Abstract
Hundreds of thousands of people worldwide live or work in close proximity to steel mills. Integrated steel production generates chemical pollution containing compounds that can induce genetic damage (1, 2). Previous investigations of herring gulls in the Great Lakes demonstrated elevated DNA mutation rates near steel mills (3, 4) but could not determine the importance of airborne or aquatic routes of contaminant exposure, or eliminate possible confounding factors such as nutritional status and disease burden. To address these issues experimentally, we exposed laboratory mice in situ to ambient air in a polluted industrial area near steel mills. Heritable mutation frequency at tandem-repeat DNA loci in mice exposed 1 km downwind from two integrated steel mills was 1.5- to 2.0-fold elevated compared with those at a reference site 30 km away. This statistically significant elevation was due primarily to an increase in mutations inherited through the paternal germline. Our results indicate that human and wildlife populations in proximity to integrated steel mills may be at risk of developing germline mutations more frequently because of the inhalation of airborne chemical mutagens.
Integrated steel mills produce chemical mutagens that contaminate atmospheric and aquatic environments (1, 2), and may pose a genetic hazard to humans and wildlife. Herring gulls (Larus argentatus) nesting near steel mills on the Great Lakes were shown to have higher germline mutation rates at minisatellite DNA loci than those at rural sites (3), and mutation frequency increased with colony proximity to integrated steel mills (4). It was postulated that inhaled airborne contaminants emitted from steel mills, such as polycyclic aromatic compounds, were largely responsible for mutation induction; however, contaminants in the aquatic food web and differences in disease and nutritional status among gull colonies could not be eliminated as contributing factors. Therefore, the role of air pollution in producing germline mutations and the risk to humans living near steel mills could not be determined.
Rodent expanded simple tandem repeat (ESTR) DNA consists of 4- to 6-bp repeat units in long tandem arrays that are unstable in the germline and tend to mutate by insertion or deletion of a number of repeat units (5–7). Laboratory studies have demonstrated that murine ESTR loci are susceptible to germline mutations induced by chemical (8) or radioactive (9, 10) mutagens, and therefore may be useful tools for environmental contamination studies. The use of sentinel laboratory animals exposed in situ is a powerful experimental approach for assessing air pollution hazards because it combines the controlled elements of laboratory studies with direct exposure to ambient pollution levels (11, 12). Here, we compare germline ESTR mutation rates in laboratory mice exposed to ambient air at an industrial site near integrated steel mills to those exposed at a rural reference location, with the objective of testing inhalation of industrial air pollution as a route of chemical mutagen exposure.
Materials and Methods
Environmental Exposure.
We housed groups of laboratory mice at two field sites: 1 km downwind from two integrated steel mills in Hamilton Harbor (43° 15′ N, 79° 51′ W), a polluted industrial area on western Lake Ontario (steel), and simultaneously at a rural reference location 30 km away (rural). The steel site was chosen based on elevated germline mutation rates in local herring gull colonies (3, 4) and the high levels of chemical mutagens, such as polycyclic aromatic hydrocarbons (PAH), present in the air (1).
At each site, 20 male and 20 female outbred Swiss–Webster mice, 6–8 weeks old (Charles River Breeding Laboratories), were housed five same-sex individuals per cage inside of identical 2.4 × 2.4 × 1.8-m vinyl utility sheds. Sections of shed walls (1.2 × 1.6 m) were replaced with hardware cloth (1.5 × 1.5-cm mesh), which allowed flow-through of ambient air. We erected both sheds on the same compass orientation in partial shade to keep sun exposure time and direction constant at each site. We placed mouse cages on a single shelving unit inside of each shed such that they were not exposed to direct wind or sunlight. Exposures lasted the 10 weeks from September 10 to November 21, 1999, during which mice at both sites were given commercial mouse chow and bottled water from the same source.
Minimum temperature in each shed was partially controlled using electric heaters. We monitored temperature range on 53 of the 70 days of exposure by using a maximum/minimum thermometer at the rural site and data from a nearby weather station at the steel site. Average maximum/minimum temperatures were 18.6 ± 4.7/6.9 ± 3.8°C and 16.8 ± 5.1/8.5 ± 4.4°C for the rural and steel sites, respectively. The temperature did not fall below −3°C or rise above 29°C at either site.
Mouse Breeding.
Following in situ exposure, mice were returned to the animal care facility at McMaster University, where they were given unique tail tattoos that identified individuals and exposure location. To ensure that fertilizations resulted from mature sperm that developed from 2n-spermatogonia beginning during in situ exposure, mice were held in same-sex groups for 6 weeks posttreatment (13–15). Breeding pairs were then assigned randomly within each group. We monitored females daily beginning 18 days postpairing, and recorded date of delivery and litter size, as well as mass of pups at four intervals during the first 5 days of growth. Tail tissue was sampled from complete families when the pups were 5 days old. All animal procedures were approved by the McMaster University Animal Research Ethics Board following the guidelines of the Canadian Council on Animal Care.
Genetic Analysis.
Genomic DNA was extracted from tail tissue of both parents and three to six pups from each mouse family by using a standard phenol/chloroform procedure. DNA (6 μg) was digested to completion with HaeIII, size-fractionated in 42-cm-long 0.8% agarose gels for 42–50 h (1.5–2.5 V/cm), and transferred to nylon membrane by Southern blotting (Hybond-XL, Amersham Pharmacia). DNA fingerprints were generated by sequential hybridization with 32P-labeled synthetic ESTR probes Ms6-hm (5), Hm-2 (6), and MMS10 (7), and visualized by autoradiography. Blots were completely stripped of probe DNA between hybridizations by using 42°C 0.4 M NaOH, followed by boiling 0.1% SDS. All samples were run with 30 ng of digested λ phage DNA as an in-lane size standard (16).
Fingerprint bands in offspring that deviated by 1.0 mm or more relative to the parental progenitor, as determined using the in-lane size standard, were scored as mutations (17). All bands co-detected by both a single and multilocus ESTR probe were included in the single-locus mutation rate only. Mutation events resulting in identical mutant bands shared among littermates (clustered mutations), or extra single-locus bands (somatic mutations during embryogenesis), were not included in our analyses. Scoring was performed without knowledge of exposure location and verified by an independent observer. Mutation rates were calculated as the number of mutant bands out of the total scored and compared using a one-tailed Fisher's exact probability test. Parentage of all pups was confirmed with band sharing using multilocus probe MMS10.
Results
Twenty breeding pairs were established from mice exposed at each field site. Seventeen pairs of steel mice and 19 pairs of rural mice successfully produced offspring (85% and 95% breeding success for steel and rural, respectively). Litters born to steel parents had 1.7 fewer pups on average than rural (mean litter size 7.9 ± 2.6 and 9.6 ± 3.0 pups for steel and rural, respectively; Mann–Whitney, n1 = 19, n2 = 17, U = 230.5, P = 0.07). The mass of pups from each group did not differ significantly at any time over the first 5 days of growth (data not shown).
Single and multilocus ESTR probes detected a 1.5- and 2.0-fold elevation in germline mutation frequency in the steel group over the rural group, respectively (Table 1). Examples of mutant bands detected with ESTR single-locus probe Hm-2 are shown in Fig. 1. Multiallelism and high heterozygosity at ESTR single loci Ms6-hm and Hm-2 allowed us to determine the parental origin of mutant bands in all cases. Paternal mutations were 1.6 times more frequent in steel mice than rural mice, making up most of the overall elevation in the steel group (Table 2). Maternal mutation rates did not differ significantly between the two sites.
Table 1.
Germline DNA mutation rates in sentinel mice exposed in situ
| Site | Probe | Pups scored | Bands scored | Mutant bands | Mutation rate per band ± SE | Fisher's exact test P value |
|---|---|---|---|---|---|---|
| Rural | Ms6-hm | 110 | 234 | 51 | 0.22 ± 0.03 | |
| Hm-2 | 96 | 150 | 23 | 0.16 ± 0.03 | ||
| Total single locus: | 384 | 74 | 0.19 ± 0.02 | |||
| MMS10 | 110 | 1,851 | 51 | 0.03 ± 0.00 | ||
| Steel | Ms6-hm | 94 | 188 | 50 | 0.27 ± 0.03 | 0.06 |
| Hm-2 | 75 | 96 | 30 | 0.31 ± 0.05 | 0.01 | |
| Total single locus: | 284 | 80 | 0.28 ± 0.03 | 0.01 | ||
| MMS10 | 94 | 1,523 | 93 | 0.06 ± 0.01 | <0.01 |
The mutation rates presented do not include 7 and 12 instances of identical mutant bands shared among two or more littermates (clustered mutations) in the rural and steel groups, respectively. The frequency of this type of mutation event did not differ between groups (two-tailed Fisher's exact test, P = 0.28).
SE = [p(1 − p)/n]0.5, where p = observed mutation rate.
Probability value for a one-tailed Fisher's exact test comparing rural and steel mutation.
Hm-2 fragments were too small to be detected in a number of parents, resulting in a reduction in the number of pups and bands screened for mutations at this locus.
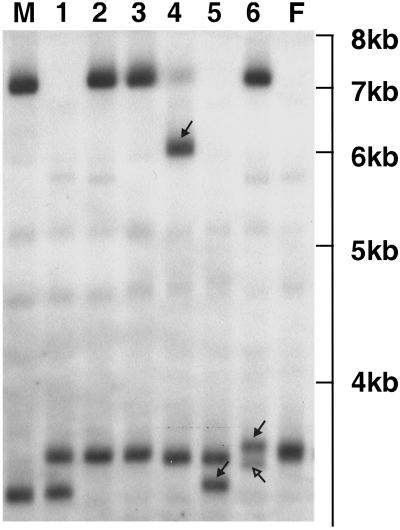
Fig 1.
DNA profile of a mouse family exposed at the steel site at ESTR locus Hm-2. The parents are labeled M (male) and F (female); pups are marked 1–6 (size range is indicated in kb). Three germline mutations are identified with solid arrows. The paternal alleles in pups 4 and 5 have undergone a large reduction and a small gain in size, respectively. The maternal allele in pup 6 has undergone a germline mutation event resulting in a small increase in size (solid arrow), as well as a somatic mutation during embryogenesis that produced a less intense extra band (open arrow). Somatic mutation events were not included in our analyses.
Table 2.
Paternal and maternal germline ESTR mutation rates in sentinel mice exposed in situ
| Site | Band origin | Mutant bands | Bands scored | Mutation rate* per band ± SE† | P value‡ |
|---|---|---|---|---|---|
| Rural | Paternal | 38 | 192 | 0.20 ± 0.03 | |
| Maternal | 36 | 192 | 0.19 ± 0.03 | ||
| Steel | Paternal | 46 | 142 | 0.32 ± 0.04 | 0.01 |
| Maternal | 34 | 142 | 0.24 ± 0.04 | 0.07 |
Data for single loci Ms6-hm and Hm-2 are presented combined. Footnotes are as in Table 1.
Discussion
We placed laboratory mice in a high-risk area for induced germline mutations as identified during herring gull studies (3, 4), and controlled for sources of environmental mutagens other than airborne emissions. Identical husbandry conditions for mice in both treatment groups during the study eliminated differences in nutritional and developmental history of the animals (average mass of adults did not differ between sites). We therefore attribute the effect on inherited mutations in the offspring of sentinel mice directly to variation in air quality between the steel and rural field sites.
Few other studies have been able to establish a significant relationship between ambient pollution levels and germline mutation induction. Hypervariable repetitive DNA markers revealed elevated mutation rates in human families living near radioactive contamination sites in Belarus (18, 19), Kazakhstan (20), and the Ukraine (21), as well as barn swallows nesting near Chernobyl (22). These sites, however, are unusual in that the source of mutagenic pollution was either a nuclear reactor accident (18, 19, 20) or bomb testing (22), which are uncommon events in most human-populated areas. Elevated minisatellite mutation rates in herring gulls nesting near steel mills on the Great Lakes (3, 4) and our findings here are currently the only examples of heritable mutation induction from chemical pollution sources. In contrast to previous studies, urban and industrial air pollution has the potential to affect many people in most countries. Taken in concert with our observation of a marginal decrease in litter size in steel mice, which may be indicative of dominant lethal mutations (23), our findings suggest that there is an urgent need to investigate the genetic consequences associated with exposure to chemical pollution through the inhalation of urban and industrial air.
The highest germline mutation rate we observed in sentinel mice was in males exposed at the steel site. The timing of spermatogenesis in mice and the 6-week delay in breeding following in situ exposure indicate that premeiotic male germ cells (10, 24) are sensitive to airborne emissions near steel mills. This period during male gametogenesis is also sensitive to ESTR mutations induced by ionizing radiation (10, 25). The fact that the male germline mutation rate was significantly elevated in our study after only a 10-week exposure is reason for concern. Globally, hundreds of thousands of humans live or work in industrial areas near steel mills and are incidentally exposed to airborne emissions. These populations may be at risk of increased heritable mutation frequency through exposed fathers. In addition, steelworkers in certain positions inside plants can be exposed to airborne emissions at much higher levels than outdoor ambient (26). Many more men than women work in steel mills, raising the possibility of heritable mutation as a previously undocumented occupational hazard.
In contrast, the slight elevation in maternal mutation rate we detected at the steel site was not statistically significant. It would be premature, based on this, to conclude that the female germline is not at risk from air pollution exposure. Relatively little is known about the induction of heritable mutations in female germ cells; however, mutation induction in mature oocytes has been demonstrated using the specific locus test with both radioactive and chemical mutagens (reviewed in refs. 23, 27, and 28). To our knowledge, direct ESTR mutation induction in the female germline by using any sort of mutagen has never been investigated (see refs. 29 and 30 for examples of indirect maternal ESTR mutation induction). We suggest that future research address the issue of ESTR mutation induction in females. Of particular interest would be long-term, low-dose exposure of adults, and embryonic exposures encompassing gametogenesis.
For management purposes, it would be most pertinent to identify important chemical mutagens and restrict their release into the air. At the present time, however, we can only state that some portion of the air in Hamilton Harbor (steel site) caused elevated heritable mutation frequency. PAHs are produced in large quantities by integrated steel mills (31), tend to be associated with breathable particulate matter (32), and are among the most genotoxic components of urban air pollution (33). Total PAH concentration in Hamilton Harbor air (average for 1999, 117.6 ng/m3) was similar to that found to cause genotoxic effects in human white blood cells (32), and >50 times higher than that of the county containing the reference site (F. Dobroff, Ontario Ministry of the Environment, personal communication). In addition, extracts of particulate matter containing PAHs in Hamilton air were shown to be mutagenic in a dose-dependent manner by using the Ames test (1). We suggest that PAHs from incomplete coal combustion during steel production are a likely candidate group for causing elevated germline mutation rates in the steel mice exposed in our study.
Integrated steel mills in Hamilton are not the only local source of PAHs and other toxic airborne emissions. A nearby major commuter highway, diesel-powered industrial vehicles, and the surrounding population of >640,000 humans also contribute to air pollution in our study area. It is important to note, however, that Yauk et al. (4) examined germline mutations in a herring gull colony in Toronto, a major city of 3 million people that has a high volume of traffic but no integrated steel mills. They found the mutation rate in the Toronto colony to be intermediate between rural and steel sites, suggesting that steel mills contribute significantly to germline mutation induction.
The possible impacts on human health associated with increased mutation rates in repetitive DNA sequences are not known; however, ESTR mutations show a dose–response relationship to radiation exposure, and have a similar doubling dose to coding regions of DNA (10). It is therefore likely that a relationship exists between mutation frequency at ESTR loci and coding regions that affect phenotype. In addition, ESTR mutations are induced through unknown mechanisms that may alter DNA replication, recombination, or repair, and thus adversely affect the entire genome. Our results suggest that a thorough investigation of the genetic hazards associated with occupational and incidental exposure to contaminated air in urban and industrial areas is warranted.
Acknowledgments
We thank Dr. B. N. White for guidance during genetic analyses, Dr. D. Davidson for helpful comments on an early draft of this manuscript, and V. Kjoss for technical support. We also thank K. and G. Gourlay and the Hamilton Harbor Commissioners/Port Authority for property access, Dr. G. Douglas for facilitating the Toxic Substances Research Initiative grant, and F. Dobroff for temperature and air quality information. This research was supported by funds from the Toxic Substances Research Initiative of the federal government of Canada.
Abbreviations
ESTR, expanded simple tandem repeat
PAH, polycyclic aromatic hydrocarbons
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Legzdins A. E., McCarry, B. E. & Bryant, D. W. (1994) Polycyclic Aromat. Compd. 5, 157-165. [Google Scholar]
- 2.Marvin C. H., Allan, L., McCarry, B. E. & Bryant, D. W. (1993) Environ. Mol. Mutagen. 22, 61-70. [DOI] [PubMed] [Google Scholar]
- 3.Yauk C. L. & Quinn, J. S. (1996) Proc. Natl. Acad. Sci. USA 93, 12137-12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yauk C. L., Fox, G. A., McCarry, B. E. & Quinn, J. S. (2000) Mutat. Res. 452, 211-218. [DOI] [PubMed] [Google Scholar]
- 5.Kelly R., Bulfield, G., Collick, A., Gibbs, M. & Jeffreys, A. J. (1989) Genomics 5, 844-856. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs M., Collick, A., Kelly, R. & Jeffreys, A. J. (1993) Genomics 17, 121-128. [DOI] [PubMed] [Google Scholar]
- 7.Bois P., Williamson, J., Brown, J., Dubrova, Y. E. & Jeffreys, A. J. (1998) Genomics 49, 122-128. [DOI] [PubMed] [Google Scholar]
- 8.Hedenskog M., Sjogren, M., Cederberg, H. & Rannug, U. (1997) Environ. Mol. Mutagen. 30, 254-259. [PubMed] [Google Scholar]
- 9.Dubrova Y. E., Jeffreys, A. J. & Malashenko, A. M. (1993) Nat. Genet. 5, 92-94. [DOI] [PubMed] [Google Scholar]
- 10.Dubrova Y. E., Plumb, M., Brown, J., Fennelly, J., Bois, P., Goodhead, D. & Jeffreys, A. J. (1998) Proc. Natl. Acad. Sci. USA 95, 6251-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichrtova E., Foltinova, J. & Takac, L. (1995) J. Aerosol Med. 8, 233-241. [DOI] [PubMed] [Google Scholar]
- 12.Reymao M. S., Cury, P. M., Lichtenfels, A. J., Lemos, M., Battlehner, C. N., Conceicao, G. M., Capelozzi, V. L., Montes, G. S., Junior, M. F., Martins, M. A., et al. (1997) Environ. Res. 74, 150-158. [DOI] [PubMed] [Google Scholar]
- 13.Leblond C. P. & Clermont, Y. (1952) Am. J. Anat. 90, 167-215. [DOI] [PubMed] [Google Scholar]
- 14.Oakberg E. F. (1956) Am. J. Anat. 99, 391-409. [DOI] [PubMed] [Google Scholar]
- 15.Oakberg E. F. (1956) Am. J. Anat. 99, 507-516. [DOI] [PubMed] [Google Scholar]
- 16.Galbraith D. A., Boag, P. T., Gibbs, H. L. & White, B. N. (1991) Electrophoresis 12, 210-220. [DOI] [PubMed] [Google Scholar]
- 17.Yauk C. L., Dubrova, Y. E., Grant, G. R. & Jeffreys, A. J. (2002) Mutat. Res. 500, 147-156. [DOI] [PubMed] [Google Scholar]
- 18.Dubrova Y. E., Nesterov, V. N., Krouchinsky, N. G., Ostapenko, V. A., Neumann, R., Neil, D. L. & Jeffreys, A. J. (1996) Nature 380, 683-686. [DOI] [PubMed] [Google Scholar]
- 19.Dubrova Y. E., Nesterov, V. N., Krouchinsky, N. G., Ostapenko, V. A., Vergnaud, G., Giraudeau, F., Buard, J. & Jeffreys, A. J. (1997) Mutat. Res. 381, 267-278. [DOI] [PubMed] [Google Scholar]
- 20.Dubrova Y. E., Bersimbaev, R. I., Djansugurova, L. B., Tankimanova, M. K., Mamyrbaeva, Z. Z., Mustonen, R., Lindholm, C., Hulten, M. & Salomaa, S. (2002) Science 295, 1037. [DOI] [PubMed] [Google Scholar]
- 21.Dubrova Y. E., Grant, G., Chumak, A. A., Stezhka, V. A. & Karakasian, A. N. (2002) Am. J. Hum. Genet. 71, 801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellegren H., Lindgren, G., Primmer, C. R. & Moller, A. P. (1997) Nature 389, 593-596. [DOI] [PubMed] [Google Scholar]
- 23.Ehling U. H. (1989) Mutat. Res. 212, 43-53. [DOI] [PubMed] [Google Scholar]
- 24.Allen J. W., Ehling, U. H., Moore, M. M. & Lewis, S. E. (1995) Mutat. Res. 330, 219-231. [DOI] [PubMed] [Google Scholar]
- 25.Dubrova Y. E. & Plumb, M. (2002) Mutat. Res. 499, 143-150. [DOI] [PubMed] [Google Scholar]
- 26.Strunk P., Ortlett, K., Heinz, H., Rossbach, B. & Angerer, J. (2002) Int. Arch. Occup. Environ. Health 75, 354-358. [DOI] [PubMed] [Google Scholar]
- 27.Russell L. B. & Russell, W. L. (1992) Mutat. Res. 296, 107-127. [DOI] [PubMed] [Google Scholar]
- 28.Wilson G. N. (1992) Mutat. Res. 296, 157-165. [DOI] [PubMed] [Google Scholar]
- 29.Niwa O. & Kominami, R. (2001) Proc. Natl. Acad. Sci. USA 98, 1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber R., Plumb, M. A., Boulton, E., Roux, I. & Dubrova, Y. E. (2002) Proc. Natl. Acad. Sci. USA 99, 6877-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgaz S., Demircigil, G. C., Karahalil, B. & Karakaya, A. E. (2002) Chemosphere 47, 57-64. [DOI] [PubMed] [Google Scholar]
- 32.Lewtas J., Walsh, D., Williams, R. & Dobias, L. (1997) Mutat. Res. 378, 51-63. [DOI] [PubMed] [Google Scholar]
- 33.Kyrtopoulos S. A., Georgiadis, P., Autrup, H., Demopoulos, N., Farmer, P., Haugen, A., Katsouyanni, K., Lambert, B., Ovrebo, S., Sram, R., et al. (2001) Mutat. Res. 496, 207-228. [DOI] [PubMed] [Google Scholar]