two-hybrid system. (A) Saccharomyces cerevisiae strain AH109 was co-transformed with pGBKT7/pACT2, pGBKT7/pACT2-polι, pGBKT7-polη/pACT2 and pGBKT7-polη/pACT2-polι. A representative colony from each transformation was grown overnight at 27°C in selective medium and a sample was spotted on to a DOBA-Trp-Leu-His-Ade plate and incubated at 27°C for 3 days. (B) Determination of the minimal region of polι that interacts with polη. Saccharomyces cerevisiae strain AH109 was co-transformed with pGBKT7-polη/pACT2, or (1) pGBKT7-polη/pACT2-polι, (2) pGBKT7-polη/pAR218 (N-terminal 492 residues of polι), (3) pGBKT7-polη/pAR216 (N-terminal 278 residues of polι) and (4) pGBKT7-polη/pAR220 (C-terminal 224 residues of polι). (C) Far-western analysis of the interaction between polι and polη. A Coomassie blue-stained SDS–polyacrylamide gel showing the expression and expected size of the GST–polι fusion proteins. A 1 µg aliquot of GST (lane 1) was used as negative control; GST–polι (lane 2); GST–Δpolι (492–715) (lane 3). The protein band indicated with an asterisk is a degradation product of the GST–Δpolι (492–715) fusion protein. Far-western blots of equivalent samples after transfer to nitrocellulose membrane and incubation of the immobilized proteins with 35S-labelled full-length polη (lanes 4–6), polη (352–713) (lanes 5–9) and polη (595–713) (lanes 10–12). (D) GST pull-down assay demonstrating that polη interacts with the C-terminal region of polι. In vitro translated 35S-labelled polη protein was incubated with glutathione–Sepharose beads and equal amounts of GST or GST–Δpolι (492–715) as indicated in Materials and methods. Bound proteins were eluted, and resolved by 4–20% SDS–PAGE. A portion of the in vitro translated 35S-labelled polη corresponding to 10% of the labelled protein in the binding reaction was loaded as input (lane 1). The results show that polη binds GST–Δpolι (492–715) (lane 3) but not GST alone (lane 2). (E) Sf9 cells were infected with baculovirus supernatants containing GST–polι, His6-polη or both. Lysates were extracted with glutathione–Sepharose beads, and the extracted proteins analysed by SDS–PAGE and western blotting. Blots were probed with anti-polη (top) or anti-polι (middle) To check the amounts of polη in the lysates, parallel samples were extracted with nickel– agarose beads and analysed for the amount of polη by western blotting.
In the second series of experiments, we constructed GST fusion proteins containing either full-length polι or the C-terminal 224 amino acids that interact with polη in the two-hybrid assay. The proteins were electrophoresed in SDS–polyacrylamide gels, transferred to membranes and probed with 35S-labelled in vitro translated polη in far-western assays (Figure 4C). The results demonstrate that polη interacts with both full-length GST–polι (lane 5) and GST–polι (492–715) (lane 6), whereas no interaction was seen with GST alone (lane 4). In order to determine which region of polη interacted with polι, we repeated the far-western analysis using fragments of polη containing either the C-terminal 362 amino acids (352–713) or the C-terminal 119 amino acids (595–713) as radiolabelled probes. Polη (352–713) interacted with polι as well as the full-length protein (Figure 4C, lanes 7–9), whereas polη (595–713) gave a much weaker signal (lanes 10–12). We conclude that the interaction takes place between the C-terminal 224 amino acids of polι and a region of polη between amino acids 352 and 595, although we cannot exclude that residues 595–713 are also required.
In the third set of experiments, the same GST–polι (492–715) construct was used in a ‘pull-down’ assay. In vitro translated 35S-labelled polη was incubated with glutathione–Sepharose beads coupled with either GST or GST–polι (492–715). The bound proteins were resolved by SDS–PAGE. Figure 4D shows that in vitro translated polη was indeed bound to the beads coupled with GST–polι (492–715), but not with GST alone. In these assays, the bound protein represents ∼10% of the total input protein.
In the final series of experiments, we looked for direct interaction of polη and polι inside cells using co-immunoprecipitation. We were unable to detect co-immunoprecipitation inside human cells, so we overexpressed both proteins in insect cells using baculovirus constructs, in which polη was tagged with His6 and polι was tagged with GST. After infection of Sf9 cells with both constructs, extracts were either incubated with glutathione–Sepharose beads to extract polι, or passed down a nickel column to extract polη, and interacting proteins were analysed by western blotting using appropriate antibodies. The results are shown in Figure 4E. Polη was found associated with GST–polι on the beads (lane 3), but not in controls without GST–polι (lane 2). The bottom panel shows that similar amounts of polη were present in both extracts. The amount of polη associated with polι represents a small proportion of the total polη in the insect cell extract, suggesting that under these assay conditions, the polη–polι interaction is not particularly robust.
Taken together, these experiments provide convincing evidence that polη and polι interact physically, and this correlates with their tight co-localization within nuclear foci.
Deletion analysis of polι
In contrast to polη, the primary sequence of polι contains no clear NLS. To identify the regions of polι required for nuclear localization and UV relocalization, a series of GFP-tagged deletion mutants was constructed, and their localization is shown in Figure 5.
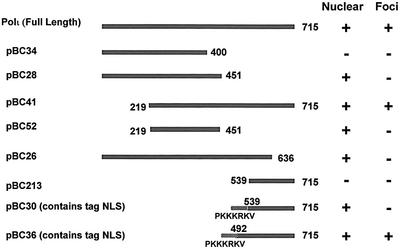
Fig. 5. Deletion analysis of polι localization. Polι deletion constructs were made as described in Materials and methods. The fragments were cloned downstream of the eGFP tag and transfected into MRC5 cells. The cells were UV irradiated (7 J/m2) 24 h later and, after a further 8 h, the cells were fixed and analysed for nuclear location and focus formation as indicated.
Nuclear localization. As shown in Figure 5, pBC28, which contains a deletion of the C-terminus from amino acid 451 to 715, was located in the cell nucleus but did not relocalize into foci after UVC irradiation. Thus, the last 265 amino acids of polι are not required for transport of the protein into the nucleus but are required for foci formation. However, using pBC34, in which the C-terminal deletion was increased to 317 amino acids (400–715), the protein was no longer nuclear. On removal of the N-terminal 218 amino acids (pBC41), the protein was still nuclear (and was able to relocalize into foci), and the construct containing amino acids 219–451 (pBC52) was sufficient to confer nuclear localization. These results indicate that the sequences necessary for nuclear localization are between amino acids 219 and 451 and are likely to be between amino acids 400 and 451.
Foci formation. Foci formation was not affected by the removal of the first 218 amino acids (pBC41) but was abolished by removal of the last 80 amino acids (pBC26). To investigate the role of C-terminal sequences in foci formation, we added an artifical NLS [corresponding to the SV40 large T antigen NLS (PKKKRKV)] to the last 175 amino acids of polι (pBC30). This construct, though clearly nuclear, did not form foci after UV irradiation. When we added the same artifical NLS to the last 224 amino acids of polι (pBC36), the result was different. In this case, the protein was able to relocalize into foci. These results indicate that the domain required for foci formation is distinct from the NLS. The foci formation domain requires the last 80 amino acids as well as sequences located between amino acids 490 and 539.
Discussion
Recent studies indicate that human cells possess four Y-family DNA polymerases (polη, polι, polκ and Rev1) along with a B-family polymerase, polζ (Nelson et al., 1996), which, based on in vitro replication assays (for a review, see Goodman, 2002), all appear capable of participating (with varying efficiencies) in lesion bypass. An important question that needs to be addressed is how each of these polymerases is physically recruited to any particular DNA lesion in vivo and, once there, how the cell determines which polymerase should be employed to bypass the lesion.
Tight co-ordination of polη and polι in the nucleus
In our previous work, we showed that in human cells, polη, which is required for translesion synthesis past UV photoproducts in DNA, is localized in replication factories (Kannouche et al., 2001). Following DNA damage, the polη-containing foci accumulate as replicating forks stall at the sites of DNA damage. In the present work, we have shown that the localization pattern of human polι is very similar to that of polη. In both undamaged cells and cells damaged either uniformly or at specific sites in the nucleus, the localization patterns are the same for both polymerases (Figure 2). We have shown, using time-lapse microscopy, that polη and polι accumulate at unrepaired lesions during DNA replication at the same time and with the same changes in intensity. This strongly suggests that the assembly of the two paralogues into replication foci is tightly co-ordinated. This important result indicates that the recruitment of these different polymerases is not sequential in the nucleus. A complex containing both polymerases is likely to be pre-formed in the nucleoplasm before it relocalizes in replication factories. We have been obliged to use overexpression in all our experiments, as none of our antibodies is able to detect endogenous levels of polymerases. However, we have previously presented arguments which strongly suggest that our results reflect the localization patterns of endogenous proteins (Kannouche et al., 2001).
How does polι relocalize into intranuclear foci?
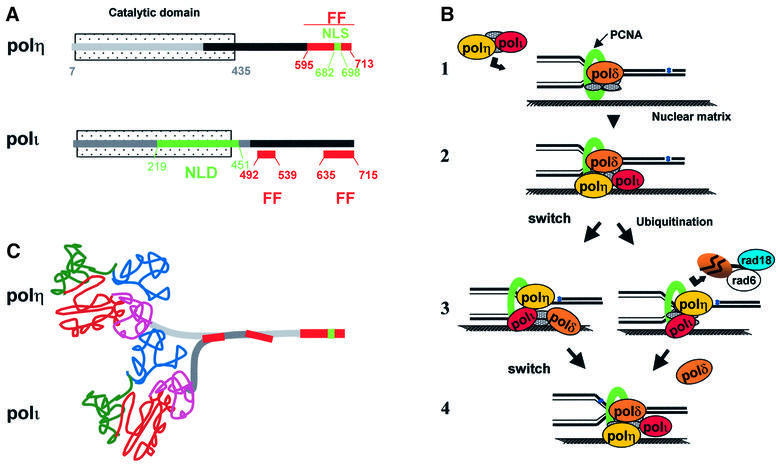
Figure 6A shows the different domains that we have identified in polη and polι. We previously showed that the C-terminal 119 amino acids of polη (595–713) were sufficient for localization into replication foci (Kannouche et al., 2001). This domain contains a putative C2H2 zinc finger, a bipartite NLS and a potential PCNA-binding site. This site recently has been shown to bind PCNA and, in doing so, stimulates the catalytic activity of polη in vitro (Haracska et al., 2001a). We have shown that the zinc finger, NLS and PCNA-binding motifs are all required for efficient localization into nuclear foci (results to be published elsewhere). Polι has neither a zinc finger nor an NLS, but has a potential PCNA-binding site. Like polη, the in vitro catalytic activity of polι is stimulated in the presence of PCNA (together with RFC and RPA) (Haracska et al., 2001b). Such findings prompted Haracska et al. to suggest that the interaction between polι and PCNA targets polι to the replication machinery (Haracska et al., 2001b). We find, however, that in XP-V cells, even though PCNA assembles into replication foci, which accumulate after UV irradiation, polι does not. Although our data do not exclude a role for PCNA in the localization of polι into replication foci, they strongly suggest that it is polη which targets polι into these foci, since (i) polι directly interacts with polη (Figure 4); (ii) the C-terminal 224 amino acids (492–715) of polι are sufficient both for interaction with polη and for localization into replication foci (Figure 6A); and (iii) in polη-defective XP-V cells, the relocalization of polι into foci is drastically diminished (Figure 3).
Fig. 6. Interactions between pol η and polι, and involvement in TLS. (A) Domain structures of polη and polι. FF, domains required for foci formation; NLS, classical nuclear localization signal; NLD, nuclear localization domain. The black bar indicates regions of interaction between the two polymerases. (B) Involvement of polymerases in bypass of damage. (1) The structure shows a replication fork attached to a nuclear matrix structure in a replication factory, with the DNA being pulled through the fork. Polη and polι are either co-localized in the factory and get recruited to the replisome or, in (2), they are an intrinsic part of the replisome, possibly by interacting with PCNA. (3) When polδ is blocked by damage, it either disengages from the primer terminus or is removed following modification, allowing polη or polι to bypass the damage. In (4), polδ is restored to the replication fork after the damage has been bypassed. (C) Scheme for interaction of polymerases. The depicted N-terminal catalytic domain is drawn in schematic form from the crystal structure of a Y-family DNA polymerase from Sulfolobus solfataricus (Ling et al., 2001), with the finger, palm, thumb and little finger domains indicated in blue, red, green and purple, respectively.
Figure 6B shows several possible mechanisms for polymerase–polymerase interactions taking place at the replication fork. Polη and polι are localized together in the replication factories, but within the factory they could either be within the body of the factory and recruited to the replisome when required, as indicated in Figure 6B,1, or they may actually be part of the replisome complex (Figure 6B,2). When the replisome encounters a lesion, there may be a switch from replicative to TLS polymerase (Figure 6B,3, left) or, alternatively, the replicative polδ may be modified by ubiquitylation (Figure 6B,3, right) and possibly degraded, and then replaced by a TLS poly merase. Since the Rad6/Rad18 E2 ubiquitin-conjugating enzyme is known to be absolutely required for TLS, at least in S.cerevisiae (Broomfield et al., 2001), it could perform the proposed ubiquitylation reaction. The TLS polymerases are inherently distributive so that, shortly after lesion bypass occurs, they would dissociate from the primer template and be replaced by polδ, either by re-engaging the ‘switched out’ polymerase or by incorporation of a new polδ molecule into the replisome (Figure 6B,4). The model of removing polδ by ubiquitylation is reminiscent of that proposed to remove RNA polymerase II blocked at lesions to enable transcription-coupled repair to take place (Ratner et al., 1998). A related model whereby the polymerase switching is mediated by ubiquitylation of PCNA has been proposed by Hoege et al. (2002).
Polι interacts with the central region of polη
A model showing how polη and polι might interact is shown in Figure 6C. Polη contains three distinct functional domains. Based on the three-dimensional structure of yeast polη, the N-terminal 435 amino acids comprise the polymerase catalytic site (Trincao et al., 2001). The C-terminal 119 amino acids (595–713) are involved in nuclear and foci localization (Kannouche et al., 2001), but no function has as yet been attributed to the intervening sequences (435–595). We have now shown (Figure 4C) that the region of polη that interacts with polι is likely to be contained within this domain. Interestingly, this sequence is not conserved in S.cerevisiae polη (Rad30), indicating that this domain is not necessary in yeast. This suggests that it could be involved in interaction with other proteins, which, like polι, are only present in higher eukaryotes.
Domain structure of polι
Despite the absence of any obvious NLS consensus motif in the amino acid sequence of polι, we observed that its nuclear localization (as opposed to relocalization into foci) is not dependent on polη. The mechanism of nuclear accumulation remains to be determined. Although polη and polι are paralogues, contain a similar N-terminal domain that comprises the polymerase catalytic site and show an identical nuclear localization into foci, the organization of their C-terminal domains is completely different. Whereas in polη the C-terminal 119 amino acids were sufficient for nuclear localization and foci formation (Kannouche et al., 2001), localization of polι in the nucleus requires sequences within amino acids 219–451, and the domain involved in foci formation may be bipartite: sequences within amino acids 492–539 and 636–715 (Figure 6A).
The role of polι in human cells
Although several studies have been carried out on the activities of polι in vitro (Johnson et al., 2000b; Tissier et al., 2000a; Vaisman and Woodgate, 2001; Vaisman et al., 2001; Zhang et al., 2001), there has been much speculation, but as yet no convincing evidence, as to its role in vivo. Our findings that the localization of polι is tightly co-ordinated with that of polη suggest that polι, like polη, is involved in TLS past DNA damage. Like other members of the Y-family, it appears able to accommodate a variety of DNA lesions in its active site but, as yet, no lesion has been identified that polι can bypass efficiently without the assistance of another polymerase. Its role in the bypass of UV photoproducts is particularly unclear due to conflicting reports on the ability of the enzyme to incorporate bases opposite T–T CPDs or T–T 6–4 photoproducts in vitro (Johnson et al., 2000b; Tissier et al., 2000a; Haracska et al., 2001b; Zhang et al., 2001). As polη is able to bypass T–T CPDs efficiently (Masutani et al., 2000; Matsuda et al., 2000), it is unlikely that polι plays a major role in TLS past this photoproduct in normal cells. However, polη and polι appear to possess roughly similar abilities to incorporate a base opposite the 3′ T of a T–T 6–4 photoproduct (Masutani et al., 2000; Tissier et al., 2000a; Zhang et al., 2000a). Neither polymerase can bypass the lesion unassisted. If, however, the (mis)incorporated bases can, as shown in vitro, be extended by another polymerase such as polζ, it is possible that polι may play a role in the bypass of T–T 6–4 photoproducts. Last, but not least, all the in vitro studies assaying the ability of Y-family polymerases to bypass UV photoproducts have been performed using only T–T photoproducts, since C-containing photoproducts are inherently unstable. It is possible that polι might be involved in the bypass of C-containing photoproducts. It is also likely that together with polζ, polι may similarly be involved in TLS past other adducts that normally block DNA replication.
In XP-V cells lacking polη, a back-up process is able to synthesize intact DNA molecules, albeit at reduced efficiency (Lehmann et al., 1975). This process is highly mutagenic as XP-V cells are hypermutable to UV (Maher et al., 1976). Presumably, in XP-V cells, another polymerase substitutes for polη and bypasses the UV photoproducts with low efficiency and low fidelity. polι is a candidate for the alternative polymerase, as neither polκ nor Rev1 appear able to incorporate bases opposite UV photoproducts (Johnson et al., 2000a; Ohashi et al., 2000; Zhang et al., 2000b, 2002). However, our observation that polι focus formation is dramatically reduced in XP-V cells would appear inconsistent with such a hypothesis. The possibility remains, however, that the reduced number of polι-containing foci might be sufficient to carry out some TLS past UV photoproducts, albeit with reduced efficiency and fidelity, thus conferring the hypermutable phenotype observed in XP-V cells.
Finally, our observation that polι is located in replication foci in undamaged cells suggests that it may also play a general role in the maintenance of genome integrity during DNA replication. This might occur though the incorporation of guanine opposite uracil resulting from deamination of cytosine (Vaisman and Woodgate, 2001), or opposite the AP site generated during the subsequent base excision repair of the uracil.
Materials and methods
Cell lines and culture conditions
The SV40-transformed human fibroblasts MRC5V1 (normal) and XP30RO(sv) (XP-V) (also designated GM3617) (Cleaver et al., 1999) were grown in Eagle’s minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS). The designations of the cell lines are abbreviated to MRC5 and XP30RO. Transfection using Fugene, isolation of stable clones and UV irradiation were carried out as described previously (Kannouche et al., 2001).
Construction of expression vectors
pCDNA-polη was constructed as previously described (Kannouche et al., 2001). Full-length cDNA encoding polι was obtained by PCR using pJM296 (McDonald et al., 1999) as template, with Pfu DNA polymerase and primers I-A and I-B (Table I).
Table I. Primers used in deletion constructs.
| Primer name | Sequence |
|---|---|
| I-A | 5′-GATATCATGGAACTGGCGGACGTGGGGGCG-3′ |
| I-B | 5′-GGATCCTTATTTATGTCCAATGTGGAAATC-3′ |
| I-C | 5′-CTCGAGTGGCTACTGGACAGGATCGA-3′ |
| I-D | 5′-GGATCCCTAATGTGTTAATGGCTTAAAAAATGA-3′ |
| I-E | 5′-CTCGAGTGGAACTGGCGGACGTGGGGGCG-3′ |
| I-F | 5′-GGATCCTTATTTATGTCCAATGTGGAAATC-3′ |
| I1 | 5′-CTCGAGCTCGAACTGGCGGACGTGGGGGCG-3′ |
| I2 | 5′-GGATCCTTATTTATGTCCAATGTGGAAATC-3′ |
| I3 | 5′-CTCGAGCTCGAACTGGCGGACGTGGGGGCG-3′ |
| I4 | 5′-GGATCCTGGCATCTTCACATTCACCATATTTCGAAAAAG-3′ |
| I5 | 5′-CTCGAGCCAAAAAAGAAGCGCAAGGTCGCCTCTAGAGCAGTATTATCT-3′ |
| I6 | 5′-GGATCCTTATTTATGTCCAATGTGGAAATC-3′ |
| I7 | 5′-CTCGAGCCAAAAAAGAAGCGCAAGGTCAATGAATTCCCACTCTGTTCACTTCC-3′ |
| I8 | 5′-GGATCCTTATTTATGTCCAATGTGGAAATC-3′ |
| I9 | 5′-CATTCATTTCCAAACCTGCAGAGTGAGCAAC-3′ |
| I10 | 5′-GTTGCTCACTCTGCAGGTTTGGAAATGAATG-3′ |
The PCR product was digested with EcoRV and BamHI and inserted into the EcoRV–BamHI sites of pCDNA3.1zeo downstream of the CMV promoter (Invitrogen), producing the plasmid pCDNA-polι.
peGFP-polη was produced as previously described and was able to correct the UV sensitivity of polη-defective XP-V cells (Kannouche et al., 2001). To generate peYFP-polη, polη cDNA lacking the ATG initiator codon was amplified by PCR using pCDNA-polη as template, Pfu DNA polymerase and the primers I-C and I-D (Table I). The product was digested with XhoI and BamHI and inserted into the XhoI–BamHI sites of the peYFP–C1 vector. PeCFP-polι was produced in a similar way, using pCDNA-polι as template and the primers I-E and I-F.
A series of eGFP-tagged deletion mutants of polι was generated to study the cellular organization of polι. All polι deletion mutants were cloned in-frame downstream of the eGFP cDNA in peGFP-C3.1. Polι cDNA fragments were generated using PCR with primers (see Table I) containing an XhoI site at the 5′ end and a BamHI site at the 3′ end. The PCR products were digested with XhoI and BamHI and cloned into peGFP-C3 digested with the same enzymes. For full-length polι (pBC21), we used primers I1 and I2. Plasmid pBC28 was obtained by digesting pBC21 with XmnI and BamHI to remove a 1 kb fragment of polι cDNA from the 3′ end of the open reading frame (ORF). The ends were made blunt with the Klenow enyzme and vector was religated. Plasmids pBC34, pBC30 and pBC36 were constructed using pBC21 as template and PCR with primers I3 and I4 (5′ 1200 bp), I5 and I6 (3′ 531 bp) and I7 and I8 (3′ 672 bp), respectively. To construct plasmid pBC26, a PstI site at nucleotide 1913 of the cDNA was introduced by site-directed mutagenesis. First, plasmid pBC21 was used as template, and primers I9 and I10. The resulting plasmid pBC25 contained a silent change with a PstI site in the cDNA sequence at nucleotide 1913. pBC26 was obtained by digesting pBC25 with PstI and BamHI to remove a 0.2 kb fragment of polι cDNA located in the 3′ end of the ORF. The ends were made blunt with the Klenow enyzme and the vector was religated. All PCR products were checked by DNA sequencing.
Live cell microscopy and image analysis
peCFP-polι (0.1 µg/ml) and peYFP-polη (0.1 µg/ml) were microinjected into MRC5 cells. After 8 h, cells were irradiated with 10 J/m2. For monitoring living cell cultures, MRC5 cells were incubated in a phenol red-free medium including 10% FCS. Fluorescence images of cell nuclei were acquired on an Axiovert TM 135 microscope (Carl Zeiss) equipped with a 100× NA 1.4 objective lens and an Orca ER CCD camera (Hamamatsu) using Acquisition Manager (Kinetic Imaging). Real time excitation measurements were made by alternating excitation at 440 ± 12.5 and 500 ± 10 nm, and detection of CFP and YFP emission at 465 ± 12 and 530 ± 15 nm, respectively. A CFP/YFP filter set (Omega XF 135) was used with excitation and emission filter wheels. The separate pairs of excitation and emission filters suppress the cross-talk, and a stationary shared dichroic mirror ensures that there is no lateral shift between the two fluorescent channels. Integrated intensity of either channel in cell nuclei did not show systematic decline. Therefore, we concluded that bleaching was either insignificant or was compensated by continuing production of the proteins. Foci in cell nuclei were detected by Sobel image enhancement followed by thresholding in every individual frame of a movie, and their positions and integrated intensities were calculated using intensity values from the original images. These data were used to track an individual focus automatically through space and time. Processing of images and data was performed in Mathematica (Wolfram Research), and the images and plots were finalized using Illustrator (Adobe).
Immunofluorescence microscopy and western blotting
Fixation of the cells and immunofluorescence microscopy and western blotting were carried out as described previously (Kannouche et al., 2001). Polι protein was detected using a rabbit polyclonal antibody directed against a keyhole limpet haemocyanin (KLH)-conjugated peptide corresponding to the extreme C-terminal 15 amino acid residues (AEWKRTGSDFHIGHK) of polι. The antibody was diluted 1:500 for immunofluorescence and 1:4000 for western blotting. Polη protein was detected using a rabbit polyclonal antibody (Kannouche et al., 2001) diluted 1:2500. PC10 monoclonal anti-PCNA protein was from Santa Cruz Biotechnology. For immunofluorescence, all antibodies were diluted in phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA). No foci were observed in cells transfected with empty vectors, and no immunofluorescent signals were observed without primary antibody.
Two-hybrid interactions
An interaction between human polη and polι was demonstrated in vivo using the S.cerevisiae two-hybrid Matchmaker III system (Clontech, Palo Alto, CA). The human polη gene was cloned as an ∼2.5 kb NdeI–XhoI fragment from pET21-polη into the NdeI–SalI-digested GAL4 DNA-binding domain plasmid, pGBKT7, to generate pAR204. The full-length human polι gene was cloned into the pACT2 activation domain vector by ligating an ∼2.5 kb NcoI–SalI fragment from pJM299 (Tissier et al., 2000b) into the NcoI–XhoI-digested vector to generate pAR116. Both pAR116 and pAR204 were then transformed into the S.cerevisiae strain AH109. Transformants were selected on DOBA-Trp-Leu (Bio 101) plates. Colonies were subsequently replica plated on DOBA-Trp-Leu-His-Ade + X-α-Gal (Bio 101, Vista, CA) plates, to confirm the activation of the reporter genes.
Deletion derivatives of the pACT2-polι plasmid, pAR116, were constructed as follows. Plasmid pAR216 containing the N-terminal 278 residues from polι was generated by ligating a 0.9 kb NcoI–XhoI fragment from pAR124 (A.R.Fernández de Henestrosa and R.Woodgate, in preparation) into the similarly digested pACT2 vector. pAR218, containing the N-terminal 492 amino acids of polι, was constructed by cloning a 1.5 kb NcoI–EcoRI fragment from pAR110 (A.R.Fernández de Henestrosa and R.Woodgate, in preparation) into the similarly digested pACT2. Finally, a plasmid, pAR220, expressing the C-terminal 224 amino acids of polι, was constructed by cloning a 1 kb NcoI–DraI fragment from pAR130 (A.R.Fernández de Henestrosa and R.Woodgate, in preparation) into pACT2. Analysis was as described above.
In vitro transcription/translation of polη
In vitro transcription/translation of full-length polη (1–713) and two N-terminal truncated proteins, polη (352–713) and polη (595–713), was performed using a TNT-coupled lysate system (Promega) according to the manufacturer’s instructions. The expression vectors encoding the full-length polη (pGBKT7-polη) and its truncated versions (cDNAs cloned in pET29a) were added separately to the reaction mixtures and then incubated for 90 min at 30°C in the presence of [35S]methionine. Reaction products were analysed directly by SDS–PAGE and used in both far-western and GST pull-down assays.
GST fusion proteins
Full-length GST–polι was purified from Sf9 insect cells infected with pJM299 baculovirus, as previously reported (Tissier et al., 2000b). A plasmid, pAR208, expressing GST fused to the C-terminal 224 residues of polι was constructed by cloning a 0.7 kb EcoRI–SalI fragment encoding the C-terminus of polι from pAR110 (a pGBKT7 derivative expressing full-length polι; A.R.Fernández de Henestrosa and R.Woodgate, in preparation), into the similarly digested vector, pGEX4T1 (Amersham Pharmacia Biotech). Extracts were made from 500 ml of exponentially growing DH5α/pGEX4T1 or DH5α/pAR208 cells that had been induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. Both the wild-type GST protein and the GST–polι (492–715) protein were subsequently purified by glutathione–Sepharose affinity chromatography following the manufacturer’s instructions (Amersham Pharmacia Biotech).
Far-western blot analysis
Purified GST fusion proteins were separated by 4–20% SDS–PAGE (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Invitrogen). Membranes were incubated overnight with 35S-labelled Polη protein at 4°C and then washed three times for a total time of 14 min at 4°C, dried briefly and scanned with a FujiFilm FLA-3000 phosphoimager.
GST pull-down assay
Equal amounts of GST or GST–polι (492–715) (∼100 µg) coupled to glutathione–agarose beads (15 µl) were mixed with 35S-labelled polη in 200 µl of binding buffer (PBS, 0.5% BSA and 0.2% Triton X-100), containing ‘Complete’ protease inhibitors (Roche Molecular Biologicals, Indianapolis, IN) for 15 min at 37°C. The beads were subsequently washed four times with binding buffer and the bound proteins separated on a 4–20% SDS–polyacrylamide gel.
Co-infection in Sf9 insect cells and pull-downs of polι and polη
Recombinant virus expressing polι tagged with GST at its N-terminus was made as previously described (Tissier et al., 2000b).Virus expressing polη tagged with His6 at its C-terminus was kindly provided by Fumio Hanaoka (Masutani et al., 2000). Sf9 cells were infected or co-infected with baculovirus expressing GST–polι, His6-polη or both. In brief, the cells were harvested 72 h after infection, washed with ice-cold PBS, resuspended in 8 vols of ice-cold buffer A (20 mM sodium phosphate pH 7.3, 10% glycerol, 10 mM β-mercaptoethanol, 300 mM NaCl, 1% NP-40, 1× protease inhibitor cocktail) and incubated on ice for 30 min with occasional agitation. The suspension was centrifuged at 12 000 g for 50 min.
GST pull-down experiments. The supernatants were incubated with glutathione–agarose beads (Amersham Pharmacia Biotech) equilibrated with buffer A for 2 h at 4°C. Beads were washed three times with 10 vols of buffer A, and the bound proteins were detected by western blotting.
Ni2+ pull-down experiments. The supernatants were adjusted to 20 mM imidazole and incubated with Ni-NTA–agarose (Qiagen) for 2 h at 4°C. Beads were washed three times with 10 vols of buffer A plus 20 mM imidazole, resuspended in the last wash, and subsequently packed into a disposable column. The bound proteins were eluted with buffer A containing 250 mM imidazole. Fractions were then analysed by western blotting.
Acknowledgments
Acknowledgements
We would like to thank Fumio Hanaoka for the His6-tagged polη baculovirus. This work was supported in part by the NIH Intramural Research Program (A.R.F., A.V. and R.W.), by AICR grant 99-063 (A.R.L. and P.K.), and MRC Programme grant G1132 (A.R.L.).
References
- Arlett C.F., Harcourt,S.A. and Broughton,B.C. (1975) The influence of caffeine on cell survival in excision-proficient and excision-deficient xeroderma pigmentosum and normal human cell strains following ultraviolet light irradiation. Mutat. Res., 33, 341–346. [DOI] [PubMed] [Google Scholar]
- Broomfield S., Hryciw,T. and Xiao,W. (2001) DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res., 486, 167–184. [DOI] [PubMed] [Google Scholar]
- Cleaver J.E. et al. (1999) Increased ultraviolet sensitivity and chromosomal instability related to p53 function in the xeroderma pigmentosum variant. Cancer Res., 59, 1102–1108. [PubMed] [Google Scholar]
- Friedberg E.C., Wagner,R. and Radman,M. (2002) Specialized DNA polymerases, cellular survival and the genesis of mutations. Science, 296, 1627–1630. [DOI] [PubMed] [Google Scholar]
- Goodman M.F. (2002) Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu. Rev. Biochem., 71, 17–50. [DOI] [PubMed] [Google Scholar]
- Guo D., Wu,X., Rajpal,D.K., Taylor,J.S. and Wang,Z. (2001) Translesion synthesis by yeast DNA polymerase ζ from templates containing lesions of ultraviolet radiation and acetylaminofluorene. Nucleic Acids Res., 29, 2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L., Johnson,R.E., Unk,I., Phillips,B., Hurwitz,J., Prakash,L. and Prakash,S. (2001a) Physical and functional interactions of human DNA polymerase η with PCNA. Mol. Cell. Biol., 21, 7199–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L., Johnson,R.E., Unk,I., Phillips,B.B., Hurwitz,J., Prakash,L. and Prakash,S. (2001b) Targeting of human DNA polymerase ι to the replication machinery via interaction with PCNA. Proc. Natl Acad. Sci. USA, 98, 14256–14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C., Pfander,B., Moldovan,G.-L., Pyrolowakis,G. and Jentsch,S. (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature, 419, 135–141. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Kondratick,C.M., Prakash,S. and Prakash,L. (1999) hRAD30 mutations in the variant form of xeroderma pigmentosum. Science, 285, 263–265. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Prakash,S. and Prakash,L. (2000a) The human DINB1 gene encodes the DNA polymerase Polθ. Proc. Natl Acad. Sci. USA, 97, 3838–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.E., Washington,M.T., Haracska,L., Prakash,S. and Prakash,L. (2000b) Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature, 406, 1015–1019. [DOI] [PubMed] [Google Scholar]
- Kannouche P., Broughton,B.C., Volker,M., Hanaoka,F., Mullenders,L.H.F. and Lehmann,A.R. (2001) Domain structure, localization and function of DNA polymerase η, defective in xeroderma pigmentosum variant cells. Genes Dev., 15, 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A.R., Kirk-Bell,S., Arlett,C.F., Paterson,M.C., Lohman,P.H.M., de Weerd-Kastelein,E.A. and Bootsma,D. (1975) Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc. Natl Acad. Sci. USA, 72, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H., Boudsocq,F., Woodgate,R. and Yang,W. (2001) Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell, 107, 91–102. [DOI] [PubMed] [Google Scholar]
- Maher V.M., Ouellette,L.M., Curren,R.D. and McCormick,J.J. (1976) Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature, 261, 593–595. [DOI] [PubMed] [Google Scholar]
- Masutani C. et al. (1999) The human XPV (xeroderma pigmentosum variant) gene encodes human polymerase η. Nature, 399, 700–704. [DOI] [PubMed] [Google Scholar]
- Masutani C., Kusumoto,R., Iwai,S. and Hanaoka,F. (2000) Accurate translesion synthesis by human DNA polymerase η. EMBO J., 19, 3100–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Bebenek,K., Masutani,C., Hanaoka,F. and Kunkel,T. (2000) Low fidelity DNA synthesis by human DNA polymerase-η. Nature, 404, 1011–1013. [DOI] [PubMed] [Google Scholar]
- McDonald J.P., Rapic-Otrin,V., Epstein,J.A., Broughton,B.C., Wang,X., Lehmann,A.R., Wolgemuth,D.J. and Woodgate,R. (1999) Novel human and mouse homologs of Saccharomyces cerevisiae DNA polymerase η. Genomics, 60, 20–30. [DOI] [PubMed] [Google Scholar]
- McDonald J.P., Tissier,A., Frank,E.G., Iwai,S., Hanaoka,F. and Woodgate,R. (2001) DNA polymerase ι and related Rad30-like enzymes. Philos. Trans. R. Soc. Lond. B Biol. Sci., 356, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.R., Lawrence,C.W. and Hinkle,D.C. (1996) Thymine–thymine dimer bypass by yeast DNA polymerase ζ. Science, 272, 1646–1649. [DOI] [PubMed] [Google Scholar]
- Ohashi E., Ogi,T., Kusumoto,R., Iwai,S., Masutani,C., Hanaoka,F. and Ohmori,H. (2000) Error-prone bypass of certain DNA lesions by the human DNA polymerase κ. Genes Dev., 14, 1589–1594. [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. et al. (2001) The Y-family of DNA polymerases. Mol. Cell, 8, 7–8. [DOI] [PubMed] [Google Scholar]
- Ratner J.N., Balasubramanian,B., Corden,J., Warren,S.L. and Bregman,D.B. (1998) Ultraviolet radiation-induced ubiquitination and proteasomal degradation of the large subunit of RNA polymerase II. Implications for transcription-coupled DNA repair. J. Biol. Chem., 273, 5184–5189. [DOI] [PubMed] [Google Scholar]
- Tissier A., Frank,E.G., McDonald,J.P., Iwai,S., Hanaoka,F. and Woodgate,R. (2000a) Misinsertion and bypass of thymine–thymine dimers by human DNA polymerase ι. EMBO J., 19, 5259–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier A., McDonald,J.P., Frank,E.G. and Woodgate,R. (2000b) Polι, a remarkably error-prone human DNA polymerase. Genes Dev., 14, 1642–1650. [PMC free article] [PubMed] [Google Scholar]
- Trincao J., Johnson,R.E., Escalante,C.R., Prakash,S., Prakash,L. and Aggarwal,A.K. (2001) Structure of the catalytic core of S.cerevisiae DNA polymerase η: implications for translesion DNA synthesis. Mol. Cell, 8, 417–426. [DOI] [PubMed] [Google Scholar]
- Vaisman A. and Woodgate,R. (2001) Unique misinsertion specificity of polι may decrease the mutagenic potential of deaminated cytosines. EMBO J., 20, 6520–6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisman A., Tissier,A., Frank,E.G., Goodman,M.F. and Woodgate,R. (2001) Human DNA polymerase ι promiscuous mismatch extension. J. Biol. Chem., 276, 30615–30622. [DOI] [PubMed] [Google Scholar]
- Volker M. et al. (2001) Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell, 8, 213–224. [DOI] [PubMed] [Google Scholar]
- Woodgate R. (1999) A plethora of lesion-replicating DNA polymerases. Genes Dev., 13, 2191–2195. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yuan,F., Wu,X., Rechkoblit,O., Taylor,J.S., Geacintov,N.E. and Wang,Z. (2000a) Error-prone lesion bypass by human DNA polymerase η. Nucleic Acids Res., 28, 4717–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yuan,F., Wu,X., Wang,M., Rechkoblit,O., Taylor,J.S., Geacintov,N.E. and Wang,Z. (2000b) Error-free and error-prone lesion bypass by human DNA polymerase κ in vitro. Nucleic Acids Res., 28, 4138–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yuan,F., Wu,X. and Wang,Z. (2000c) Preferential incorporation of G opposite template T by the low-fidelity human DNA polymerase ι. Mol. Cell. Biol., 20, 7099–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yuan,F., Wu,X., Taylor,J.S. and Wang,Z. (2001) Response of human DNA polymerase ι to DNA lesions. Nucleic Acids Res., 29, 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu,X., Rechkoblit,O., Geacintov,N.E., Taylor,J.S. and Wang,Z. (2002) Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res., 30, 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]