Abstract
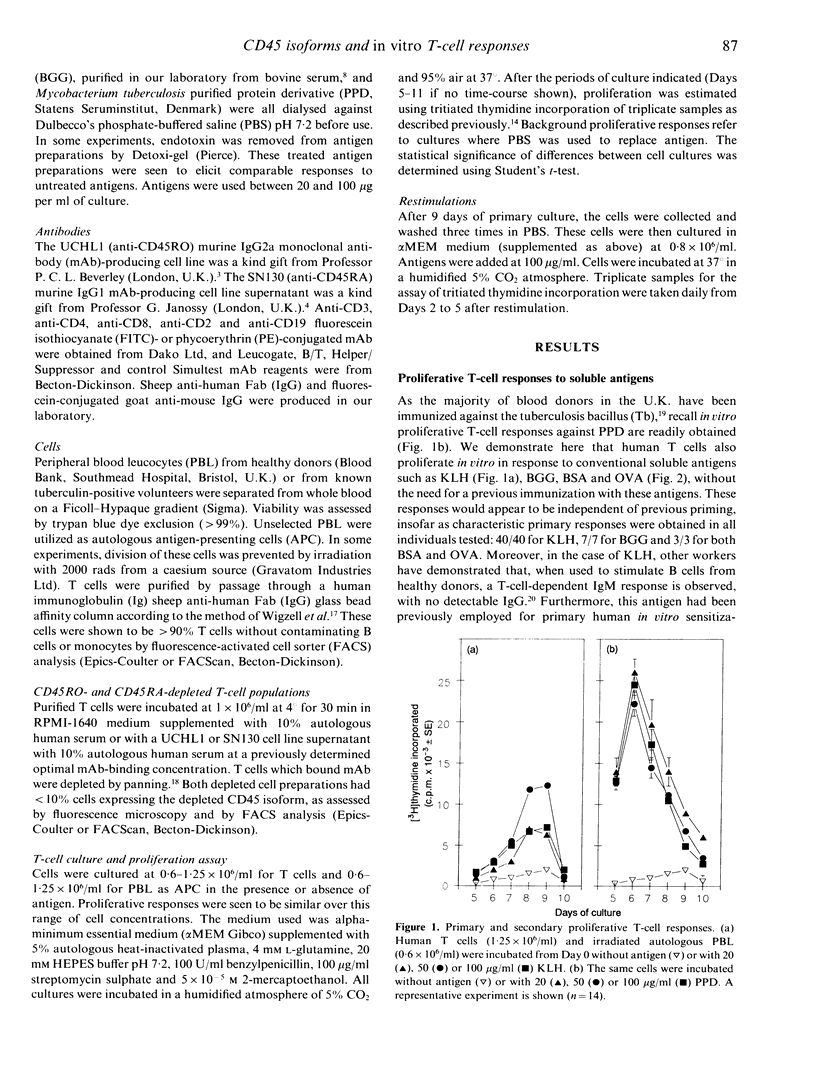
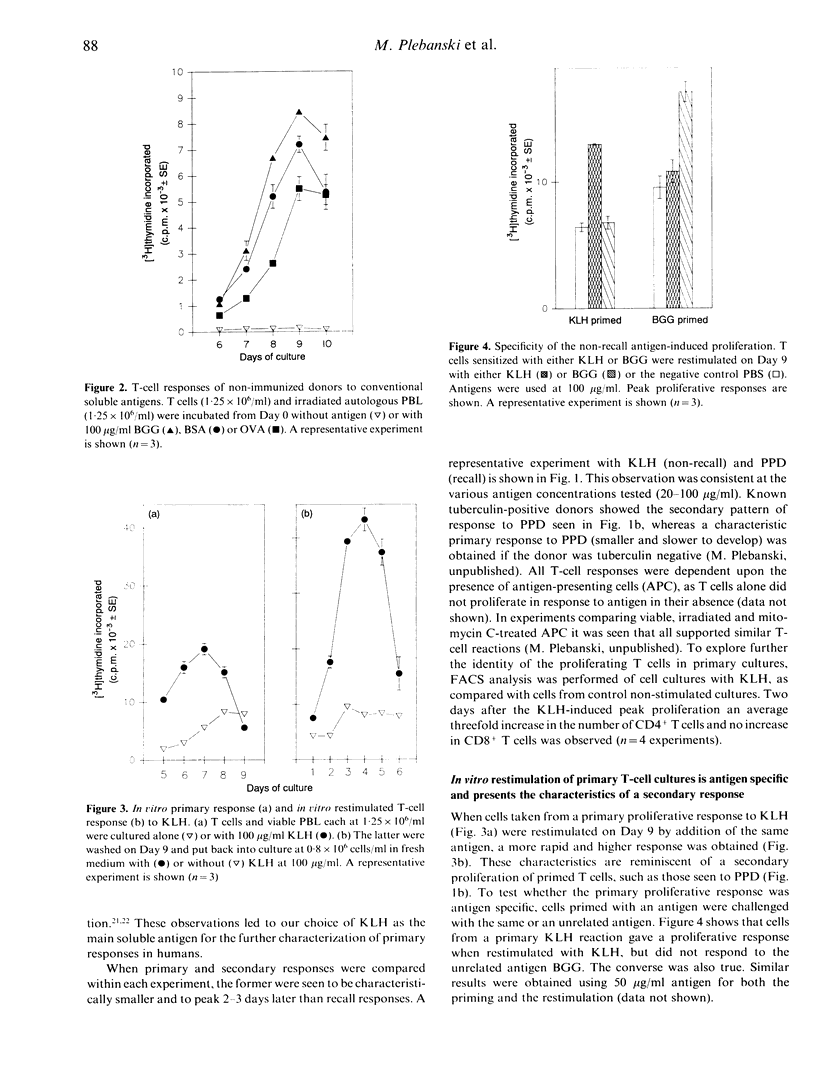
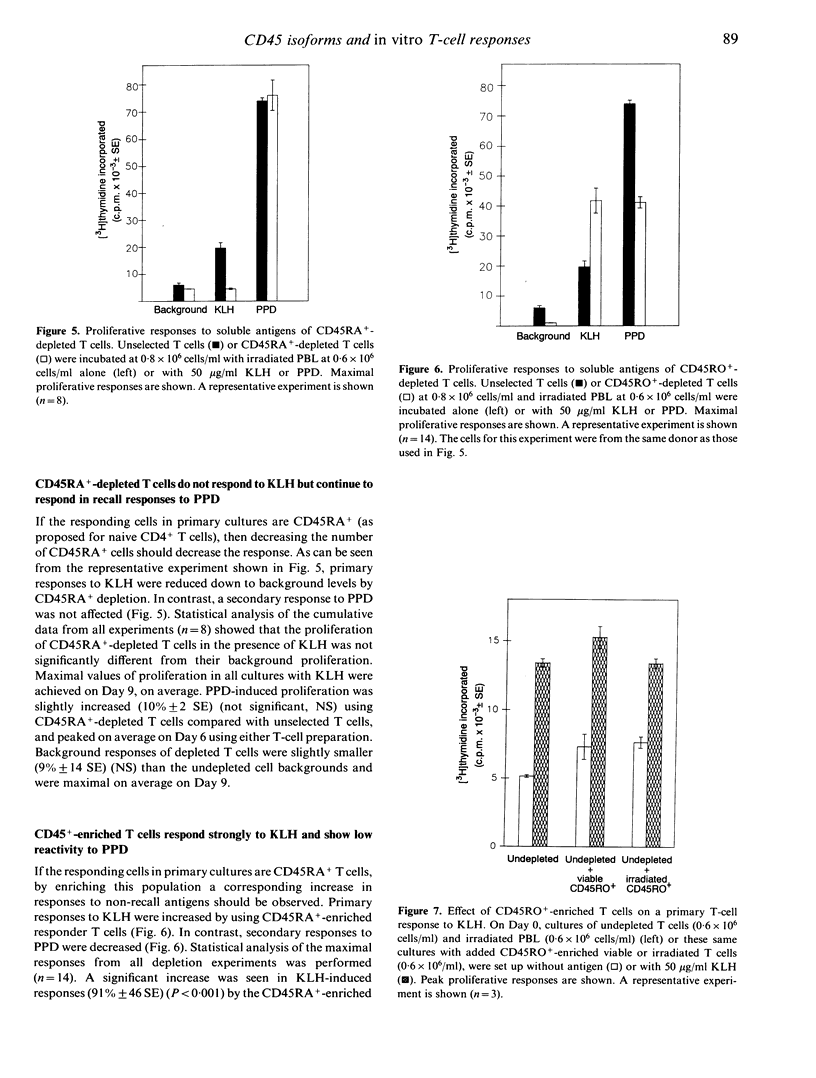
A culture system has been developed which consistently supports in vitro proliferative responses to conventional soluble antigens by human CD4+ T cells from non-immunized donors. T cells exposed to an antigen in primary cultures could be restimulated in vitro in an antigen-specific manner to give secondary responses with greater magnitudes and a more rapid onset than the initial reaction. To characterize further the responding T-cell population in primary compared with secondary reactions, T cells were depleted of CD45RA+ or CD45RO+ cells and stimulated with recall and non-recall antigens. It was found that the soluble non-recall antigen keyhole limpet haemocyanin did not stimulate CD45RO+ T cells, yet induced strong proliferative responses from CD45RA+ T cells. Conversely, it was confirmed that human CD45RO+ T cells respond to the recall antigen-purified protein derivative from Mycobacterium tuberculosis. Cell mixing experiments indicated that CD45RO+ T cells are unlikely to have any suppressive effect on the reactivity of CD45RA+ cells to non-recall antigens. These data provide new support for the hypothesis that CD45RA+ represents the naive and CD45RO+ the memory phenotype of human CD4+ T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Is T-cell memory maintained by crossreactive stimulation? Immunol Today. 1990 Jun;11(6):203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- Burtles S. S., Taylor R. B., Hooper D. C. Bovine gamma globulin-specific CD4+ T cells are retained by bovine gamma-globulin-tolerant mice. Eur J Immunol. 1990 Jun;20(6):1273–1279. doi: 10.1002/eji.1830200612. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Luqman M., Rojo J., Yagi J., Baron J. L., Woods A., Janeway C. A., Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990 Oct;20(10):2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- Hensen E. J., Elferink B. G. Primary sensitisation and restimulation of human lymphocytes with soluble antigen in vitro. Nature. 1979 Jan 18;277(5693):223–225. doi: 10.1038/277223a0. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Taylor R. B. Specific helper T cell reactivity against autologous erythrocytes implies that self tolerance need not depend on clonal deletion. Eur J Immunol. 1987 Jun;17(6):797–802. doi: 10.1002/eji.1830170610. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Young J. L., Elson C. J., Taylor R. B. Murine T cells reactive against autologous erythrocytes: evidence for in vitro and in vivo priming with mouse and rat red blood cells. Cell Immunol. 1987 Apr 15;106(1):53–61. doi: 10.1016/0008-8749(87)90149-3. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Lombardi G., Batchelor J. R., Reinsmoen N., Bach F. H. The molecular basis of alloreactivity. Immunol Today. 1990 Mar;11(3):83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Lee W. T., Yin X. M., Vitetta E. S. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J Immunol. 1990 May 1;144(9):3288–3295. [PubMed] [Google Scholar]
- Merkenschlager M., Ikeda H., Wilkinson D., Beverly P. C., Trowsdale J., Fisher A. G., Altmann D. M. Allorecognition of HLA-DR and -DQ transfectants by human CD45RA and CD45R0 CD4 T cells: repertoire analysis and activation requirements. Eur J Immunol. 1991 Jan;21(1):79–88. doi: 10.1002/eji.1830210113. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F. Primary in vitro anti-KLH antibody formation by peripheral blood lymphocytes in man: detection with a radioimmunoassay. J Immunol. 1981 Aug;127(2):514–517. [PubMed] [Google Scholar]
- Rodey G. E., Luehrman L. K., Thomas D. W. In vitro primary immunization of human peripheral blood lymphocytes to KLH: evidence for HLA-D region restriction. J Immunol. 1979 Nov;123(5):2250–2254. [PubMed] [Google Scholar]
- Rothstein D. M., Yamada A., Schlossman S. F., Morimoto C. Cyclic regulation of CD45 isoform expression in a long term human CD4+CD45RA+ T cell line. J Immunol. 1991 Feb 15;146(4):1175–1183. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Cooper M. D., Clement L. T. Human lymphocyte differentiation antigens HB-10 and HB-11. II. Differential production of B cell growth and differentiation factors by distinct helper T cell subpopulations. J Immunol. 1985 May;134(5):2989–2994. [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Wallace D. L., Beverley P. C. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990 Mar;69(3):460–467. [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to a soluble protein antigen in vitro. Immunology. 1990 Nov;71(3):411–416. [PMC free article] [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to herpes simplex virus in vitro. Immunology. 1991 Jan;72(1):34–39. [PMC free article] [PubMed] [Google Scholar]


