Abstract
Much of the work conducted on adult stem cells has focused on mesenchymal stem cells (MSCs) found within the bone marrow stroma. Adipose tissue, like bone marrow, is derived from the embryonic mesenchyme and contains a stroma that is easily isolated. Preliminary studies have recently identified a putative stem cell population within the adipose stromal compartment. This cell population, termed processed lipoaspirate (PLA) cells, can be isolated from human lipoaspirates and, like MSCs, differentiate toward the osteogenic, adipogenic, myogenic, and chondrogenic lineages. To confirm whether adipose tissue contains stem cells, the PLA population and multiple clonal isolates were analyzed using several molecular and biochemical approaches. PLA cells expressed multiple CD marker antigens similar to those observed on MSCs. Mesodermal lineage induction of PLA cells and clones resulted in the expression of multiple lineage-specific genes and proteins. Furthermore, biochemical analysis also confirmed lineage-specific activity. In addition to mesodermal capacity, PLA cells and clones differentiated into putative neurogenic cells, exhibiting a neuronal-like morphology and expressing several proteins consistent with the neuronal phenotype. Finally, PLA cells exhibited unique characteristics distinct from those seen in MSCs, including differences in CD marker profile and gene expression.
INTRODUCTION
Stem cells are a population possessing 1) self-renewal capacity, 2) long-term viability, and 3) multilineage potential. The multilineage potential of embryonic stem cells and adult stem cells from the bone marrow has been characterized extensively. Although embryonic stem cell potential is enormous, many ethical and political issues accompany their use. Therefore, adult stem cells from the bone marrow stroma (i.e., mesenchymal stem cells, MSCs) have been proposed as an alternative source. Originally identified as a source of osteoprogenitor cells, MSCs differentiate into adipocytes, chondrocytes, osteoblasts, and myoblasts in vitro (Hauner et al., 1987; Grigoradis et al., 1988; Wakitani et al., 1995; Ferrari et al., 1998; Johnstone et al., 1998; Pittenger et al., 1999) and undergo differentiation in vivo (Benayahu et al., 1989; Bruder et al., 1998a), making these stem cells promising candidates for mesodermal defect repair and disease management. However, the clinical use of MSCs has presented problems, including pain, morbidity, and low cell number upon harvest. This has led many researchers to investigate alternate sources for MSCs.
Adipose tissue, like bone marrow, is derived from the mesenchyme and contains a supportive stroma that is easily isolated. Based on this, adipose tissue may represent a source of stem cells that could have far-reaching effects on several fields. We have previously identified a putative stem cell population within human lipoaspirates (Zuk et al., 2001). This cell population, called processed lipoaspirate (PLA) cells, can be isolated from adipose tissue in significant numbers and exhibits stable growth and proliferation kinetics in culture. Moreover, PLA cells, like MSCs, differentiate in vitro toward the osteogenic, adipogenic, myogenic, and chondrogenic lineages when treated with established lineage-specific factors. The multilineage differentiation capacity of PLA cells led us to speculate that a population of multipotent stem cells, comparable with MSCs, can be isolated from human adipose tissue.
To confirm whether PLA cells represent a stem cell population, we conducted an extensive molecular and biochemical characterization of the PLA population and several clonal isolates, termed adipose-derived stem cells (ADSCs). PLA cells expressed several CD marker antigens similar to those observed on MSC controls. Induction of PLA cells and clones toward multiple mesodermal lineages resulted in the expression of several lineage-specific genes and proteins similar to those observed in induced MSC controls and lineage-committed precursor cell lines. Moreover, established biochemical assays confirmed lineage-specific metabolic activity in induced PLA populations. In addition to mesodermal capacity, PLA cells and clones differentiated into putative neurogenic cells exhibiting a neuronal-like morphology and expressing several proteins consistent with the neuronal phenotype. Finally, PLA cells exhibited unique characteristics distinct from that seen in MSCs, including differences in CD marker and gene expression profiles. In conclusion, the results presented in this study suggest that adipose tissue may be an additional source of unique, pluripotent stem cells with multi-germline potential.
MATERIALS AND METHODS
Cell Culture and Differentiation
PLA cells were obtained from raw human lipoaspirates and cultured as described in a previous study (Zuk et al., 2001). Briefly, raw lipoaspirates were washed extensively with sterile phosphate-buffered saline (PBS) to remove contaminating debris and red blood cells. Washed aspirates were treated with 0.075% collagenase (type I; Sigma-Aldrich, St. Louis, MO) in PBS for 30 min at 37°C with gentle agitation. The collagenase was inactivated with an equal volume of DMEM/10% fetal bovine serum (FBS) and the infranatant centrifuged for 10 min at low speed. The cellular pellet was resuspended in DMEM/10% FBS and filtered through a 100-μm mesh filter to remove debris. The filtrate was centrifuged as detailed above and plated onto conventional tissue culture plates in control medium (Table 1). Normal human osteoblasts (NHOst), normal human chondrocytes from the knee (NHCK), and a population of MSCs from human bone marrow were purchased from Clonetics (Walkersville, MD) and maintained in commercial medium. The murine 3T3-L1 preadipocyte cell line (Green and Meuth, 1974) was obtained from American Type Culture Collection (Manassas, VA). NHOst, PLA cells, and 3T3-L1 cells were treated with mesenchymal lineage-specific media as outlined in Table 1. MSCs were induced using commercial control medium supplemented with the growth factors outlined in Table 1. NHOst and NHCK cells were induced using commercially available induction media (Clonetics).
Table 1.
Lineage-specific differentiation induced by media supplementation
| Medium | Media | Serum | Supplementation |
|---|---|---|---|
| Control | DMEM | FBS (10%) | 1% antibiotic/antimycotic |
| Adipogenic (AM) | DMEM | FBS (10%) | 0.5 mM isobutyl-methylxanthine (IBMX), 1 μM dexamethasone, 10 μM insulin, 200 μM indomethacin, 1% antibiotic/antimycotic |
| Osteogenic (OM/VD) | DMEM | FBS (10%) | 0.01μM 1,25-dihydroxyvitamin D3 *, 50 μM ascorbate-2-phosphate, 10 mM β-glycerophosphate, 1% antibiotic/antimycotic |
| Chondrogenic (CM) | DMEM | FBS (1%) | 6.25 μg/ml insulin, 10 ng/ml TGFβ1, 50 nM ascorbate-2-phosphate, 1% antibiotic/antimycotic |
| Myogenic (MM) | DMEM | FBS (10%), HS (5%) | 50 μM hydrocortisone, 1% antibiotic/antimycotic |
| Neurogenic (NM) | DMEM | none | 5–10 mM β-mercaptoethanol |
0.1 μM dexamethasone can be used as a replacement of 0.01 μM vitamin D.
Antibodies
The antibodies and commercial sources used in this study are indicated in online Table S1.
Flow Cytometry
PLA cells and MSCs were cultured in control medium 72 h before analysis. Flow cytometry with a FACscan argon laser cytometer (BD Biosciences, San Jose, CA) was performed according to a previous study (Zuk et al., 2001). Briefly, cells were harvested in 0.25% trypsin/EDTA and fixed for 30 min in ice-cold 2% formaldehyde. The fixed cells were washed in flow cytometry buffer (PBS, 2% FBS, 0.2% Tween 20) and incubated for 30 min in flow cytometry buffer containing fluorescein isothiocyanate-conjugated monoclonal antibodies to SH3, STRO-1, and the following CD antigens: 13, 14, 16, 31, 34, 44, 45, 49d, 56, 62e, 71, 90, 104, 105, and 106. PLA cells and MSCs were stained with a phycoerythrin-conjugated nonspecific IgG to assess background fluorescence.
Histology, Immunohistochemistry, and Indirect Immunofluorescence
Indirect Immunofluorescence.
PLA cells and MSCs were processed as described previously (Zuk et al., 2001) by using monoclonal antibodies to specific CD markers and lineage-specific proteins (online Table S1).
Histology and Immunohistochemistry.
Differentiated PLA cells and clones were processed as described previously (Zuk et al., 2001) by using the following histological assays: alkaline phosphatase (AP) (osteogenesis), Oil Red O (adipogenesis), and Alcian blue (AB) (chondrogenesis). Chondrogenic PLA cells and clones were examined for collagen type 2 (CNII), keratan sulfate, and chondroitin-4-sulfate expression by immunohistochemistry as described previously (Zuk et al., 2001). Neurogenic PLA cells and clones were examined by immunohistochemistry for the expression of neural-specific proteins.
Spectrophotometric Assays
AP.
Triplicate samples of PLA cells were differentiated in osteogenic medium (OM) for up to 6 wk. Cells were washed with PBS, harvested, and AP enzyme activity was assayed using a commercial AP enzyme kit according to the method of Beresford et al. (1986). AP activity was expressed as nanomoles of p-nitrophenol produced per minute per microgram of protein. Differentiated MSCs were assayed as a positive control, whereas non-induced PLA cells were assayed as a negative control. Values are expressed as the mean ± SD. A Student's t test (paired) was performed to determine statistical significance between induced and control samples.
Total Calcium.
Triplicate samples of PLA cells were differentiated in OM for up to 6 wk. Cells were washed with PBS (no Ca2+, no Mg2+) and harvested in 0.1 N HCl. Cells were extracted in 0.1 N HCl at 4°C for a minimum of 4 h and centrifuged for 5 min at 10,000 × g. Total calcium in the supernatant was determined using a commercial kit (#587; Sigma-Aldrich) and expressed as millimolar Ca2+ per microgram of protein. Differentiated MSCs were assayed as a positive control, whereas non-induced PLA cells were assayed as a negative control. Values are expressed as the mean ± SD. A Student's t test (paired) was performed to determine statistical significance between induced and control samples.
Glycerol-3-Phosphate Dehydrogenase (GPDH).
Triplicate samples of PLA cells were differentiated in adipogenic medium (AM) for up to 5 wk. GPDH activity was assayed according to the method of Wise and Green (1979). One unit of GPDH was defined as the oxidation of 1 nmol of NADH per minute. GPDH activity was expressed as units of GPDH per microgram. Differentiated 3T3-L1 cells were assayed as a positive control, whereas non-induced PLA cells were assayed as a negative control. Values are expressed as the mean ± SD. A Student's t test (paired) was performed to determine statistical significance between induced and control samples.
Dimethyldimethylene Blue.
Triplicate samples of PLA cells were differentiated in chondrogenic medium (CM) for up to 3 wk by using established high-density micromass protocols (Reddi, 1982). PLA nodules were harvested and assayed for sulfated proteoglycans, according to an established method (Farndale et al., 1986). Proteoglycan levels were expressed as micrograms of sulfated proteoglycan per microgram of protein. Non-induced PLA cells were assayed as a negative control. Values are expressed as the mean ± SD. A Student's t test (paired) was performed to determine statistical significance between induced and control samples.
Reverse-Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
PLA cells were induced toward five lineages, as outlined in Table 1, for defined time periods. Total cellular RNA was isolated and reverse transcribed using conventional protocols. PCR amplification was performed using the primer sets outlined in online Table S2. All primer sequences were determined using established GenBank sequences. Duplicate PCR reactions were amplified using primers designed β-actin as a control for assessing PCR efficiency and for subsequent analysis by agarose gel electrophoresis. The sequence of each PCR product was confirmed using automated sequencing. Non-induced PLA cells were examined as a negative control. Lineage-specific cell lines (NHOst, 3T3-L1, and NHCK) were analyzed as positive controls for the osteogenic, adipogenic, and chondrogenic lineages, respectively. Total human skeletal muscle and brain RNA (Ambion, Austin, TX) were reverse transcribed and amplified by PCR as positive controls for the myogenic and neurogenic lineages, respectively.
Quantitative Real-Time PCR
PLA cells were maintained in noninductive control medium for 3 wk or were induced toward the osteogenic and adipogenic lineages for 1 and 3 wk. The expression of core-binding factor alpha-1 (CBFA-1) and AP was quantitated for osteogenic PLA cells, whereas the expression of lipoprotein lipase (LPL) was quantitated for adipogenic samples. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and probe (5′ JOE and 3′ TAMRA) were purchased from Applied Biosystems (Foster City, CA). Total cellular RNA was isolated and reversed transcribed using the TaqMan Gold RT-PCR kit for real-time PCR (Applied Biosystems). Quantitative real-time PCR was performed using this kit according to the manufacturer and an ABI 7700 Prism Sequence Detection system. Primer and probe sequences were designed by the UCLA Sequencing Core Facility and synthesized by BioSource (Camarillo, CA). All probes were designed with a 5′ fluorogenic probe 6FAM and a 3′ quencher TAMRA. The expression of human GAPDH was used to normalize gene expression levels.
Western Blotting
PLA cells were differentiated toward the osteogenic lineage for 7 and 28 d, washed in PBS, and lysed in 1% SDS. Equivalent amounts of protein in each lysate were resolved by denaturing PAGE (SDS-PAGE) and analyzed using standard immunoblotting protocols. Lysates were examined for the expression of osteopontin (OP), osteonectin (ON), AP, collagen type I (CNI), vitamin D receptor (VDR), and retinoid X receptor α (RXRα). Expression of the transferrin receptor (TfR) was used as an internal control for quantitation. Expression of α-actin was used as a qualitative control for the Western blot procedure only. Non-induced PLA cells were also analyzed as a negative control. To quantitate, protein levels were normalized with respect to the TfR and expressed relative to undifferentiated PLA controls.
Neurogenic Differentiation
Subconfluent PLA cells were cultured for 24 h in pre-induction medium (DMEM, 20% FBS, 1 mM β-mercaptoethanol). After pre-induction, the cells were induced for up to 9 h in neurogenic medium (NM), according to an established protocol (Woodbury et al., 2000) and analyzed by immunohistochemistry for the expression of neuronal-specific nuclei protein (NeuN), neural-specific enolase (NSE), 70-kDa neurofilament protein (NF-70), and microtubule-associated protein 2 (MAP-2) (neuronal lineage), glial acidic fibrillary protein (GFAP) (astrocyte lineage), and galactocerebroside (GalC) (oligodendrocyte lineage). Samples were also analyzed by RT-PCR (online Table S2). Finally, PLA samples were also induced in 1) NM for 9 h and maintained for 1 wk in a neural progenitor maintenance medium (NPMM) and 2) control medium supplemented with indomethacin and insulin (IIM) for up to 1 wk.
Isolation and Analysis of PLA Clones
PLA cells were plated at limiting confluence to result in isolated single cells. Cultures were maintained in control medium until the formation of well-defined colonies. The single PLA-cell derived colonies were harvested using sterile cloning rings and expanded in cloning medium (15% FBS, 1% antibiotic/antimycotic in F-12/DMEM [1:1]). Expanded clones were subcloned by limiting dilution. All clones were analyzed for osteogenic, adipogenic, chondrogenic, and neurogenic potential by immunohistochemistry. The expression of lineage-specific genes was confirmed by RT-PCR.
Online Supplementary Material
Online supplementary material includes the following:
Figure S1. Immunofluorescence analysis of PLA and MSC populations: CD marker profile.
Figure S2. Growth kinetics and histological analysis of adipo-induced PLA populations.
Figure S3. Immunofluorescence and RT-PCR analysis of adipo-induced PLA cells.
Figure S4. Growth kinetics of osteo-induced PLA cells
Figure S5. Immunofluorescence and RT-PCR analysis of osteo-induced PLA cells and MSCs.
Figure S6. Immunohistochemical and RT-PCR analysis of PLA cells and NHCK controls.
Figure S7. Immunohistochemical analysis of PLA clones.
Table S1. List of antibodies.
Table S2. List of RT-PCR oligonucleotide primers.
RESULTS
Phenotypic Characterization of PLA Populations: CD Marker Profile
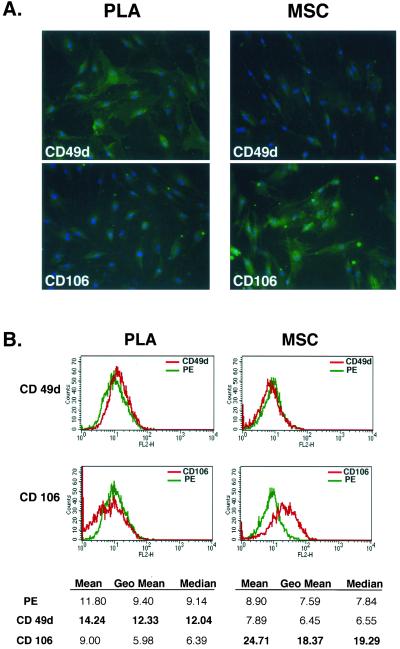
To characterize the PLA population, CD marker profile was examined and compared with a commercial population of human MSCs (Figures 1 and online S1). Both PLA and MSC cells expressed CD29, CD44, CD71, CD90, and CD105/SH2 and SH3, which together with SH2, is considered a marker for MSCs (Haynesworth et al., 1992). In addition to these markers, both PLA and MSCs expressed STRO-1 (our unpublished data), a marker used to isolated multilineage progenitors from bone marrow (Gronthos et al., 1994; Dennis et al., 2002). In contrast, no expression of the hematopoietic lineage markers CD31, CD34, and CD45 was observed in either of the cultures. Flow cytometry confirmed the immunofluorescence results, in addition to detecting the expression of CD13 and the absence of CD14, 16, 56, 61, 62E, 104, and 106 (Table 2). On immunofluorescence and flow cytometric analysis, two CD marker antigens were found to differ between PLA and MSC populations: CD49d (α4 integrin) and CD106 (VCAM). Specifically, PLA cells expressed CD49d, whereas this antigen was not observed in MSC cultures. Unlike MSCs, no expression of CD106 was observed in PLA samples.
Figure 1.
PLA cells express a unique set of CD markers. (A) PLA cells and MSCs were processed by immunofluorescence for expression of multiple CD antigens (green). Cells were costained with 4,6-diamidino-2-phenylindole to visualize nuclei (blue) and the fluorescent images combined. The differential expression of CD49d and CD106 between PLA cells and MSCs is shown (Figure S1 for remaining CD antigens). (B) Flow cytometric analysis on PLA cells and MSCs for the expression of CD49d and CD106 was performed (red). Cells stained with a fluorochrome-conjugated nonspecific IgG were examined as a control (γPE, green). The geometric mean and median values for CD49d and Cd106 are shown below. Significant differences are shown in bold.
Table 2.
Flow cytometric analysis of CD marker expression on non-induced PLA cells
| CD Antigen | Geometric Mean |
|---|---|
| CD13 | 148.88 |
| CD14 | 2.43 |
| CD16 | 2.38 |
| CD31 | 2.22 |
| CD34 | 3.55 |
| CD44 | 16.92 |
| CD45 | 2.52 |
| CD49d | 14.99 |
| CD56 | 2.66 |
| CD62E | 2.30 |
| CD71 | 3.76 |
| CD90 | 25.96 |
| CD104 | 2.31 |
| CD105 | 8.39 |
| CD106 | 2.45 |
| SH3 | 8.95 |
| STRO-1 | 31.26 |
| −ve | 2.59 |
PLA Cells Undergo Adipogenic Differentiation In Vitro
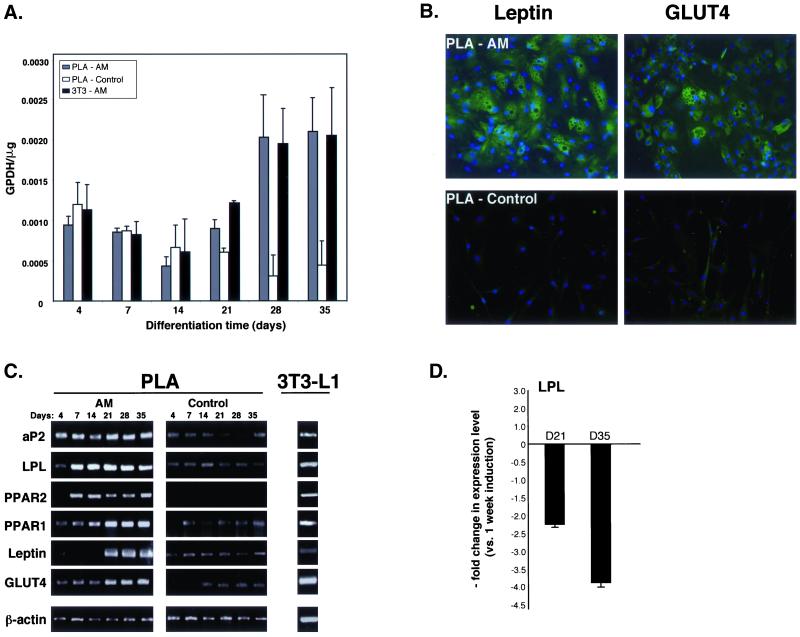
Induction of PLA cells with AM resulted in an expanded cell morphology and a time-dependent increase in intracellular Oil Red O staining, an established lipid dye (online Figure S2). Moreover, adipogenic differentiation did not result in an appreciable increase in PLA cell number and is consistent with growth arrest observed upon commitment of preadipocytes (online Figure S2). Induction of PLA cells and 3T3-L1 controls resulted in a significant up-regulation in the activity of the lipogenic enzyme GPDH (Figure 2A). However, no significant difference in GPDH activity was detected between induced PLA samples and non-induced controls until 4 wk of induction, whereupon a 6.5- and 4.7-fold increase vs. controls was measured at 28 and 35 d, respectively. Moreover, statistical analysis confirmed a significant difference between induced and control PLA samples at these time points (p < 0.01). Finally, the time-dependent increase in GPDH activity correlated with the increased percentage of lipid-filled PLA cells within adipo-induced cultures and was consistent with adipogenic differentiation by these cells.
Figure 2.
Adipogenic PLA cells express several genes and proteins consistent with adipogenic differentiation. (A) Triplicate samples of PLA cells and 3T3-L1 controls were induced for up to 5 wk in AM (PLA-AM and 3T3-AM, respectively) and assayed for GPDH activity (GPDH per microgram). Non-induced PLA cells were analyzed as a negative control (PLA-control). Values were expressed as mean ± SD. (B) PLA cells were induced in AM (PLA-AM) or maintained in non-inductive control medium (PLA-control) for 14 d. Cells were examined for the expression of GLUT4 and leptin by indirect immunofluorescence. (C) PLA cells were induced in AM or maintained in non-inductive control medium for up to 5 wk. Samples were analyzed by RT-PCR for the indicated genes. 3T3-L1 cells maintained for 2 wk in AM were analyzed as a positive control. (D) Expression of the gene LPL was quantitated by real-time PCR in PLA cells induced in control medium and AM for up to 5 wk. LPL expression levels were normalized with respect to endogenous GAPDH. LPL expression in PLA cells induced for 3 (D21) and 5 wk (D35) in AM were expressed relative to 1-wk levels.
Adipogenic induction of PLA cells also resulted in lineage-specific gene and protein expression. Immunofluorescence confirmed the expression of leptin and GLUT4 in induced PLA samples (Figure 2B), two proteins that are up-regulated in differentiating adipocytes (Tanner et al., 1992; Chen et al., 1997). Expression of these proteins seemed to be restricted to mature, lipid-filled PLA cells, as low levels were observed in cells with a fibroblastic morphology. Moreover, the expression of both leptin and GLUT4 seemed to be specific to adipogenic PLA samples as no protein expression was detected in non-induced controls. The expression of leptin and GLUT4 was also observed in lipid-filled MSCs upon adipogenic induction (online Figure S3A). Adipogenic differentiation of PLA cells was further confirmed by RT-PCR (Figure 2C). Induction of PLA cells with AM resulted in expression of the adipose-specific transcription factor peroxisome-proliferating activated receptor γ (PPARγ2). Moreover, PPARγ2 expression was specific to adipo-induced PLA cells, in addition to MSCs and induced 3T3 cells (online Figure S3B). Initial differentiation (i.e., 4 d) of the PLA and MSC populations was characterized by the absence of PPARγ2, with expression of this transcription factor appearing after 1 wk of induction and persisting throughout the remaining induction period. Expression of PPARγ1 was also detected in adipo-induced PLA cells and MSC controls. However, constitutive expression of PPARγ1 was observed in non-induced PLA cells, whereas basal expression was not observed in non-induced MSCs (online Figure S3B). In addition to the PPAR isoforms, expression of the adipogenic genes LPL and aP2 was also detected in PLA cells and MSC controls. Constitutive expression of these genes was detected in both cell populations and adipogenic induction resulted in a qualitative increase in expression level compared with non-induced controls as detected by conventional RT-PCR. LPL up-regulation in adipo-induced PLA cells was also confirmed by quantitative real-time PCR. Non-induced PLA controls expressed negligible levels of LPL and a significant up-regulation in expression was measured at day 7 upon induction, consistent with the expression of this gene during the early stages of preadipocyte differentiation (Jonasson et al., 1984). LPL levels beyond this point decreased with a two- and fourfold drop in expression being measured at day 21 and day 35 compared with day 7 levels (Figure 2D). Finally, adipogenic differentiation of PLA cells, in addition to MSC and 3T3 controls, resulted in the expression of leptin and GLUT4 mRNA. In contrast to protein expression, non-induced PLA cells expressed basal levels of leptin mRNA with adipogenic induction seeming to increase expression level late in differentiation. Finally, the adipogenic induction conditions used in this study were specific for the fat lineage and did not result in the expression of genes consistent with bone and cartilage differentiation (osteocalcin [OC] and CNII, respectively; our unpublished data).
PLA Cells Undergo Osteogenic Differentiation In Vitro
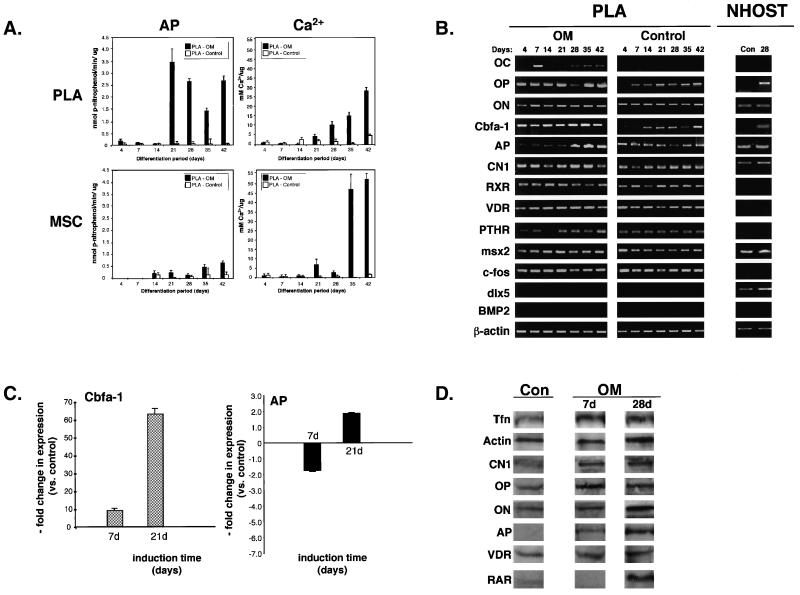
Induction of PLA cells with OM, containing dexamethasone (Table 1), resulted in the appearance of AP activity and an increase in matrix mineralization as confirmed by histology (online Figure S4). Moreover, distinct phases of PLA proliferation, matrix synthesis, and mineralization could be discerned in osteo-induced PLA cultures, consistent with results observed in osteoblast cultures (online Figure S4). However, recent work has questioned the efficacy of glucocorticoids, such as dexamethasone, in mediating osteogenesis (Cooper et al., 1999). Therefore, PLA cells were induced in OM containing 1,25-dihydroxyvitamin D3 (VD) rather than dexamethasone. To assess osteogenesis, levels of AP enzyme activity and matrix mineralization were quantitated. AP activity appeared in osteo-induced PLA and MSC samples between 2 and 3 wk of induction with PLA samples exhibiting significantly elevated AP levels compared to MSC controls at 3 wk of induction (p = 0.008; paired t test) (Figure 3A). Maximum AP levels were detected in induced PLA samples at 3 wk with an approximate 35-fold increase in activity measured from 2 to 3 wk of induction. Furthermore, the response to VD induction seemed to be time dependent, producing a distinct biphasic pattern. AP activity appeared 1 wk earlier in the MSC population and maximum levels were not observed until 6 wk. PLA cells treated with dexamethasone exhibited significantly lower levels of AP activity compared with VD-treated samples (our unpublished data). Interestingly, treatment of MSCs with dexamethasone produced increased AP levels compared with VD induction, suggesting a differential response to induction conditions between the PLA and MSC populations (our unpublished data). AP enzyme activity was negligible in non-induced PLA controls, indicating a low level of endogenous activity. Because AP activity is intimately involved in matrix calcification, extracellular calcium accumulation was measured. Consistent with osteogenesis, VD induction of PLA cells and MSC controls resulted in a time-dependent increase in matrix mineralization with matrix calcification appearing in both populations at 3 wk and maximum levels detected at 6 wk. Induction of PLA cells resulted in an approximate 30-fold increase in matrix calcification over the 6-wk treatment period. Despite the lower AP activity compared with PLA cells, induced MSCs were associated with significantly more matrix calcification, compared with induced PLA cells (p < 0.001; 35-d induction), with a 68-fold overall increase in calcium accumulation detected over the 6-wk induction period.
Figure 3.
Osteo-induced PLA cells express several osteogenic genes and proteins. (A) PLA cells and MSCs were induced for up to 6 wk in OM. Cells were assayed for AP activity and total calcium and normalized with respect to protein. Non-induced PLA cells (control) were analyzed as a negative control. Values were expressed as the mean ± SD. (B) PLA cells were cultured in OM or noninductive control medium for up to 6 wk and analyzed by RT-PCR for the indicated genes. NHOst cells maintained in control medium (Con) or OM for 4 wk (28 d) were analyzed as a positive control. (C) Expression of the genes CBFA-1 and AP was quantitated by real-time PCR in PLA cells induced in control medium and OM for up to 4 wk. Gene expression levels were normalized with respect to endogenous GAPDH and expressed relative to non-induced control levels. (D) PLA cells were cultured in OM or control medium for 7 and 28 d and analyzed by Western blotting for the expression of OP, ON, AP, RARα, the VDR, and CNI. Expression of the TfR and α-actin was assessed as internal controls.
To confirm osteogenesis, cells were examined by RT-PCR for the expression of several genes, including OC, CBFA-1, AP, ON, OP, bone morphogenic protein-2 (BMP-2), c-fos, and CNI, in addition to receptors involved in osteogenesis (parathyroid hormone receptor/PTHR, RXRα, and vitamin D receptor/VDR) and the homeodomain proteins msx2 and distal-less 5 (dlx5) (Figures 3B and online S5). The osteogenic induction conditions used in this study were specific for the bone lineage and did not result in the expression of genes consistent with fat and cartilage differentiation (our unpublished data). Expression of CBFA-1, a transcription factor that binds to the promoters of several osteogenic genes (Ducy et al., 1997), was observed at all time points in osteo-induced PLA cells, MSCs, and NHOst cells. Furthermore, CBFA-1 expression was not specific to osteo-induced cells, as basal expression was observed in non-induced PLA cells and MSCs. Quantitation of CBFA-1 expression using real-time PCR confirmed a time-dependent increase in gene expression compared with non-induced controls (Figure 3C). Initial osteogenic induction of PLA cells (i.e., 7 d) resulted in an approximate 10-fold increase in CBFA-1 expression vs. controls, whereas a dramatic 60-fold increase was measured by 3 wk of induction. Induction of PLA cells in OM containing dexamethasone rather than VD also resulted in a time-dependent increase in CBFA-1 expression vs. controls, albeit at significantly lower levels, again suggesting an inhibitory effect of this glucocorticoid on PLA osteogenesis (our unpublished data). Finally, AP expression was observed at all time points in differentiated and control PLA cells, MSCs, and NHOst cells. Quantitative real-time PCR detected a decrease in AP levels after 1 wk of induction (1.7-fold). However, continued treatment (i.e., 21 d) resulted in an approximate twofold increase in AP expression level and corresponded well with the AP enzyme assay results.
In addition to CBFA-1 and AP, expression of CNI, OP, and ON was also observed in differentiated and control PLA cells, MSC, and NHOst controls. Although expression of these genes is indicative of osteogenesis, they are not specific markers. However, expression of the bone-specific gene OC was observed in both induced PLA cells and MSC controls. OC expression in osteo-induced PLA cells seemed to be biphasic, expressed as early as day 7 of induction and at late phases of differentiation in these cells (i.e., 21–42 d), whereas no expression was detected at 14 d. No such pattern was observed in osteo-induced MSCs with relatively consistent expression levels being observed. Moreover, in contrast to MSCs, OC expression was restricted to osteogenic induction, as no basal expression was seen in PLA cells maintained in non-inductive control medium, whereas low basal OC expression was detected in non-induced MSCs. Interestingly, exposure of PLA cells to dexamethasone inhibited the expression of OC at all time points (online Figure S5). Replacement of dexamethasone with VD for the last 48 h of induction was sufficient to overcome this inhibitory effect (our unpublished data). This inhibitory effect has also been observed in rat MSCs and human bone cultures (Beresford et al., 1986; Leboy et al., 1991; Jaiswal et al., 1997) and suggests that dexamethasone may be inhibitory to PLA osteogenesis. Because the actions of VD are mediated through its receptor via heterodimerization with the retinoid receptor RXR (Westin et al., 1988), expression of these receptors was confirmed in both control and induced PLA populations at all time points, together with the PTHR. Finally, both osteo- and non-induced PLA cells, MSCs, and NHOsts expressed the transcription factor c-fos and the homeodomain protein msx2, two genes involved in osteoblast differentiation (Benson et al., 2000). However, expression of the homeodomain protein dlx5 (Jabs et al., 1993; Ryoo et al., 1997; Newberry et al., 1998; Benson et al., 2000) and BMP-2, a member of the transforming growth factor-β (TGFβ) superfamily known to mediate osteogenesis (Johnson et al., 1988; Wang et al., 1990; Lieberman et al., 1998), was differentially expressed between the PLA and MSC populations. Specifically, no dlx5 and BMP-2 were detected in non-induced and induced PLA cells, whereas expression of both genes was observed in induced MSCs and NHOst controls.
Osteogenesis by PLA cells was also confirmed at the protein level by quantitative Western blotting. Osteogenic differentiation of PLA cells did not seem to alter the general activity of PLA cells, as equivalent levels of the transferrin receptor and α-actin were seen in both osteo-induced cells and controls. As shown in Figure 3D, expression of the bone matrix proteins OP and ON was detected in both differentiated cells and non-induced controls. However, osteogenic induction was accompanied by a 1.5 fold increase in OP expression at day 7 and a 1.2-fold increase at day 28, whereas a 1.6-fold increase in ON was detected in PLA cells from day 7 to day 28. Expression of these proteins was also confirmed in PLA cells and MSC controls by indirect immunofluorescence (online Figure S5). Control and osteo-induced PLA cells also expressed CNI and an approximate twofold increase in CNI protein was measured after 4 wk of induction. Consistent with the AP enzyme assays, expression of AP was detected specifically in osteo-induced PLA samples and induction resulted in a 2.6-fold increase in AP protein level. In addition to these matrix proteins, osteo-induced PLA cells specifically expressed the retinoic acid receptor α (RARα) after 4 wk of induction and expressed the VDR both before and after induction. Interestingly, osteogenic induction resulted in a 2.2-fold decrease in VDR levels by 4 wk of induction.
PLA Cells Undergo Chondrogenic Differentiation In Vitro
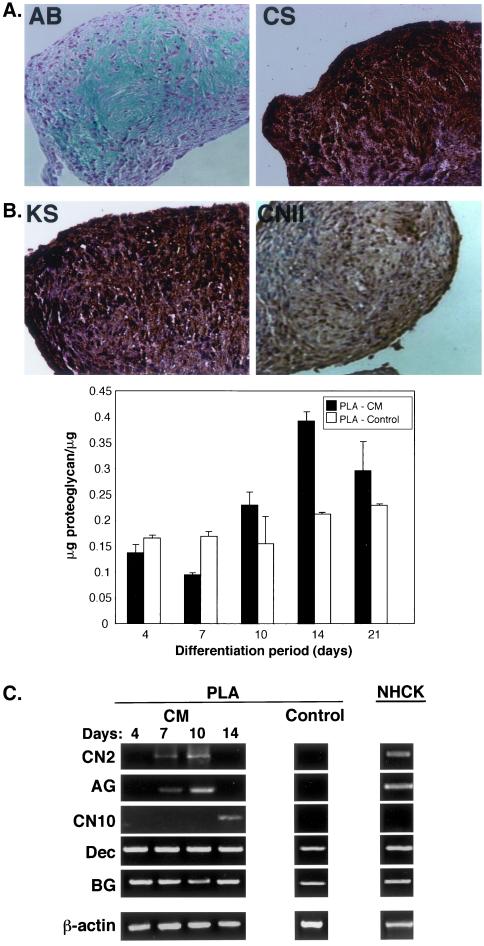
Chondrogenic induction of PLA cells, under micromass conditions, resulted in cell condensation as early as 12 h after induction and was followed by ridge and spheroid/nodule formation by 2 d (online Figure S6A). Nodules at this time point stained positively using AB, confirming the presence of sulfated proteoglycans within the matrix. Induction beyond 2 d resulted in an increase in nodule size and AB staining intensity. PLA chondrogenesis was dependent upon high cell density and induction conditions. Specifically, PLA nodule formation was dependent upon the presence of TGFβ1 and could not be induced in monolayer culture (our unpublished data). PLA nodules induced for 14 d in CM stained positively using AB, specifically expressing both keratan and chondroitin-4-sulfate (Figure 4A). Expression of the cartilagenous collagen II isoform (CNII splice variant CNIIB, mature chondrocytes shown) was also observed. Interestingly, micromass culture of MSCs in CM did not result in nodule formation and could not be used as a positive control in this study. Therefore, cells derived from human articular cartilage of the knee (NHCK) cells were used. Quantitation of sulfated proteoglycan levels revealed a time-dependent increase in cartilage-induced PLA cells up to 2 wk of induction (Figure 4B), followed by a slight decrease at 3 wk. A similar reduction was also noted in NHCK controls and may represent remodeling of the extracellular matrix (our unpublished data). Although control and induced PLA cells produced relatively equivalent levels of proteoglycan within the first 2 wk of induction, 14 d PLA nodules were associated with significantly more proteoglycan (1.8-fold more, p < 0.001), consistent with the increase in matrix synthesis associated with chondrogenic differentiation.
Figure 4.
PLA cells induced toward the chondrogenic lineage synthesize a cartilagenous matrix and express genes consistent with the chondrogenic lineage. (A) PLA cells were induced in CM under high-density conditions for 14 d. Nodules were sectioned and stained with AB, in addition to antibodies to CNII, keratan sulfate (KS), and chondroitin-4-sulfate (CS). (B) PLA cells were induced for up to 3 wk in CM (PLA-CM). Sulfated proteoglycan levels were determined and normalized with respect to protein per microgram (PG). Non-induced PLA cells (PLA-control) were analyzed as a negative control. Values were expressed as the mean ± SD. (C) PLA nodules were induced in CM for up to 14 d or maintained in non-inductive control medium for 10 d (PLA-Con). Samples were analyzed by RT-PCR for the indicated genes. NHCK cells induced for 2 wk in CM were analyzed as a positive control. Dec, decorin.
Treatment of PLA cells with CM resulted in the expression of genes consistent with chondrogenesis (Figures 4C and online S6B). CNII expression (splice variant IIB) was observed specifically in induced PLA cells and was restricted to days 7 and 10. A low level of CNII expression was also observed upon chondrogenic induction of NHCK controls. In addition, induced PLA cells also expressed the large proteoglycan aggrecan. Like CNII, aggrecan expression was restricted to days 7 and 10 and was specific to induced PLA samples. Aggrecan expression was also observed upon chondrogenic induction of NHCK controls. Chondrogenic induction of PLA nodules resulted in the specific expression of CNX, a marker of hypertrophic chondrocytes, at day 14 only. In contrast to this, little, if any, expression of CNX could be observed in NHCK controls and may be due to their derivation from articular cartilage. Induced and control PLA cells, together with induced NHCK controls, were also associated with additional collagen types, including CNI and CNIII with the majority of PLA samples examined exhibiting a restricted collagen expression pattern (day 4 only) (online Figure S6B). Induced PLA cells and NHCKs also expressed the cartilagenous proteoglycans decorin and biglycan. Expression of these genes was observed at all time points and was also seen in non-induced PLA cells. No expression of OC was seen at any time point, confirming the absence of osteogenic differentiation.
PLA Cells Undergo Myogenic Differentiation In Vitro
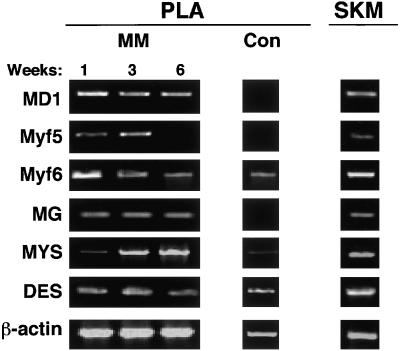
As shown in an previous study, myogenic induction of PLA cells for up to 6 wk in myogenic medium (MM) resulted in the expression of the myogenic transcription factor myod1 followed by fusion and the formation of multinucleated cells that expressed the myosin heavy chain (Mizuno et al., 2001). To further this characterization, the expression of multiple myogenic transcription factors, in addition to myod1 and myosin, was confirmed by RT-PCR. As shown in Figure 5, expression of the transcription factors myod1, myf6, and myogenin was observed at all induction points, whereas expression of myf5 was restricted to 1 and 3 wk only. Consistent with the early role of myod1 in myogenic determination, increased levels of this gene were observed at 1 wk. In addition, a qualitative increase in myf6 expression was also observed at this time point. Consistent with the terminal differentiation of myoblasts, a qualitative increase in myosin expression was observed over induction time (Figure 5B). Finally, expression of desmin, an intermediate filament protein expressed at high levels in skeletal muscle, was found at all induction points in both myo-induced and control PLA cells. Expression of these myogenic genes was also observed in human skeletal muscle controls.
Figure 5.
PLA cells induced toward the myogenic lineage express several myogenic genes. PLA cells induced in MM for up to 6 wk or maintained in control medium were analyzed by RT-PCR for the expression of the indicated myogenic genes. Total RNA prepared from human skeletal muscle (SKM) was analyzed as a positive control. DES, desmin; MD1, myod1; MG, myogenin; Myf, myogenic regulatory factor; MYS, myosin heavy chain.
PLA Cells May Undergo Neurogenic Differentiation In Vitro
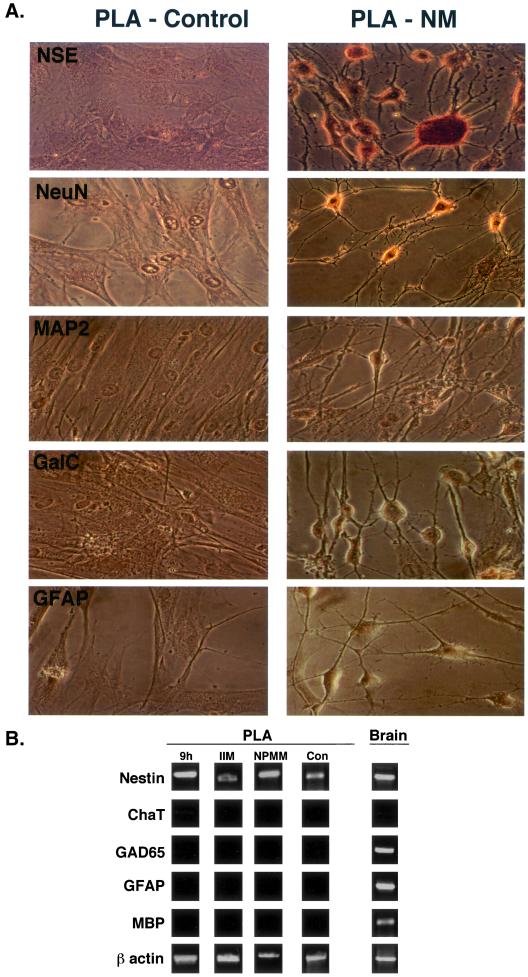
PLA cells were induced toward the neurogenic lineage using an established protocol (Woodbury et al., 2000) and assessed for the expression of neuronal markers (NSE, NeuN, and MAP-2), in addition to GFAP and GalC as markers of astrocytes and oligodendrocytes, respectively. Neurogenic induction for 30 min resulted in a change in PLA cell morphology, with 10% of the cells assuming a neuronal-like phenotype. Specifically, neuro-induced PLA cells underwent retraction, forming compact cell bodies with multiple extensions. Cell bodies became more spherical and cell processes exhibited secondary branches with increasing induction time. Sixty minutes of induction increased the proportion of neuronal-like PLA cells to 20% of the culture. Induction for 3 h increased this phenotype to a maximum of 70% and no significant increase was observed beyond this induction time. Induction in NM resulted in expression of the NSE and NeuN, consistent with the neuronal lineage (Figure 6A). The majority of the induced PLA cells in culture stained positively for NSE, and Western blotting confirmed an increase in this protein upon induction (Ashjian et al. 2003). In contrast to NSE, not all PLA cells were NeuN positive and may indicate development of a restricted subpopulation of neurogenic cells. No expression of the mature neuronal markers MAP-2 or NF-70 was observed (our unpublished data), suggesting that induced PLA cells at these time points represent an early developmental stage. In addition, no expression of GalC and the GFAP was noted, indicating that PLA cells did not differentiate into oligodendrocytes and astrocytes, respectively. Finally, control PLA cells did not express any neuronal, oligodendrocytic, or astrocytic markers, confirming the specificity of our induction conditions and staining protocol.
Figure 6.
PLA cells exhibit neurogenic capacity in vitro. (A) PLA cells were maintained in NM or control medium for 5 h (PLA-NM and PLA-control, respectively) and analyzed for expression of neural (NSE and NeuN), astrocytic (GFAP), and oligodendritic (GalC) markers. (B) PLA cells were induced in 1) NM for 9 h or 2) NM for 9 h and maintained for 1 wk in NPMM or 3) control medium supplemented with IIM for 1 wk. Samples were analyzed by RT-PCR for the indicated genes. Non-induced PLA cells (con) were analyzed as a negative control. Total RNA prepared from human brain (brain) was examined as a positive control. ChaT, choline acetyltransferase.
RT-PCR analysis confirmed the expression of nestin, an intermediate filament found in neural stem cells, in PLA cells induced for 9 h in NM (Figure 6B) (Lendahl et al., 1990). Nestin expression was also detected in non-induced PLA cells and in total RNA prepared from human brain. No expression of markers characteristic of more mature neuronal subtypes, choline acetyltransferase (Chat) or GAD65, was observed. Moreover, RT-PCR did not detect other neurogenic lineages, as no expression of GFAP (astrocytic) or myelin-basic protein (oligodendrocytic) was detected. A similar gene expression profile, including nestin, was also observed in PLA cells induced for 9 h in NM, followed by maintenance for up to 1 wk in a medium designed to maintain neurogenic precursors (NPMM). In addition, nestin expression was also found in PLA cells maintained in non-inductive control medium containing indomethacin and IBMX (IIM). Taken together, the expression of nestin, NSE, and NeuN, together with the absence of choline acetyltransferase, myelin-basic protein, or GFAP expression, suggests that PLA cells may be capable of assuming an early neuronal or neural precursor phenotype.
PLA Clonal Isolates Possess Multilineage Potential
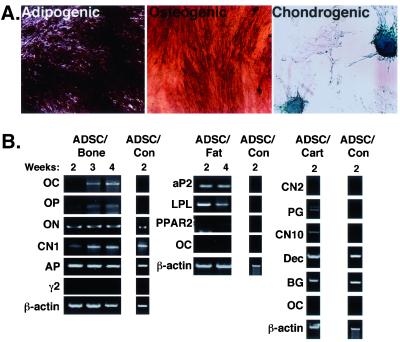
To confirm the presence of a stem cell population within adipose tissue, PLA samples were cultured at a low confluence such that the formation of single PLA cell-derived colonies was possible. Five hundred PLA clones were isolated and expanded. Thirty clones exhibited differentiation into at least one of the three mesodermal lineages examined (osteogenic, adipogenic, and chondrogenic). In addition, seven clones exhibited differentiation into all of these lineages, staining positively for AP, Oil Red O, and Alcian blue (Figures 7A and online S7). We designated these tri-lineage clones as ADSCs. Like PLA cells, ADSCs were fibroblastic in morphology and, after expansion, no evidence of other cell morphologies (e.g., endothelial and macrophages) could be observed, suggesting the homogeneity of ADSC cultures (our unpublished data). A qualitative increase in differentiation level, as measured by histological staining, was observed in all ADSC populations compared with heterogenous PLA samples (our unpublished data). Finally, isolation and expansion of tri-lineage ADSCs did not alter the CD expression profile as shown by immunofluorescence (our unpublished data). In addition to ADSCs, other PLA-derived clones exhibiting a more restricted dual-lineage potential (osteogenic/adipogenic, osteogenic/chondrogenic, and adipogenic/osteogenic) and single lineage potential (adipogenic) were also isolated (online Figure S7).
Figure 7.
PLA clones possess multi-lineage potential. (A) PLA clonal isolates were analyzed for osteogenic (alkaline phosphatase), adipogenic (Oil Red O), and chondrogenic (Alcian blue) capacity. (B) Tri-lineage clones (osteogenic, adipogenic, and chondrogenic), or ADSCs, were cultured in either OM (ADSC-bone), AM (ADSC-fat), or CM (ADSC-cartilage), in addition to control medium (ADSC-control). ADSCs were analyzed by RT-PCR for the indicated lineage-specific genes.
To confirm multi-lineage potential, ADSCs were examined like the heterogenous PLA population by RT-PCR for the expression of several lineage-specific genes. Supportive of their multi-lineage capacity, ADSCs expressed multiple genes characteristic of the osteogenic, adipogenic, and chondrogenic lineages (Figure 7B). Specifically, induction of ADSCs with OM resulted in the expression of OC, ON, OP, CNI, and AP. Adipose induction of ADSCs resulted in the specific expression of aP2 and LPL, together with a low level of PPARγ2. Finally, expression of aggrecan, CNX, decorin, and biglycan was detected upon 2 wk of chondrogenic induction. The expression patterns of these genes in ADSCs was indistinguishable from that observed in the heterogenous PLA population. Together with the immunohistochemistry data, the RT-PCR results confirm the multi-lineage capacity of ADSC isolates and suggest that the multi-lineage capacity of the PLA population is due to the presence of stem cell population.
DISCUSSION
In the present study, we confirm the multi-lineage capacity of a population of stem cells, termed PLA cells, isolated from human lipoaspirates. Preliminary studies characterized the heterogeneity and growth kinetics of this cell population and revealed that PLA cells may have multi-lineage potential (Zuk et al., 2001). The purpose of this work was twofold: 1) to confirm whether stem cells exist in adipose tissue, and 2) to compare the differentiation potential of these cells to MSCs, a well characterized stem cell population isolated from bone marrow. Our findings reveal that PLA cells are capable of multiple mesodermal lineage differentiation, as shown by the expression of several lineage-specific genes and proteins. In addition, PLA cells can also be induced to express markers consistent with a neurogenic phenotype, suggesting an ectodermal potential. Finally, mesodermal and ectodermal capacity was detected in PLA clonal isolates, suggesting that adipose tissue represents a source of adult stem cells.
PLA Cells Are Phenotypically Similar to MSCs
Characterization of MSCs has been performed using the expression of cell-specific proteins and CD markers (Bruder et al., 1998b; Conget and Minguell, 1999; Pittenger et al., 1999). Like MSCs, PLA cells expressed CD29, CD44, CD71, CD90, CD105/SH2, and SH3 and were absent for CD31, CD34, and CD45 expression (online Figure S1). Moreover, flow cytometry on PLA cells confirmed the expression of CD13, whereas no expression of CD14, 16, 56, 62e, or 104 was detected (Table 2). These results demonstrate that similar CD complements are expressed on both PLA cells and MSCs. However, distinctions in two CD markers were observed: PLA cells were positive for CD49d and negative for CD106, whereas the opposite was observed on MSCs. Expression of CD106 has been confirmed in the bone marrow stroma and, specifically, MSCs (Levesque et al., 2001) where it is functionally associated with hematopoiesis. The lack of CD106 on PLA cells is consistent with the localization of these cells to a non-hematopoietic tissue.
PLA Cells Differentiate into Bone, Fat, Cartilage, and Muscle: Multiple Mesodermal Lineage Capacity
As suggested in a previous study (Zuk et al., 2001), PLA cells seem to possess the capacity to differentiate into multiple mesodermal lineages, including bone, fat, and cartilage. This observation has led us to speculate that adipose tissue may be a source of mesodermal stem cells. The current study supports this hypothesis, characterizing the metabolic activity of several mesodermal lineages, in addition to confirming the expression of multiple lineage-specific genes and proteins.
Adipogenesis
Consistent with the initiation of the adipogenic program, adipo-induction of PLA cells resulted in a significant increase in GPDH activity, a lipogenic enzyme involved in triglyceride synthesis (Kuri-Harcuch et al., 1978). In addition to possessing metabolic activity consistent with the formation of mature adipocytes, PLA cells expressed several genes and/or proteins involved in lipid biosynthesis and storage, including 1) adipo-induced specific expression of PPARγ2, a fat-specific transcription factor that functions in preadipocyte commitment (Totonoz et al., 1994); 2) increased expression of LPL, a lipid exchange enzyme up-regulated during adipogenesis (Ailhaud et al., 1992); 3) up-regulation of aP2, a protein associated with lipid accumulation within mature adipocytes (Bernlohr et al., 1985); and 4) increased expression of both leptin and GLUT4 and restriction of these proteins to lipid-filled PLA cells. Although the expression of these genes in induced PLA cells and MSC controls was similar to 3T3 controls and suggests adipogenic differentiation, the timing of their expression does differ from lineage-committed precursors. Specifically expression of aP2 is restricted to a late phase in developing adipocytes, yet is detected early in PLA and MSC differentiation and preceded that of PPARγ2. This altered sequence of adipose gene expression in PLA cells may be due to a distinct developmental program characteristic of stem cells. Consistent with this, osteocalcin expression, an established late marker of osteoblast differentiation, is also observed early in osteogenic PLA cell and MSC populations. Alternatively, the observed gene sequence may be due to the asynchronous development of cell subpopulations within the heterogenous PLA.
Osteogenesis
Induction of PLA cells with OM supplemented with vitamin D resulted in several events supportive of osteogenesis. Specifically, AP activity and mineralization capacity increased in a time-dependent manner upon osteogenic induction of PLA cells. However, AP kinetics were not linear in induced PLA samples but assumed a biphasic pattern. Time-course studies on rat calvaria and marrow stromal cells have shown that AP peaks early, correlating with matrix mineralization and is down-regulated during terminal differentiation into osteocytes (Owen et al., 1990; Malaval et al., 1994). Moreover, a dose-dependent inhibition of AP activity by VD has been measured in mature osteosarcoma cells, an effect thought to represent the return of a cell fraction to the osteoprogenitor pool or their terminal differentiation (Majeska and Rodan, 1982). It is therefore possible that the biphasic AP enzyme pattern in PLA cells may be due to the differentiation of multiple osteoprogenitor subpopulations with distinct temporal and developmental profiles.
In addition to increased AP activity and matrix calcification, expression of multiple genes can be used to confirm osteogenic differentiation. RT-PCR confirmed the expression of the majority of the genes examined (c-fos, RXRα, VDR, PTHR, OP, ON, AP, CBFA-1, and CNI) in both non-induced and induced PLA and MSC cell populations, consistent with previous results observed in MSCs and indicative of osteogenic differentiation. Furthermore, quantitative real-time PCR confirmed increases in CBFA-1 upon the onset of osteogenic differentiation. Increases in AP were also measured later in PLA differentiation, consistent with the AP spectrophotometric assay results. In addition to increases at the gene level, Western blotting also detected increases in OP and CNI protein levels along with the specific expression of AP. Although the expression of ON, OP and the increased expression of AP and CBFA-1 is strongly suggestive of osteogenesis, these genes are not considered to be specific markers for differentiation. One such gene is OC. Although considered a late marker of osteoblast differentiation (Owen et al., 1990), OC is expressed early during osteogenesis of marrow stromal cells (Malaval et al., 1994). Consistent with this, induction of PLA cells and MSC controls resulted in early OC expression. Moreover, osteo-induction of PLA cells resulted in a biphasic OC expression pattern. This pattern, similar to AP activity, may be the response of PLA cell subpopulations at distinct developmental stages to osteogenic induction. In support of this, several other induction agents have been shown to stage-specific effects on osteogenesis, including TGFβ (Breen et al., 1994). Finally, OC expression by induced PLA cells was dependent upon osteogenic agent as OC expression was inhibited upon dexamethasone exposure, an effect not observed in MSC controls.
Chondrogenesis and Myogenesis
Chondrogenic differentiation in vitro of MSCs requires high-density culture, thus duplicating the process of cellular condensation, in addition to media supplementation. Consistent with this, high-density culture of PLA cells in CM resulted in the formation of compact nodules that exhibited many characteristics of cells differentiating toward the chondrogenic lineage. First, PLA nodules were associated with a time-dependent increase in the sulfated proteoglycans keratan- and chondroitin-sulfate, in agreement with that observed in high-density MSC cultures (Yoo et al., 1998). In addition, nodules also contained the type II collagen isoform, a collagen characteristic of cartilage (Yoo et al., 1998). Second, chondrogenic PLA nodules also expressed several genes consistent with chondrogenesis, including the following: the specific expression of CNII and the large, cartilage proteoglycan aggrecan in induced PLA samples; 2) expression of the small, leucine-rich proteoglycans decorin and biglycan; and 3) the late expression of CNX, a marker of hypertrophic chondrocytes. The expression of CNX by PLA cells may indicate possible ossification and endochondral bone formation, an event that is supported by the expression of CNI within the PLA nodule. However, expression of many collagens, including CNI, has been observed in chondrogenic MSC nodules (Yoo et al., 1998) and in high-density embryonic chick limb-bud cell aggregates (Osdoby and Caplan, 1979; Tachetti et al., 1987). Moreover, no expression of osteocalcin by chondrogenic PLA or NHCK cells was seen at any time point, confirming the absence of osteogenic differentiation within the PLA nodule.
Finally, myogenic lineage potential in PLA cells was confirmed by the expression of several transcription factors, including myf6, myf5, myod1, and myogenin and the structural proteins desmin and myosin. Determination and execution of the myogenic program in myoblast precursors is controlled at the transcription level by these same transcription factors (Atchley et al., 1994; Lassar and Musterberg, 1994), whereas terminal differentiation can be confirmed through the expression of myosin. Therefore, the expression of these genes together with previous work confirming the expression of myoD1 and myosin at the gene and protein level (Mizuno et al., 2001) is supportive of the myogenic lineage in PLA cells.
Neurogenic Induction of PLA Cells Results in Expression of Neuronal Markers: Potential Ectodermal Capacity?
Like MSCs, it is not surprising to observe the differentiation of putative stem cells from adipose tissue (i.e., PLA cells) into multiple mesodermal lineages because fat tissue, like the bone marrow stroma, is a mesodermal derivative. However, recent reports have documented the differentiation of MSCs to neural-like cells (Sanchez-Ramos et al., 2000; Woodbury et al., 2000), suggesting that adult stem cells may not be as restricted as previously thought. Recent work on MSCs undergoing early neurogenic differentiation has confirmed the expression of nestin, an intermediate filament protein thought to be expressed at high levels in neural stem cells (Lendahl et al., 1990; Sanchez-Ramos et al., 2000). Consistent with this, nestin expression was detected in non-induced PLA cells and those induced under several established neurogenic media conditions (i.e., NPMM and IIM), suggesting the assumption of a neural stem cell phenotype by PLA cells. Nestin expression has also been observed in myogenic cells, endothelial cells, and hepatic cells, indicating that it cannot be used as a marker for putative neurogenic potential. However, neurogenic induction of PLA cells also resulted in the assumption of a neuronal-like morphology and the increased expression of two neuron-specific proteins, NSE and NeuN. NeuN expression is thought to coincide with terminal differentiation of developing and post-mitotic neurons (Mullen et al., 1992), and its expression has also been used to identify neuronal development in MSCs (Sanchez-Ramos et al., 2000). Therefore, combined with the expression of early neuronal markers, such as NeuN, nestin expression may indicate potential neurogenic capacity in PLA cells. Finally, induction of PLA cells seemed to restrict their development to an early, neuronal stage as no expression of established oligodendrocyte and astrocyte markers or mature neuronal markers were observed at the gene or protein level. The absence of mature neuronal markers has also been observed in MSC cultures by several groups (Sanchez-Ramos et al., 2000; Deng et al., 2001) and may reflect the induction conditions used or the need for prolonged induction time.
PLA Clones Possess Multi-lineage Capacity: ADSCs
PLA multi-lineage differentiation may result from the commitment of multiple lineage-specific precursors rather than the presence of a pluripotent stem cell population. Therefore, the isolation of clones derived from single PLA cells is critical to their identification as stem cells. Clonal analysis isolated several tri-lineage PLA clones (ADSCs), expressing multiple osteogenic, adipogenic, and chondrogenic genes, strongly suggesting that ADSCs possess multi-potentiality and may be considered stem cells. In addition, clonal analysis also isolated samples with more restricted potentials, including dual lineage (osteogenic/adipogenic, osteogenic/chondrogenic, and adipogenic/chondrogenic) and single lineage (adipogenic only). In support of this, the isolation of restricted lineage MSC clones from transgenic mice and bone marrow has been reported (Dennis et al., 1999; Pittenger et al., 1999). Older models of mesenchymal differentiation propose that lineage progenitors are determined by the microenvironment (Friedenstein, 1990). Based on this, one would expect differentiation to be a stochastic event resulting in a random combination of phenotypes. However, a recent model has proposed the existence of a hierarchy in the MSC differentiation pathway, with the adipogenic lineage diverging early and the osteogenic lineage a default pathway (Muraglia et al., 2000). Although the isolation of osteogenic/chondrogenic PLA clones is in agreement with this model, the presence of both adipogenic/osteogenic and adipogenic/chondrogenic isolates (not previously reported in MSC populations) suggests that the differentiation of PLA stem cells follows a more random course of action.
Distinctions between PLA and MSC Populations
Analysis of PLA cells and MSCs in this study has identified many similarities between the two populations, lending support to the theory that stem cells can be found within adipose tissue. However, these similarities may also indicate that PLA cells are simply an MSC population located within the adipose compartment, perhaps the result of infiltration of MSCs from the peripheral blood supply. However, we do no believe this to be the case. First, the presence of MSCs in the peripheral blood is controversial. Moreover, if present within the peripheral blood, the number of MSCs within the bone marrow stroma is extremely low (∼1 MSC per 105 stromal cells; Rikard et al., 1994; Bruder et al., 1997; Pittenger et al., 1999) and is likely to be even lower in the peripheral blood. This low level is unlikely to give the relatively high levels of differentiation observed in this study. Second, we have observed several distinctions between PLA and MSC populations that suggest they are similar, but not identical, cell types: 1) Preliminary results on PLA cells indicate that sera screening is not necessary for their expansion and differentiation (Zuk et al., 2001), a requirement for MSCs (Lennon et al., 1996). 2) MSCs did not undergo chondrogenic or myogenic differentiation under the conditions used in this study, suggesting distinctions in differentiation capacities and/or kinetics. 3) Immunofluorescence analysis identified differences in CD marker profile between PLA and MSC populations. In contrast to MSCs, expression of CD106 was not observed on PLA cells, whereas PLA cells were found to express CD49d. 4) Distinctions between PLA and MSC populations may also extend to the gene level. For example, osteocalcin expression was restricted to PLA samples induced specifically with VD. Although treatment of MSCs with VD also induced OC expression, expression of this gene was also observed in dexamethasone-treated and non-induced MSCs, albeit at lower levels (our unpublished data; online Figure S5). In addition, PLA cells and MSCs exhibited distinctions in BMP-2 and dlx5 expression, both of which were found in induced MSCs only. Because dlx5 and BMP2 are known to mediate expression of multiple osteogenic genes, it is possible that PLA and MSC populations differ in their regulation of the osteogenic differentiation pathway. Taken together, these differences may indicate that adipose tissue contains stem cells, distinct from those found in the bone marrow stroma. However, the possibility that PLA cells are a clonal variant of circulating MSCs cannot be ruled out.
Future Directions
Stem cells are considered to be cells possessing self-replicating potential and the ability to give rise to terminally differentiated cells of multiple lineages (Hall and Watt, 1989). Until recently, the embryonic stem cell has been the “gold standard,” capable of differentiating into cells from all three embryonic germ layers (Evans and Kaufman, 1981; Shamblott et al., 1998). However, unlike embryonic stem cells, research on adult-derived stem cells (i.e., MSCs) has suggested a more restricted potential. The traditional view of adult stem cell differentiation believed that stem cell progeny progressed in a linear, irreversible manner that eliminated their stem cell propensity and restricted their fate to within a germ line. A new, evolving theory of differentiation proposes that stem cell progeny differentiates in a more graded manner, giving rise to more progressively restricted daughter cells that possess trans-germ potential. There is precedence for this belief. Clonal strains of marrow adipocytes can be directed to form bone (Bennett et al., 1991) and chondrocytes can dedifferentiate toward the osteogenic lineage (Galotto et al., 1994). Recent studies confirming the neurogenic potential of MSCs, the induction of HSCs into hepatocytes (Legasse et al., 2000) and the conversion of neurogenic precursors into muscle and blood (Bjornson et al., 1999; Galli et al., 2000) have contributed to this theory and may be the beginning of a paradigm shift.
There is a physiological need for stem cells with plasticity. However, although the mechanism of stem cell plasticity remains unknown, several examples of this phenomenon can be found at the molecular level. Several genes, including leptin, CBFA1, and PPARγ participate in more than one lineage pathway. Leptin is known to participate in both adipogenesis and osteogenesis (Chen et al., 1997; Ogeuch et al., 2000). CBFA-1 is not only constitutively expressed in marrow stromal cells but also is retained as these cells differentiate into multiple cell types (e.g., osteogenic and chondrogenic) (Satomura et al., 2000). Consistent with this, expression of both leptin and CBFA1 is observed in non-induced PLA cells and cells differentiating into multiple lineages (our unpublished data). It is possible that stem cells, unlike more committed precursors, are capable of switching phenotypes at a “late” stage of development. This plasticity, together with the ability of stem cells to cross germ layers, presents researchers with exciting possibilities and the definition of a stem cell may need to be amended. Equally exciting, is the emerging concept that stem cells may be found in multiple organs (e.g., muscle, heart, and liver) (Lucas et al., 1992; Young et al., 1995) and tissues, such as skin (Toma et al., 2001), placenta, and fat (Zuk et al., 2001). With this, there are now multiple stem cell reservoirs available for research and clinical applications. Although further characterization of the PLA population within adipose tissue and its application in vivo is necessary, the results presented in this study suggest that adipose tissue may be another source of pluripotent stem cells with multi-germline potential.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by the Wunderman Family Foundation, the American Society for Aesthetic Plastic Surgery, the Plastic Surgery Educational Foundation, and the Los Angeles Orthopaedic Hospital Foundation, and by grants from the Orthopedic Hospital Institute of Los Angeles and the National Institute of Arthritic and Musculoskeletal Diseases (NIH).
Abbreviations used:
- ADSC
adipose-derived stem cell
- AG
aggrecan
- AM
adipogenic medium
- AP
alkaline phosphatase
- BG
biglycan
- BMP-2
bone morphogenic protein-2
- CBFA-1
core-binding factor alpha 1
- CM
chondrogenic medium
- CN I
II, III, X, collagen type 1, type 2, type 3, type 10
- DES
desmin
- dlx5
distal-less 5
- GalC
galactocerebroside
- GFAP
glial fibrillary acidic protein
- GPDH
glycerol-3-phosphate dehydrogenase
- LPL
lipoprotein lipase
- MBP
myelin basic protein
- MG
myogenin
- MM
myogenic medium
- MSC
mesenchymal stem cell
- NeuN
neuronal nuclei protein
- NHCK
normal human chondrocyte from the knee
- NHOst
normal human osteoblast
- NM
neurogenic medium
- NSE
neuron-specific enolase
- OC
osteocalcin
- OM
osteogenic medium
- ON
osteonectin
- OP
osteopontin
- PLA
processed lipoaspirate
- PTHR
parathyroid hormone receptor
- PPARγ
peroxisome-proliferating activated receptor [γ]
- RXRα
retinoid X receptor α; TfR, transferrin receptor
- VD
1,25-dihydroxyvitamin D3
- VDR
vitamin D receptor
Footnotes
Online version of this article contains supplemental figures and tables. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0105. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0105.
REFERENCES
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–33. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Ashjian, R.A., El-Barbary, A.S., Edmonds, B., Dellgarte, D.A., Zhu, M., Zuck, P.A., Lorenz, H.P., Benhaim, P., and Hendrick, M.H. (2003). In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast. Reconstr. Surg. (in press). [DOI] [PubMed]
- Atchley WR, Fitch WM, Bronner-Fraser M. Molecular evolution of the MyoD family of transcription factors. Proc Natl Acad Sci USA. 1994;91:11522–11526. doi: 10.1073/pnas.91.24.11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayahu D, Kletter Y, Zipori D, Weintroub S. Bone-marrow derived stromal cell line expressing osteoblast phenotype in vitro and osteogenic capacity in vivo. J Cell Physiol. 1989;140:1–7. doi: 10.1002/jcp.1041400102. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99:131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- Benson MD, Bargeon JL, Xiao G, Thomas PE, Kim A, Cui Y, Franceschi RT. Identification of a homeodomain binding element in the bone sialoprotein gene promoter that is required for its osteoblast-selective expression. J Biol Chem. 2000;275:13907–13917. doi: 10.1074/jbc.275.18.13907. [DOI] [PubMed] [Google Scholar]
- Beresford JN, Gallagher JA, Russel RGG. 1,25-Dihydroxyvitamin D3 and human bone-derived cells in vitro: effects on alkaline phosphatase, type I collagen and proliferation. Endocrinology. 1986;119:1776–1785. doi: 10.1210/endo-119-4-1776. [DOI] [PubMed] [Google Scholar]
- Bernlohr DA, Doering TL, Kelly TJ, Lane MD. Tissue specific expression of p422 protein, a putative lipid carrier in mouse adipocytes. Biochem Biophys Res Commun. 1985;132:850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Breen EC, Ignotz RA, McCabe L, Stein JL, Stein GS, Lian JL. TGFβ alters growth and differentiation related gene expression in proliferating osteoblasts in vitro, preventing development of the mature bone phenotype. J Cell Biochem. 1994;160:323–335. doi: 10.1002/jcp.1041600214. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bruder, S.P., Jaiswal, N., Ricalton, N.S., Mosca, J.D., Kraus, K.H., and Kadiyala, S. (1998b). Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin. Orthop. S247–S256. [DOI] [PubMed]
- Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998a;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- Chen X, Hausman DB, Dean RG, Hausman GJ. Differentiation-dependent expression of obese (ob) gene by preadipocytes and adipocytes in primary cultures of porcine stromal-vascular cells. Biochim Biophys Acta. 1997;1359:136–142. doi: 10.1016/s0167-4889(97)00083-9. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Hewison M, Stewart PM. Glucocorticoid activity, inactivity and the osteoblast. J Endocrinol. 1999;163:159–164. doi: 10.1677/joe.0.1630159. [DOI] [PubMed] [Google Scholar]
- Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic cAMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Evans M, Kaufman M. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Osteogenic stem cells in the bone marrow. In: Heersche JNM, Kanis JA, editors. Bone and Mineral Research. Vol. 7. San Diego, CA: Elsevier Science; 1990. pp. 243–272. [Google Scholar]
- Galli R, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- Galotto M, Campanile G, Robino G, Cancedda FP, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res. 1994;9:1239–1249. doi: 10.1002/jbmr.5650090814. [DOI] [PubMed] [Google Scholar]
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Grigoradis A, Heersche JNM, Aubin J. Differentiation of muscle fat, cartilage and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metabol. 1987;64:832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Jabs EW, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Johnson EE, Urist MR, Finerman GAM. Repair of segmental defects of the tibia with cancellous bone grafts augmented with human bone morphogenic protein. A preliminary report. Clin Orthop. 1988;236:249–257. [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Jonasson L, Hansson GK, Bondjers G, Bengtsson G, Olivecrona T. Immunohistochemical localization of lipoprotein lipase in human adipose tissue. Atherosclerosis. 1984;51:313–326. doi: 10.1016/0021-9150(84)90179-5. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W, Wise LS, Green H. J. Biol Chem. 1978;252:2158–2160. [PubMed] [Google Scholar]
- Lassar A, Munsterberg A. Wiring diagrams: regulatory circuits and the control of skeletal myogenesis. Curr Opin Cell Biol. 1994;6:432–442. doi: 10.1016/0955-0674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991;146:370–378. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- Legasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nutr Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol. 1996;32:602–611. [Google Scholar]
- Levesque JB, Takamatsu Y, Hilsson SK, Haylock DN, Simmons RJ. Vascular cell adhesion molecule-1 (VCAM-1) is cleaved by neutrophil proteases in the bone marrow following hematopoietic cell mobilization by granulocyte colony stimulating factor. Blood. 2001;98:1289–12990. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- Lucas PA, Calcutt AF, Mulvaney DJ, Young HE, Southerland SS. Isolation of putative mesenchymal stem cells from rat embryonic and adult skeletal muscle. In Vitro Cell Biol. 1992;28:154A. [Google Scholar]
- Majeska RJ, Rodan GA. The effect of 1, 25 (OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J Biol Chem. 1982;257:3362. [PubMed] [Google Scholar]
- Malaval L, Madrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation of human processed lipoaspirate cells. Plastic Reconstr Surg. 2001;109:199–209. doi: 10.1097/00006534-200201000-00030. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:210–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Newberry EP, Latifi T, Towler DA. Reciprocal regulation of osteocalcin transcription by the homeodomain proteins msx2 and dlx5. Biochemistry. 1998;37:16360–16368. doi: 10.1021/bi981878u. [DOI] [PubMed] [Google Scholar]
- Ogeuch O, Sooranna S, Nicolaides KH, Johnson MR. The relationship between leptin concentration and bone metabolism in the human fetus. J Clin Endo Meta. 2000;85:1997–1999. doi: 10.1210/jcem.85.5.6591. [DOI] [PubMed] [Google Scholar]
- Osdoby P, Caplan AI. Osteogenesis in cultures of limb mesenchymal cells. Dev Biol. 1979;73:84–102. doi: 10.1016/0012-1606(79)90140-4. [DOI] [PubMed] [Google Scholar]
- Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Regulation of local differentiation of cartilage and bone by extracellular matrix: a cascade type mechanism. Prog Clin Biol Res. 1982;110:261–268. [PubMed] [Google Scholar]
- Rikard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, Stein GS, Stein JL, van Wijnene AJ, Lian JB. Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997;11:1681–1694. doi: 10.1210/mend.11.11.0011. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Satomura K, Kresbach P, Bianco P, Gehron-Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J Cell Biochem. 2000;78:391–403. [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from culture human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachetti C, Quarto R, Nitsch L, Hartmann DJ, Cancedda R. In vitro morphogenesis of chick embryo hypertrophic cartilage. J Cell Biol. 1987;106:999–1006. doi: 10.1083/jcb.105.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JW, Leingang KA, Mueckler MM, Glenn KC. Cell mechanism of the insulin-like effect of growth hormone in adipocytes. Rapid translocation of the HepG2 type and adipocyte/muscle glucose transporters. Biochem J. 1992;182:99–106. doi: 10.1042/bj2820099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Totonoz P, Hu E, Graves RA, Budvari AI, Speigelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Wang EA, et al. Recombinant human bone morphogenic protein induces bone formation. Proc Natl Acad Sci USA. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin S, Kurokawa R, Nolte RT, Wisely GB, Mcinerney EM, Rose DW, Milburn MV, Rosenfield MG, Glass CK. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1988;395:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979;254:273–275. [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Young HE, Mancini ML, Wright RP, Smith JC, Black AC, Jr, Reagan CR, Lucas PA. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn. 1995;202:137–144. doi: 10.1002/aja.1002020205. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang JI, Futrell WJ, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–226. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.