Abstract
This study describes the characterization of orf358, an open reading frame of previously unidentified function, in the purple bacterium Rubrivivax gelatinosus. A strain in which orf358 was disrupted exhibited a phenotype similar to the wild type under photosynthesis or low-aeration respiratory growth conditions. In contrast, under highly aerated respiratory growth conditions, the wild type still produced bacteriochlorophyll a (Bchl a), while the disrupted strain accumulated a compound that had the same absorption and fluorescence emission spectra as Mg-protoporphyrin but was less polar, suggesting that it was Mg-protoporphyrin monomethylester (MgPMe). These data indicated a blockage in Bchl a synthesis at the oxidative cyclization stage and implied the coexistence of two different mechanisms for MgPMe cyclization in R. gelatinosus, an anaerobic mechanism active under photosynthesis or low oxygenation and an aerobic mechanism active under high-oxygenation growth conditions. Based on these results as well as on sequence analysis indicating the presence of conserved putative binuclear-iron-cluster binding motifs, the designation of orf358 as acsF (for aerobic cyclization system Fe-containing subunit) is proposed. Several homologs of AcsF were found in a wide range of photosynthetic organisms, including Chlamydonomas reinhardtii Crd1 and Pharbitis nil PNZIP, suggesting that this aerobic oxidative cyclization mechanism is conserved from bacteria to plants.
Purple bacteria perform anoxygenic photosynthesis on the basis of a bacteriochlorophyll-mediated process. Their photosynthetic apparatus, related to plant photosystem II, comprises three pigment-protein complexes: the light-harvesting antennae LHI and LHII and the reaction center, associated with bacteriochlorophyll a (Bchl a) and carotenoids (13, 15, 23). The organization of the genes implicated in photosynthesis has been extensively studied for purple bacteria such as Rhodobacter capsulatus and Rhodobacter sphaeroides, for which a “photosynthetic cluster” containing most of the genes coding for the photosynthetic apparatus and the pigment biosynthesis enzymes has been described (38), as well as in other models such as Rubrivivax gelatinosus (17, 26, 27). However, the functions of some open reading frames (ORFs) of the photosynthetic cluster remain to be elucidated.
Among these uncharacterized ORFs, R. gelatinosus orf358 appeared particularly worth investigating, since several protein or putative protein homologs have been found in a wide range of photosynthetic organisms from bacteria to plants, but not in nonphotosynthetic organisms to date. Previous studies have demonstrated the implication of some of these homologs in physiological processes. In the higher plant Pharbitis nil, PNZIP mRNA expression has been shown to be limited to the leaf photosynthetic mesophyll cells, promoted in darkness, and regulated by phytochrome, which suggested a photosynthesis-related function (37). In the green alga Chlamydomonas reinhardtii, expression of Crd1 was activated in copper- or oxygen-deficient cells and was shown to affect photosystem I and light-harvesting-complex accumulation (24). However, no precise biochemical function could be attributed to these proteins.
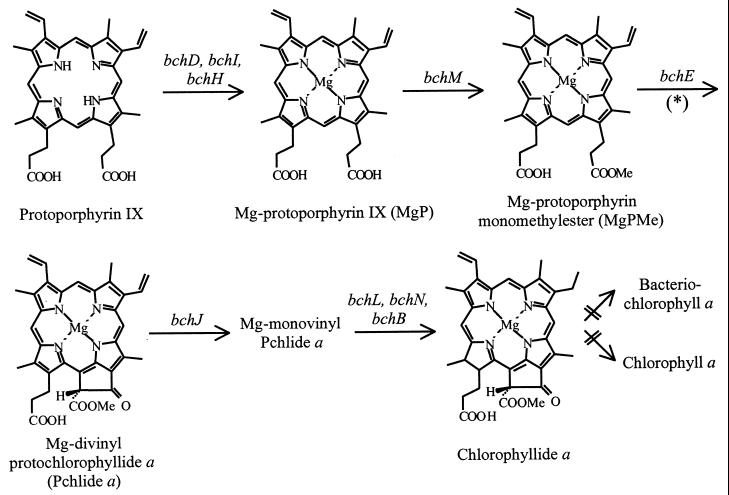
The use of R. gelatinosus as a bacterial model in which to investigate the function of such conserved ORFs presents several advantages, including the easy genetic manipulation of the strain and its ability to grow under different trophic conditions (25). In particular, the low-level expression of photosynthetic complexes and pigments under respiratory conditions makes it possible to study the effects of mutations that impair functions necessary for photosynthesis. In this study, an R. gelatinosus strain in which orf358 is disrupted, and which exhibits a severe defect in Bchl a synthesis at the oxidative cyclization step when grown under aerobiosis, is described. Bchl a is the most widely distributed bacteriochlorin pigment, occurring in most photosynthetic bacteria. The early steps of Bchl a synthesis up to chlorophyllide a are common to the biosynthesis pathway of chlorophyll (Chl) a, a pigment present in all organisms capable of oxygenic photosynthesis (Fig. 1). The chelation of Mg in protoporphyrin IX through an enzymatic process catalyzed by the products of the bchD, bchI, and bchH genes to form Mg-protoporphyrin IX (MgP) is the first step specifically committed to Bchl a synthesis. This step is oxygen regulated and constitutes a specific on-off switch for Bchl a synthesis (5). Methylation of MgP to form MgP monomethylester (MgPMe) is catalyzed by the product of bchM. MgPMe is the substrate for the oxidative cyclase responsible for the formation of the isocyclic ring V of protochlorophyllide (Pchlide) a (Fig. 1). To date, only the bchE gene of R. capsulatus has been demonstrated to be involved in this reaction, which requires a cobalamin (vitamin B12) cofactor (10, 16, 38). In plant etioplasts, C. reinhardtii chloroplasts, and Synechocystis sp. strain PCC 6803 extracts, evidence has been obtained for an Fe-, NADPH-, and O2-requiring step during isocyclic ring formation (7, 8, 11, 12, 31). Several studies have proposed that the addition of a hydroxyl during the closure reaction of the Chl isocyclic ring may involve a mixed-function oxidase that utilizes dioxygen as a substrate (11, 34). In purple bacteria, H2O has been shown to be the source of oxygen for the cyclization reaction in R. sphaeroides cells shifted from respiratory to photosynthetic growth conditions (28). In addition, two different cyclization mechanisms have been shown to coexist in Rhodovulum sulfidophilum where both an oxygenase and a hydratase operate to form the 13(1)-oxo group of the Bchl a isocyclic ring (29).
FIG. 1.
Biosynthesis pathway from protoporphyrin IX to chlorophyllide a. Bacterial genes coding for biosynthesis enzyme subunits are indicated by an asterisk. Isocyclic ring formation may proceed via an aerobic and/or an anaerobic mechanism, depending on the organism considered, involving various intermediates not detailed here (for a review, see reference 3).
The study presented here demonstrates that disruption of orf358 in R. gelatinosus causes the blockage of Bchl a synthesis at the stage of MgPMe oxidative cyclization and accumulation of protoporphyrin IX and MgPMe when the cultures are grown under aerobic conditions. The diversity of phenotypes observed under different growth conditions in the orf358 disruption strain supports the coexistence of an anaerobic and an aerobic mechanism for oxidative cyclization in this bacterium. Evidence is given for a crucial role of ORF358 in the aerobic mechanism as a putative binuclear-iron-cluster O2-activating protein, and sequence analysis suggests that this aerobic oxidative cyclization mechanism is conserved from bacteria to plants. According to these results, we propose to rename orf358, designating it acsF, for aerobic cyclase system Fe-containing subunit.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Escherichia coli was grown at 37°C on Luria-Bertani (LB) medium (30). R. gelatinosus strain S1 (32) and SIX9Km were grown at 30°C, either anaerobically in the light in 8-ml filled glass tubes (photosynthetic conditions), in the dark in malate (ML) medium (1) in 50-ml flasks filled with 50 ml of medium (low-oxygenation conditions), or in 250-ml flasks containing 20 ml of medium (high-oxygenation conditions). Shaking was performed at 150 rpm. Antibiotics were used at the following concentrations: chloramphenicol, 10 μg/ml; ampicillin, 100 μg/ml; kanamycin, 10 μg/ml; tetracycline, 10 μg/ml. Bacterial strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| E. coli XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi-1 relA1 lac F′ [proAB+lacIqlacZΔM15 Tn10 (Tcr)] | Stratagene |
| R. gelatinosus | ||
| S1 | Wild type | 32 |
| SIX9Km | Interposon strain (orf227::Km at StuI) | This work |
| SIX9Km+pCm358 | SIX9Km bearing the replicative plasmid pCm358 | This work |
| SIX9Km+pTc | SIX9Km bearing the replicative plasmid pBBR1MCS3 | This work |
| Plasmids | ||
| pUC4K | Plasmid bearing the Km cartridge (Apr Kmr) | Pharmacia |
| pBluescript KS(+) | Cloning vector | Stratagene |
| pBBR1MCS1 | Cloning vector (bom+ Cmr) | 19 |
| pBBR1MCS3 (pTc) | Cloning vector (bom+ Tcr) | 19 |
| pBBR1MCS4 | Cloning vector (bom+ Apr) for R. gelatinosus S1 genomic DNA library | 19 |
| pCm358 | A 1.69 kb HindIII-XmnI fragment from pKS1-21 comprising orf154 and orf358 is inserted at HindIII/SmaI in pBBR1MCS1 | This work |
| pB1-21 | pBBR1MCS4-based vector, isolated from our genomic DNA library, contains a 10-kb insert including crtB | This work |
| pSO67 | pBluescript KS(+) where a 1.34-kb HindIII-BglII fragment containing orf154 and most of orf358 is inserted at HindIII/BamHI | This work |
| pSO67Km | Suicide vector derived from pSO67, where the Km cartridge is inserted at StuI to disrupt orf358 | This work |
Km, kanamycin resistance cartridge; Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; Tcr, tetracycline resistant.
Molecular biology techniques.
Standard methods were used according to the work of Sambrook et al. (30), unless otherwise indicated. Plasmid DNAs were purified using a QIAprep spin miniprep kit (Qiagen) or a Quantum prep plasmid midiprep kit (Bio-Rad). DNA was treated with restriction enzymes and other nucleic acid-modifying enzymes (Klenow fragment, alkaline phosphatase, T4 DNA polymerase, T4 DNA ligase) according to the manufacturer’s specifications. DNA fragments were analyzed on agarose gels, and different restriction fragments were purified using the GeneClean kit (Bio 101).
Genomic DNA library construction.
An R. gelatinosus S1 genomic DNA library was constructed by partial digestion of purified genomic DNA by the Sau3A restriction enzyme. Fragments ranging from 5 to 15 kb were purified and ligated to pBBR1MCS4 (Apr) opened by BamHI. E. coli XL1-Blue cells were transformed with the ligation product and plated on LB medium supplemented with ampicillin and containing isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). A total of 3,400 white clones were cultured independently and pooled (20 clones to a pool) to form a library of 170 pools numbered from 1 to 170. Those pools were then grouped by 10’s to form 17 pools named A to Q (A comprises pools 1 to 10, and so forth; Q comprises pools 161 to 170).
Library screening for orf358.
A 1-μg plasmid preparation from each library pool was digested by HindIII, electrophoresed on a 1% agarose Tris-borate-EDTA gel, and transferred to a Hybond N+ membrane, as indicated by Amersham. A crtB probe labeled with [α-32P]dCTP by nick translation was used to perform Southern hybridization. Cells from pools hybridizing the crtB probe were diluted and plated to obtain isolated colonies for PCR screening using the oligonucleotides 5′-CGACCATCAGGCGTTCCG and 5′-GCACCAGCGCGCCGAAG, which amplify a 377-bp fragment located in the 3′ end of orf276. PCRs were carried out using Taq DNA polymerase from Appligene according to the manufacturer’s instructions, excepted that dimethyl sulfoxide was added to a 10% final concentration. Hybridization was carried out at 65°C.
Gene transfer in R. gelatinosus.
Plasmid DNA was introduced in R. gelatinosus cells by electroporation as previously described (25). Electroporated cells were diluted in 10 ml of ML medium and incubated overnight in darkness at 30°C. Serial dilutions were plated on nonselective ML plates to assess cell survival and on selective ML plates to obtain transformants. For gene disruption, two different antibiotic resistance markers were used to distinguish between double- and single-crossover events, namely, the ampicillin resistance gene of pBluescript KS(+) and the kanamycin resistance borne by the cartridge inserted in the gene to be disrupted.
Preparation of pigment extracts.
Cells were harvested in the late-exponential-growth phase, when the optical density of the culture reached 0.8 at 680 nm. Pigments were extracted from cell pellets by adding 20 volumes of ice-cold acetone-methanol (7:2, vol/vol), resuspending, and eliminating cell debris by centrifugation. Further extractions were carried out until the cell debris pellet was completely depigmented. The solvent and residual water were then totally evaporated. The dried pigments were resolubilized in a small volume of acetone-methanol (7:2, vol/vol) and concentrated by evaporation.
Spectroscopic analyses.
Absorption spectroscopy was performed with a CARY 500 spectrophotometer on cells resuspended in 60% sucrose, pigment extracts, or high-performance liquid chromatography (HPLC) fractions. Fluorescence emission spectra were recorded on a Fluorescence Spectrometer LS50B (Perkin-Elmer) on HPLC fractions.
HPLC analysis.
Twenty microliters of a pigment preparation or a standard Mg-protoporphyrin IX solution was injected on a 5-μm Kromasil C18 column (inner diameter, 4.6 mm; length, 250 mm) and eluted at a 1-ml/min solvent flow rate, according to a previously described method (14). The HPLC solvent was acetonitrile-methanol-dichloromethane (75:15:15, vol/vol/vol). Detection was carried out at 436 nm, and collected fractions were further analyzed by absorption and/or fluorescence emission measurements.
Nucleotide sequence accession number.
The sequence of orf358 from R. gelatinosus strain S1 has been deposited in GenBank under accession number AY057871.
RESULTS
Cloning and sequence analysis of the orf358 genomic region in R. gelatinosus.
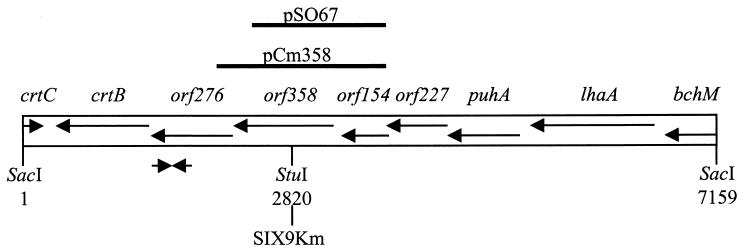
A previous study on R. gelatinosus strain IL-144 reported orf358 to be a few kilobases away from the carotenoid biosynthesis crtB gene (17). Based on this information and on sequences previously obtained in our laboratory in the crtB region (26), a screen was performed on the genomic DNA library of R. gelatinosus strain S1 constructed in our laboratory. This led to the identification of pB1-21 (Table 1). Sequence analysis of a 7.15-kb SacI fragment from pB1-21 revealed the presence of genes whose function had been previously identified and of several putative ORFs for which homologs were found in other organisms, in agreement with the results obtained for R. gelatinosus strain IL-144 (2, 9, 17, 36, 37, 38). Following the putative transcriptional orientation of this region, these genes and ORFs are, in order, the 3′ end of the Mg-protoprophyrin IX methyltransferase bchM gene; the lhaA gene (previously known as F1696), whose protein product plays a role in the assembly of light harvesting complex I; the structural gene puhA, encoding the reaction center H subunit; orf227; orf154; orf358; orf276; the phytoene synthase gene crtB; and finally, in the opposite direction, the 3′ end of the hydratase-encoding crtC gene (Fig. 2).
FIG. 2.
Physical map of the 7.2-kb orf358-containing SacI fragment of R. gelatinosus. Genes and ORFs are represented by arrows and named above in italics. Solid lines above the map represent inserts used to construct pSO67 and pCm358. Converging arrows represent the oligonucleotides used in the PCR screen. The restriction enzyme site used to insert the Km cartridge into pSO67 to construct SIX9Km is indicated below.
Construction of the orf358 disruption strain SIX9Km.
In order to study the physiological role of orf358, a disrupted strain was constructed by insertional mutagenesis using the kanamycin resistance (Km) cartridge, which exerts no polar effect on the transcription of genes located downstream of its insertion site. A 1.34-kb HindIII-BglII fragment containing orf154 and most of orf358 was obtained from pB1-21 and cloned into pBluescript KS(+) to construct pSO67 (Table 1). Insertion of the Km cartridge at the StuI site of orf358 in pSO67 led to the suicide vector pSO67Km. Wild-type (WT) cells electroporated with pSO67Km were plated on selective medium to select transformants resulting from double-crossover events. The resulting disrupted strain was called SIX9Km (Fig. 2). SIX9Km was able to grow in liquid ML medium with kanamycin under photosynthetic or respiratory conditions, indicating that disruption of orf358 is not lethal for R. gelatinosus.
Investigation of SIX9Km photosynthetic and respiratory phenotypes.
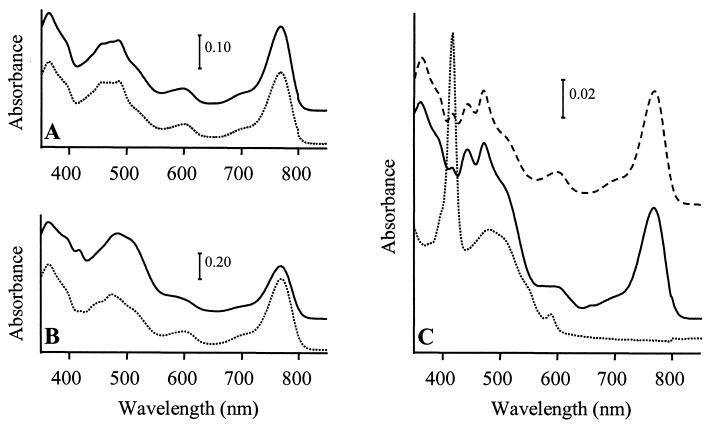
The growth properties of SIX9Km and its ability to assemble spectral complexes and synthesize pigments were investigated in comparison to the WT under various growth conditions (see Materials and Methods). Under photosynthesis, both strains had a generation time of 3 h. They assembled the same amount of photosynthetic complexes under photosynthetic and low-oxygenation conditions, but SIX9Km assembled no complexes under high-oxygenation conditions while the WT still had small amounts (data not shown). Analysis of acetone-methanol pigment extracts from the WT and SIX9Km showed identical spectra for the two strains under photosynthetic or low-oxygenation conditions, with absorption at 770 nm for Bchl a, 420 to 530 nm for carotenoids, and 365 nm for the Bchl a Soret absorption band (Fig. 3A and B). Under high oxygenation, the WT spectrum still presented the same characteristics, although the total amounts of pigments were decreased due to culture conditions. In contrast, no Bchl a could be detected in highly oxygenated SIX9Km, while a peak appeared at 416 nm (Fig. 3C). Addition of 5 to 15 μg of vitamin B12 (cyanocobalamin)/ml to the medium during SIX9Km growth did not modify its phenotype. Northern blot hybridization was performed on total RNAs from highly oxygenated WT and SIX9Km to determine the level of expression of the puf operon, which codes for the LHI and three of the reaction center apoproteins. Similar levels of pufBA and pufBALMC transcripts were found in the two strains (data not shown). Thus, the lack of photosynthetic complex formation in SIX9Km was not attributed to a decreased expression of puf in comparison to the WT level, but rather to a pleiotropic effect of the lack of Bchl a on photosynthetic-complex assembly.
FIG. 3.
Absorption spectra of pigment extracts from R. gelatinosus cultures grown under photosynthetic conditions (A), darkness and low oxygenation (B), or darkness and high oxygenation (C) and harvested when cultures reached an optical density of 0.8 at 680 nm. Solid lines, WT; dotted lines, SIX9Km; dashed lines, SIX9Km/pCm358. Zero levels were shifted for better viewing.
Functional complementation of SIX9Km respiratory phenotype.
pCm358, a chloramphenicol-resistant replicative plasmid allowing expression of a functional orf358 (Table 1), was introduced in SIX9Km by electroporation. The resulting SIX9Km/pCm358 strain assembled spectral complexes and was able to synthesize Bchl a under high oxygenation, as shown by its pigment absorption spectrum, which was similar to that of the WT (Fig. 3C). When pCm358 was cured of SIX9Km/pCm358 by transformation with the tetracycline-resistant plasmid pBBR1MCS3 (pTc), the resulting SIX9Km/pTc strain exhibited a phenotype identical to that of SIX9Km. These experiments confirmed that the Bchl-less phenotype of SIX9Km grown under high oxygenation was strictly due to the loss of orf358 function.
HPLC analysis of pigments from highly oxygenated WT and SIX9Km.
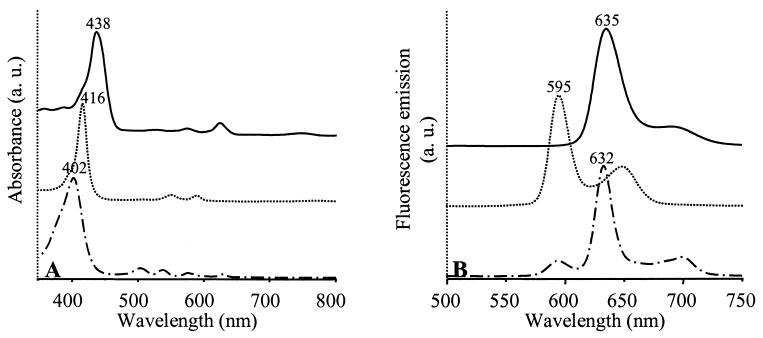
The pigment contents of the WT and SIX9Km grown under high oxygenation were analyzed by HPLC (Table 2). Isolated carotenoid fractions were identified on the basis of their retention times and UV/visual (UV/Vis) absorption spectra. Bchl a precursors eluted earlier than carotenoids. Two fractions were isolated in SIX9Km and another was isolated in the WT; these were analyzed by UV/Vis absorbance (Fig. 4A) and fluorescence emission (Fig. 4B). In the WT, the Bchl a precursor isolated in fraction 2 was identified as protochlorophyllide a. In SIX9Km, fraction 1 was identified as Mg-protoporphyrin or its monomethylester (MgPMe), since esterification does not modify the spectral properties of MgP, and fraction 3 was identified as protoporphyrin IX.
TABLE 2.
HPLC analysis of pigments extracted from R. gelatinosus WT or SIX9Km culturesa
| Fraction no. | RTb (min) | λmax in HPLC solventc | Compound identificationd | Detectione in:
|
|
|---|---|---|---|---|---|
| WT | SIX9Km | ||||
| 1 | 2.7 | 416, 552, 590 | MgPMe | − | + |
| 2 | 3.0 | 438, 576, 626 | Protochlorophyllide a | + | − |
| 3 | 4.2 | 402, 504, 538, 574, 630 | Protoporphyrin IX | − | + |
| 4 | 5.5 | 530 (560) | Diketospirilloxanthin | + | + |
| 5 | 6 | 482 (508) | OH-spheroidenone | + | + |
| 6 | 10.8 | 770 | Bchl a | + | − |
| 7 | 11.2 | 418, 442, 472 | OH-neurosporene | + | − |
| 8 | 12.3 | 482 (508) | Spheroidenone | + | + |
| 9 | 15.0 | 430, 458, 488 | Spheroidene | − | ± |
| 10 | 22.0 | 418, 442, 472 | Neurosporene | ± | ± |
Cultures were grown under high oxygenation in the dark. The HPLC solvent was acetonitrile-methanol-dichloromethane (75:15:15, vol/vol/vol).
RT, retention time.
Determined in the UV/Vis range. Values in parentheses correspond to shoulders on the spectra.
+, detected; ±, detected in trace amounts; −, not detected.
FIG. 4.
Spectroscopic analysis of Bchl a precursors purified by HPLC from WT or SIX9Km cultures grown under high oxygenation. (A) UV/Vis absorption spectra; (B) fluorescence emission spectra. The absorbance λmax were used as excitation wavelengths for fluorescence measurements. Solid lines, WT fraction 2; dotted and dashed lines, SIX9Km fractions 1 and 3, respectively. The solvent is acetonitrile-methanol-dichloromethane (75:15/:5, vol/vol/vol). Fraction numbers refer to Table 2. Zero levels were shifted for better viewing.
Determination of the esterification level of fraction 1 MgP.
The nature of fraction 1 was determined by HPLC by comparison to a solution of standard MgP (Fig. 5). Retention times were 1.7 min for MgP and 2.7 min for fraction 1, indicating that fraction 1 was less polar than MgP. Fraction 1 was thus proposed to be MgPMe. These results led to the conclusion that disruption of orf358 causes a blockage in Bchl a synthesis at the MgPMe oxidative cyclization step (Fig. 1) when the disrupted strain SIX9Km is grown in the dark under high oxygenation, suggesting that orf358 codes for an enzymatic subunit involved in an aerobic cyclization mechanism.
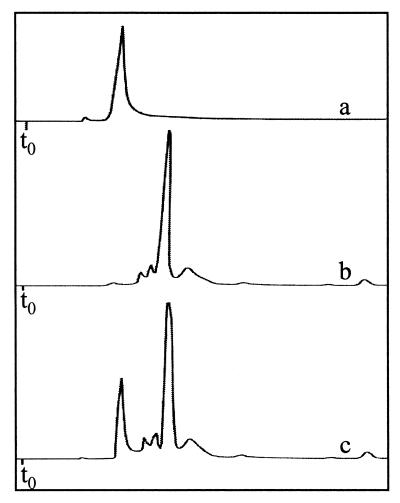
FIG. 5.
Comparison of the HPLC retention time of a standard solution of MgP (trace a) to that of the SIX9Km fraction 1 compound (trace b). Trace c shows the coinjection of the two samples. The HPLC solvent is acetonitrile-methanol-dichloromethane (75:15:15, vol/vol/vol).
DISCUSSION
This study described the disruption of orf358, an ORF of previously unidentified function, in the purple bacterium R. gelatinosus. The phenotype of the disrupted strain SIX9Km was characterized under different trophic conditions. The SIX9Km phenotype was similar to that of the WT under photosynthetic and low-oxygenation conditions. In contrast, when the strains were grown under high oxygenation, the WT still synthesized pigments and assembled photosynthetic complexes while SIX9Km no longer synthesized Bchl a and therefore could not assemble photosynthetic complexes. A compound identified as MgPMe accumulated in SIX9Km under high oxygenation, indicating that the conversion of MgPMe to Pchlide a was blocked. These experiments demonstrated that orf358 function is necessary to the cyclization of MgPMe to form Pchlide a under high-oxygenation conditions but not under low-oxygenation or photosynthetic conditions. This implies that at least two different cyclization mechanisms exist in R. gelatinosus, an aerobic mechanism involving ORF358 and a second, anaerobic mechanism, active under low oxygenation and photosynthesis. These results are in agreement with studies that demonstrated the existence of an anaerobic cyclization process in R. sphaeroides cultures shifted from respiratory to photosynthetic growth conditions, where the oxygen atoms incorporated in Bchl a evolved from water, as well as in R. sulfidophilum, where both an oxygenase and a hydratase operated to form the 13(1)-oxo group of the Bchl a isocyclic ring (28, 29). In our experiments, the anaerobic cyclization mechanism was unable to compensate for the loss of the aerobic mechanism in SIX9Km under high-oxygenation conditions. To date, only the bchE gene of R. capsulatus had been demonstrated to be involved in the oxidative cyclization reaction (10). Cloning and disrupting bchE in R. gelatinosus will be particularly interesting, in order to determine if BchE is involved in the aerobic as well as the anaerobic mechanism and to study its expression pattern in R. gelatinosus in relation to growth conditions, since repression of bchE expression under high-oxygenation conditions might explain why the aerobic mechanism is the only one to operate in this case.
Previous sequence analysis of some ORF358 homologs revealed the presence of a leucine zipper and of two copies of a primary sequence motif, (D/E)ExxH (24, 37). This latter motif is characteristic of monooxygenases, a class of metalloproteins including, for instance, the enzymes methane monooxygenase and ribonucleotide reductase, where two copies of the motif (D/E)x(28–37)DExRH provide all of the protein-derived ligands necessary to bind a binuclear-iron cluster involved in their dioxygen activation function (35). These multimeric enzymes incorporate one oxygen in the substrate and reduce the other to H2O in the presence of a reducing agent (NADPH or NADH). Careful analysis of the ORF358 putative protein sequence aligned with nine of its homologs shows that two complete Ex(29–35)DExRH motifs are present (Fig. 6). The existence of this putative binuclear-iron cluster in ORF358 was correlated to various studies which suggested the involvement of iron in the enzymatic transformation of the side chain of MgPMe (8, 11, 12, 31). In R. sphaeroides, a decrease in Bchl a synthesis accompanied by increased production of MgPMe was observed in cells cultured in low-iron media (18). Metal chelators inhibited Pchlide a formation in cucumber etioplasts, C. reinhardtii chloroplasts, and Synechocystis sp. strain PCC 6803 extracts, and this inhibition could be reversed by the addition of Fe2+ (8, 11). It was proposed that iron would be needed for the synthesis of an Fe-containing protein, the role of which might be to hydroxylate the β-carbon of the 6-methylpropionate side chain. The use of inhibitors showed that this putative iron-containing enzyme did not belong to the class of iron-sulfur cluster or heme-containing proteins (12, 33). Conversion of MgPMe to Pchlide a in plants was also shown to be stimulated by NADPH and dependent on O2 (12).
FIG. 6.
Comparison of the acsF (orf358) putative protein sequence to various homologs. The alignment was generated using ClustalW (http://www2.ebi.ac.uk/clustalw). N- and C-terminal parts of the proteins are not shown. R.g., Rubrivivax gelatinosus (accession number AY057871); Rba. sph., Rhodobacter sphaeroides (AF195122); Synecho., Synechocystis sp. strain PCC 6803 (D90899, D90912); Cyanidium, Cyanidium caldarium (AF022186); Chlamy., Chlamydomonas reinhardtii (AF226628); Porphyra, Porphyra purpurea (PPU38804). O. sativa, Oryza sativa (AP000815); P. nil, Pharbitis nil (INU37437); A. thal, Arabidopsis thaliana (AL138655). The conserved amino acids constituting the putative iron-binding Ex(29–35)DExRH motifs are boldfaced.
A seductive possibility is that ORF358 and its homologs form a conserved family of putative binuclear-iron-cluster-containing oxygenase subunits responsible for the introduction of an oxygen atom during the closure reaction of the isocyclic ring in Bchl and Chl. According to this, we suggest replacing the term orf358 with a new gene name corresponding to its proposed function, acsF, standing for aerobic cyclase system Fe-containing subunit. In addition, AcsF may be involved in a regulatory pathway, as suggested by experiments which demonstrated the light-regulated expression of PNZIP mRNA in P. nil and the fact that MgP and MgPMe act as regulatory factors of chloroplast origin in C. reinhardtii (20, 21, 37). Taken together, these experiments suggest that control of AcsF expression by light, or perhaps by oxygen in other organisms, would influence the efficiency of the oxidative cyclization reaction and control the levels of the precursors MgP and MgPMe and thus of their regulatory activities. If such a pathway could be demonstrated in R. gelatinosus, it would be interesting to determine the nature of the regulatory signal (light or oxygen) and of the target genes.
In conclusion, this study made it possible to identify the first component of the putative aerobic oxidative cyclase in R. gelatinosus, AcsF. The role of the glutamate and histidine residues predicted to act as a ligand for iron in the Ex(29–35)DExRH motifs in AcsF will have to be confirmed by directed mutagenesis, and the actual presence of iron in this protein should be demonstrated by use of purified preparations. The conservation of the AcsF-family of oxygenases among photosynthetic organisms suggests also that this aerobic oxidative cyclization mechanism is conserved from bacteria to plants. Further biochemical studies on R. gelatinosus AcsF should indicate if this protein is membrane bound or soluble and should help to identify other polypeptide components possibly involved in this reaction.
ADDENDUM
The coinjection of SIX9Km fraction 1 with standard MgPMe (kindly provided by D. Bollivar, Illinois Wesleyan University) on our HPLC system showed that the two compounds coeluted, confirming our results.
Acknowledgments
We are grateful to Wolfhart Rüdiger and Ulrike Oster of the Botanisches Institut—Universität München (Munich, Germany) for kindly providing us with MgP. We also thank Linda Sperling and Sylviane Liotenberg for critical reading of the manuscript.
REFERENCES
- 1.Agalidis, I., E. Rivas, and F. Reiss-Husson. 1990. Reaction center-light harvesting B875 complex from Rhodocyclus gelatinosus: characterization and identification of quinones. Photosynth. Res. 23:249–255. [DOI] [PubMed] [Google Scholar]
- 2.Aklujkar, M., A. L. Harmer, R. C. Prince, and J. T. Beatty. 2000. The orf162b sequence of Rhodobacter capsulatus encodes a protein required for optimal levels of photosynthetic pigment-protein complexes. J. Bacteriol. 182:5440–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beale, S. I. 1999. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 60:43–73. [Google Scholar]
- 4.Belanger, F. C., and C. A. Rebeiz. 1980. Chloroplast biogenesis. Detection of divinyl protochlorophyllide in higher plants. J. Biol. Chem. 255:1266–1272. [PubMed] [Google Scholar]
- 5.Biel, A. J. 1992. Oxygen-regulated steps in the Rhodobacter capsulatus tetrapyrrole biosynthetic pathway. J. Bacteriol. 174:5272–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biel, A. J., and B. L. Marrs. 1983. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodobacter capsulatus in response to oxygen. J. Bacteriol. 156:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollivar, D. W., and S. I. Beale. 1995. Formation of the isocyclic ring of chlorophyll by isolated Chlamydomonas reinhardtii chloroplasts. Photosynth. Res. 43:113–124. [DOI] [PubMed] [Google Scholar]
- 8.Bollivar, D. W., and S. I. Beale. 1996. The chlorophyll biosynthetic enzyme Mg-protoporphyrin IX monomethyl ester (oxidative) cyclase. Characterization and partial purification from Chlamydomonas reinhardtii and Synechocystis sp. PCC 6803. Plant Physiol. 112:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollivar, D. W., Z. Y. Jiang, C. E. Bauer, and S. I. Beale. 1994. Heterologous expression of the bchM gene product from Rhodobacter capsulatus and demonstration that it encodes S-adenosyl-l-methionine:Mg-protoporphyrin IX methyltransferase. J. Bacteriol. 176:5290–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollivar, D. W., J. Y. Suzuki, J. T. Beatty, J. M. Dobrovolsky, and C. E. Bauer. 1994. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J. Mol. Biol. 237:622–640. [DOI] [PubMed] [Google Scholar]
- 11.Chereskin, B. M., and P. A. Castelfranco. 1982. Effects of iron and oxygen on chlorophyll biosynthesis. II. Observations on the biosynthetic pathway in isolated etiochloroplasts. Plant Physiol. 68:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chereskin, B. M., Y.-S. Wong, and P. A. Castelfranco. 1982. In vitro synthesis of the chlorophyll isocyclic ring. Plant Physiol. 70:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogdell, R. J., N. W. Isaacs, T. D. Howard, K. McLuskey, N. J. Fraser, and S. M. Prince. 1999. How photosynthetic bacteria harvest solar energy. J. Bacteriol. 181:3869–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Leenhers, A. P., and H. J. Nelis. 1992. Profiling and quantitation of carotenoids by high-performance liquid chromatography and photodiode array detection. Methods Enzymol. 213:251–265. [Google Scholar]
- 15.Drews, G., and J. R. Golecki. 1995. Structure, molecular organization, and biosynthesis of membranes of purple bacteria, p.231–257. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 16.Gough, S. P., B. O. Petersen, and J. O. Duus. 2000. Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor. Proc. Natl. Acad. Sci. USA 97:6908–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi, N., J. Harada, S. Nagashima, K. Matsuura, K. Shimada, and K. V. P. Nagashima. 2001. Horizontal transfer of photosynthesis gene cluster and operon re-arrangement in purple bacteria. J. Mol. Evol. 52:333–341. [DOI] [PubMed] [Google Scholar]
- 18.Jones, O. T. G. 1963. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas sphaeroides. Biochem. J. 86:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800–802. [PubMed] [Google Scholar]
- 20.Kropat, J., U. Olster, W. Rüdiger, and C. F. Beck. 2000. Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 24:523–531. [DOI] [PubMed] [Google Scholar]
- 21.Kropat, J., U. Oster, W. Rüdiger, and C. F. Beck. 1997. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl. Acad. Sci. USA 94:14168–14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkum, A. W. D. 1991. The evolution of chlorophylls, p.367–383. In H. Scheer (ed.), Chlorophylls. CRC Press, Inc., Boca Raton, Fla.
- 23.Lockhart, P. J., M. A. Steel, and A. W. D. Larkum. 1996. Gene duplication and the evolution of photosynthetic reaction center proteins. FEBS Lett. 385:193–196. [DOI] [PubMed] [Google Scholar]
- 24.Moseley, J., J. Quinn, M. Eriksson, and S. Merchant. 2000. The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J. 19:2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouchane, S., M. Picaud, F. Reiss-Husson, C. Vernotte, and C. Astier. 1996. Development of genetic transfers for Rubrivivax gelatinosus S1. Construction, characterization and complementation of a puf operon deletion strain. Mol. Gen. Genet. 252:379–385. [DOI] [PubMed] [Google Scholar]
- 26.Ouchane, S., M. Picaud, C. Vernotte, and C. Astier. 1997. Photooxidative stress stimulates illegitimate recombination and mutability in carotenoid-less mutants of Rubrivivax gelatinosus. EMBO J. 16:4777–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouchane, S., M. Picaud, C. Vernotte, F. Reiss-Husson, and C. Astier. 1997. Pleiotropic effects of puf interposon mutagenesis on carotenoid biosynthesis in Rubrivivax gelatinosus. A new gene organization in purple bacteria. J. Biol. Chem. 272:1670–1676. [DOI] [PubMed] [Google Scholar]
- 28.Porra, R. J., W. Schäfer, I. Katheder, and H. Scheer. 1995. The derivation of the oxygen atoms of the 131-oxo and 3-acetyl groups of bacteriochlorophyll a from water in Rhodobacter sphaeroides cells adapting from respiratory to photosynthetic conditions: evidence for an anaerobic pathway for the formation of isocyclic ring E. FEBS Lett. 371:21–24. [DOI] [PubMed] [Google Scholar]
- 29.Porra, R. J., M. Urzinger, J. Winkler, C. Bubenzer, and H. Scheer. 1998. Biosynthesis of the 3-acetyl and 13(1)-oxo groups of bacteriochlorophyll a in the facultative aerobic bacterium, Rhodovulum sulfidophilum—the presence of both oxygenase and hydratase pathways for isocyclic ring formation. Eur. J. Biochem. 257:185–191. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Spiller, S. C., A. M. Castelfranco, and P. A. Castelfranco. 1982. Effects of iron and oxygen on chlorophyll biosynthesis. I. In vivo observations on iron- and oxygen-deficient plants. Plant Physiol. 69:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uffen, R. L. 1976. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc. Natl. Acad. Sci. USA 73:3298–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker, C. J., K. E. Mansfield, I. N. Rezzano, C. M. Hanamoto, K. M. Smith, and P. A. Castelfranco. 1988. The magnesium-protoporphyrin IX (oxidative) cyclase subunit. Biochem. J. 255:685–692. [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, C. J., K. E. Mansfield, K. M. Smith, and P. A. Castelfranco. 1989. Incorporation of atmospheric oxygen into the carbonyl functionality of the protochlorophyllide isocyclic ring. Biochem. J. 257:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallar, B. J., and J. D. Lipscomb. 1996. Dioxygen activation by enzymes containing binuclear non-heme iron clusters. Chem. Rev. 96:2625–2657. [DOI] [PubMed] [Google Scholar]
- 36.Youvan, D. C., E. J. Bylina, M. Alberti, H. Begusch, and J. E. Hearst. 1984. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell 37:949–957. [DOI] [PubMed] [Google Scholar]
- 37.Zheng, C. C., R. Porat, P. Lu, and S. D. O’Neill. 1998. PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and a circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol. 116:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zsebo, K. M., and J. E. Hearst. 1984. Genetic-physical mapping of a photosynthetic gene cluster from Rhodopseudomonas capsulata. Cell 37:937–947. [DOI] [PubMed] [Google Scholar]