Abstract
1 The study was designed to determine the mechanism by which vigabatrin causes a fall in plasma phenytoin concentrations when added to the drug therapy of eight epileptic patients.
2 Total plasma phenytoin concentration was measured before and at intervals during 5 weeks' treatment with vigabatrin.
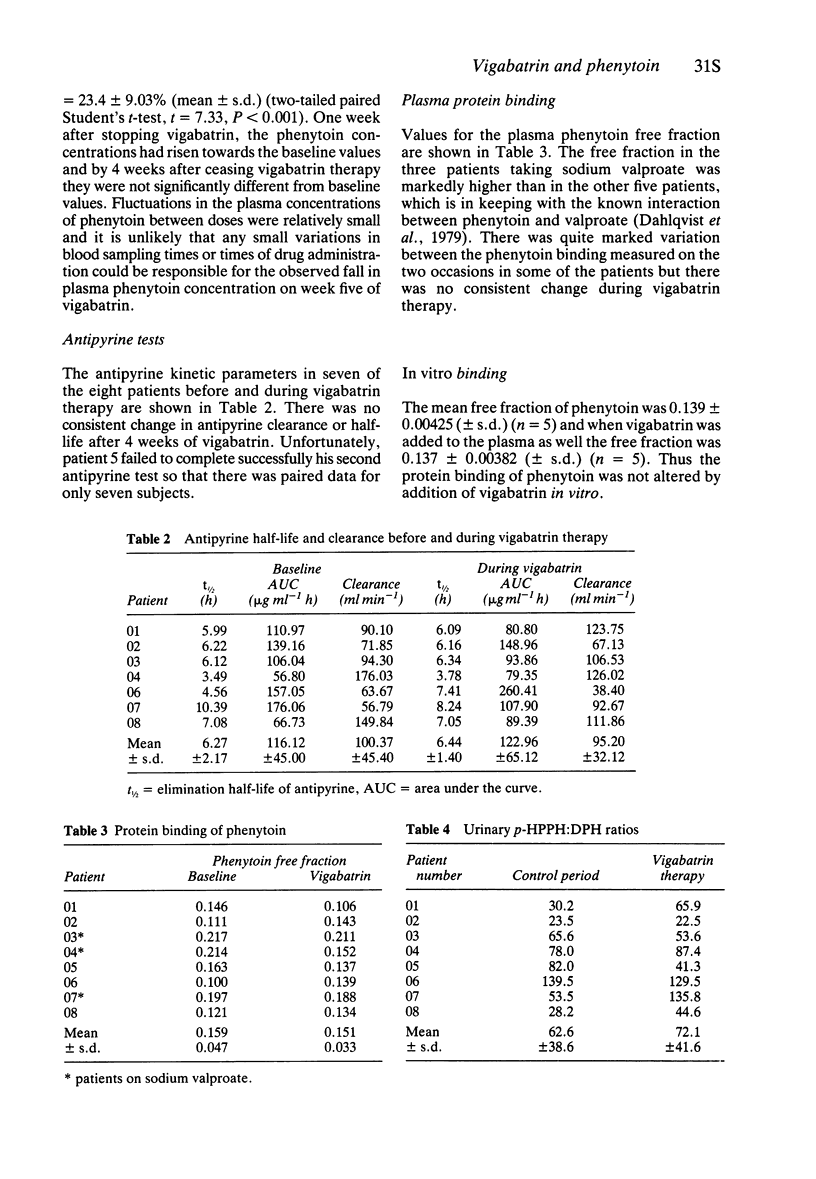
3 Plasma protein binding of phenytoin, the urinary ratio of phenytoin to 5-(p-hydroxyphenyl)-5-phenylhydantoin, and antipyrine clearance were measured before and at the end of treatment period.
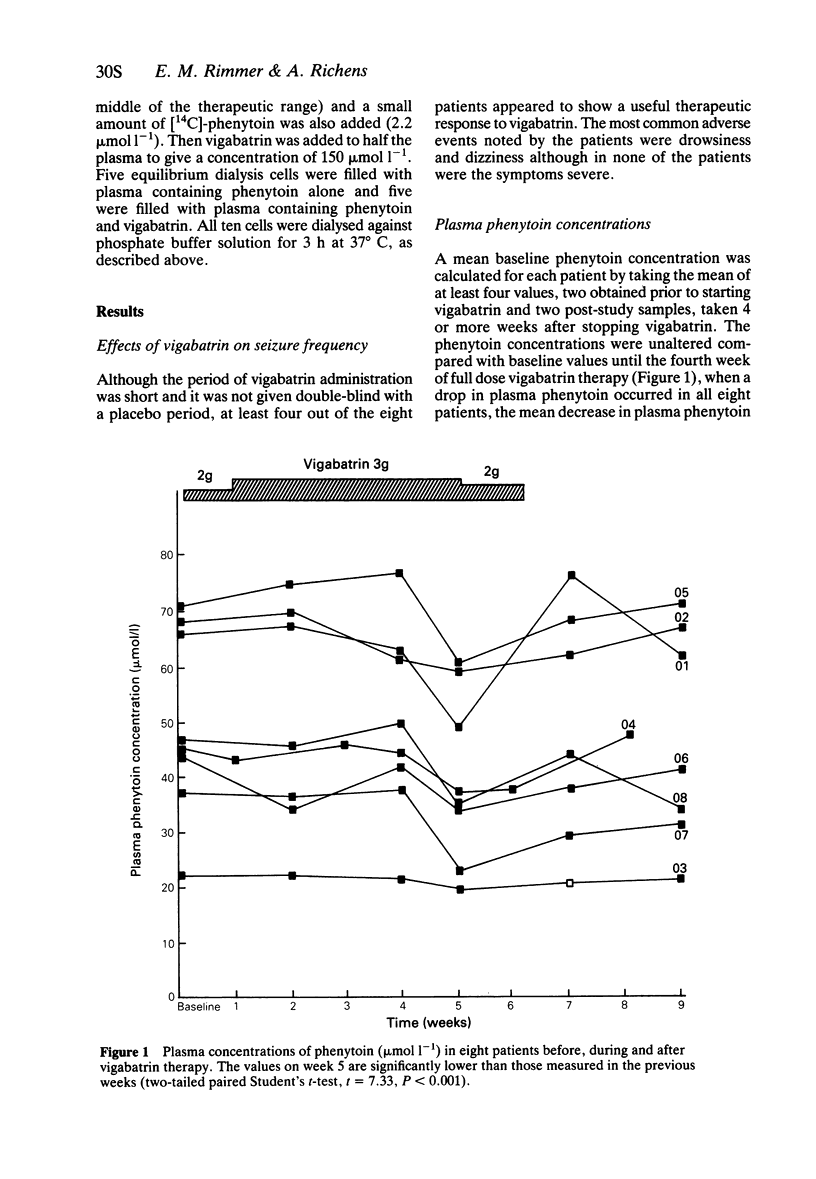
4 Mean plasma phenytoin concentration fell significantly by 23% during the fifth week.
5 No change was found in any of the other measures.
6 Although an interaction between phenytoin and vigabatrin has been confirmed, the mechanism has not been elucidated.
Keywords: vigabatrin, phenytoin, interactions, protein binding
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner F., Hooper W. D., Sutherland J. M., Eadie M. J., Tyrer J. H. The renal handling of diphenylhydantoin and 5-(p-hydroxyphenyl)-5-phenylhydantoin. Clin Pharmacol Ther. 1973 Sep-Oct;14(5):791–796. doi: 10.1002/cpt1973145791. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Mattson R. H., Penry J. K., Smith D. B., Treiman D. M., Wilder B. J., Ben-Menachem E., Napoliello M. J., Sherry K. M., Szabo G. K. Vigabatrin for refractory complex partial seizures: multicenter single-blind study with long-term follow-up. Neurology. 1987 Feb;37(2):184–189. doi: 10.1212/wnl.37.2.184. [DOI] [PubMed] [Google Scholar]
- Dahlqvist R., Borgå O., Rane A., Walsh Z., Sjöqvist F. Decreased plasma protein binding of phenytoin in patients on valproic acid. Br J Clin Pharmacol. 1979 Dec;8(6):547–552. doi: 10.1111/j.1365-2125.1979.tb01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. S., Mucklow J. C., Murray S., Davies D. S. Assessment of antipyrine kinetics by measurement in saliva. Br J Clin Pharmacol. 1976 Apr;3(2):321–325. doi: 10.1111/j.1365-2125.1976.tb00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Abernethy D. R., Divoll M. Kinetics of low-dose intravenous antipyrine: use of liquid chromatography. Int J Clin Pharmacol Ther Toxicol. 1983 Feb;21(2):51–55. [PubMed] [Google Scholar]
- Karlén B., Garle M., Rane A., Gutova M., Lindborg B. Assay of the major (4-hydroxylated) metabolites of diphenylhydantoin in human urine. Eur J Clin Pharmacol. 1975 Jun 13;8(5):359–363. doi: 10.1007/BF00562663. [DOI] [PubMed] [Google Scholar]
- Park B. K. Assessment of the drug metabolism capacity of the liver. Br J Clin Pharmacol. 1982 Nov;14(5):631–651. doi: 10.1111/j.1365-2125.1982.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E., Hedges A., Makki K. A., Ruprah M., Wilson J. F., Richens A. A comparative study of the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol. 1984 Sep;18(3):401–410. doi: 10.1111/j.1365-2125.1984.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer E. M., Buss D. C., Routledge P. A., Richens A. Should we routinely measure free plasma phenytoin concentration? Br J Clin Pharmacol. 1984 Jan;17(1):99–102. doi: 10.1111/j.1365-2125.1984.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer E. M., Richens A. Double-blind study of gamma-vinyl GABA in patients with refractory epilepsy. Lancet. 1984 Jan 28;1(8370):189–190. doi: 10.1016/s0140-6736(84)92112-3. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979 Sep;26(3):275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]
- Wang S. T., Peter F. The Abbott TDx fluorescence polarization immunoassay and liquid chromatography compared for five anticonvulsant drugs in serum. Clin Chem. 1985 Mar;31(3):493–494. [PubMed] [Google Scholar]


