Abstract
The AraC homolog ToxT coordinately regulates virulence gene expression in Vibrio cholerae. ToxT is required for transcriptional activation of the genes encoding cholera toxin and the toxin coregulated pilus, among others. In this work we focused on the interaction of ToxT with the tcpA promoter and investigated the mechanism of ToxT-dependent transcriptional activation at tcpA. Deletion analysis showed that a region from −95 to +2 was sufficient for ToxT binding and activation, both of which were simultaneously lost when the deletion was extended to −63. A collection of point mutations generated by error-prone PCR revealed two small regions required for ToxT-dependent transactivation. Binding studies performed with representative mutations showed that the two regions define sites at which ToxT binds to the tcpA promoter region, most likely as a dimer. Results obtained by using a rpoA truncation mutation showed that ToxT-dependent activation at tcpA involves the C-terminal domain of the RNA polymerase alpha subunit. A model of ToxT-dependent transcriptional activation at tcpA is proposed, in which ToxT interacts with two A-rich regions of tcpA centered at −72 and −51 and requires the alpha C-terminal domain of RNA polymerase.
Vibrio cholerae is the etiological agent of the life-threatening diarrheal disease cholera. This bacterium inhabits an aquatic environmental niche when it is not infecting a human host and is acquired through ingestion of contaminated food or drinking water. V. cholerae colonizes the upper intestine of humans via the toxin coregulated pilus (TCP) (25, 63), one of the organism's two main virulence factors. The second major virulence factor is cholera toxin (CT), which is responsible for the efflux of fluids into the intestine that results in diarrhea (33). Expression of both TCP and CT is regulated by various environmental factors, such as temperature, pH, osmolarity, bile, CO2, and amino acids (reviewed in references 9 and 60). This regulation by external stimuli ensures that TCP and toxin are expressed only when they are needed (i.e., in the host). The genes required for TCP biogenesis are located on the Vibrio pathogenicity island (15, 34). The CT subunits are encoded on a separate genetic element, the lysogenic CTXφ bacteriophage (68). These two elements are thought to have been acquired separately through horizontal gene transfer, and it has been shown that CTXφ uses TCP as its receptor (68).
Regulation of genes encoding TCP and CT is controlled at the level of transcription by a regulatory cascade referred to as the ToxR virulence regulon (9, 12, 60). ToxR is a transmembrane protein with homology to the OmpR family of DNA binding proteins (44) that, along with its partner ToxS, is encoded within the ancestral V. cholerae genome. ToxR directly binds to DNA (44), while ToxS enhances the ability of ToxR to form a transcriptionally active complex (11, 41, 52). The toxRS operon is expressed constitutively under most growth conditions. ToxR and ToxS have other roles in V. cholerae besides virulence gene regulation (10, 43). One example is regulation of the relative abundance of the porins OmpU and OmpT. Transcription of ompU is positively regulated by ToxR, while ompT is repressed by ToxR. This ToxR-dependent porin modulation has recently been shown to have an important role in pathogenesis (54). When ompT is expressed in a ToxR+ strain in place of ompU, the levels of ctx and tcp transcription decrease and colonization is reduced. It has been proposed that the importance of porin regulation led to the recruitment of ToxR as a regulator of the acquired pathogenicity island virulence genes, such as tcp and ctx (54).
A second pair of membrane-bound proteins, TcpP and TcpH, have homology to ToxR and ToxS, respectively, and are thought to function in a similar manner (22). TcpP and TcpH are encoded on the TCP-ACF pathogenicity island, and their expression is influenced by factors such as temperature and pH in a manner that parallels what happens in tcp and ctx gene expression (6). Transcription of the tcpPH operon is dependent on two regulators which are encoded on the ancestral genome, AphA and the LysR homolog AphB (35, 62).
ToxRS and TcpPH function cooperatively to activate expression of another TCP-ACF-encoded activator, ToxT (12, 22). ToxT is required for activation of the tcp and ctx operons (3, 7), as well as other genes in the virulence regulon, such as tcpI, acfA, tagA, and tagD (12; Taylor, unpublished data). ToxT also regulates its own expression through an autoregulatory loop (3, 70). ToxT therefore functions as a coordinate regulator of virulence gene expression in V. cholerae by activating genes encoded on both the TCP-ACF element and the CTX element in response to environmental cues. In addition to the series of activators involved in this regulatory cascade, the global regulators cyclic AMP receptor protein and H-NS have a negative influence on virulence gene expression in V. cholerae (49, 61). Both of these regulators influence expression of a number of genes in the regulon. H-NS has been shown to function directly at the ctx, toxT, and tcpA promoters (49). ToxT overrides the negative effects of these regulators directly at the tcpA and ctx promoters (49).
ToxT is a member of the AraC/XylS family of transcriptional regulators (26, 50), a family made up almost exclusively of activator proteins. AraC/XylS family members are characterized by a conserved stretch of amino acids at the C terminus, which constitutes the DNA binding domain (reviewed in references 18 and 38). This region of homology contains two helix-turn-helix (HTH) motifs, and crystal structures of AraC family members have shown that these HTH motifs interact with the DNA helix in the major groove (36, 55). Many AraC family members have binding sites that are adjacent to or overlap the −35 region, and interactions between AraC family members and RNA polymerase have been suggested (1, 18, 27, 28, 30, 31, 38). The N terminus of AraC family members is not conserved and has been shown for some family members to be involved in dimerization and binding of small effector molecules (18, 38).
Proteins in the AraC/XylS family fall into three main groups depending on the types of genes that they regulate. Family members that regulate carbon metabolism, such as AraC of Escherichia coli, are known to respond to small effector molecules that bind to the N-terminal domain of the protein. In the case of AraC, the effector molecule is arabinose. The members of a second group of the AraC family regulate genes involved in stress response. These proteins include SoxS, Rob, and MarA, and they have been shown to function as monomers (38). A third group, including ToxT, Rns of enterotoxigenic E. coli (45), BfpT (PerA) of enteropathogenic E. coli (66), and ExsA of Pseudomonas aeruginosa (29), regulate virulence gene expression. These virulence regulators may respond to physical cues, such as temperature and pH; only one virulence regulator of the AraC/XylS family, UreR, has been shown to directly respond to an effector molecule, urea (65). Recently, the effect of bile on regulation of virulence genes in V. cholerae has been investigated (20, 57). The addition of bile to growth media repressed transcription of tcp and ctx but, paradoxically, induced transcription of toxT. This suggests that bile may directly influence the transcriptional activity of the ToxT protein itself, perhaps by binding to the N-terminal domain (57).
Several promoters in V. cholerae that are regulated by ToxT have been characterized, and there is no obvious consensus sequence that can be proposed as the ToxT binding site. Many AraC/XylS family members recognize asymmetric sequences (18, 45), which makes it difficult to predict the binding sites of these proteins. We have focused most of our work in this regard on defining the cis-active requirements for ToxT interaction at the tcp operon promoter and investigating the mechanism of ToxT-dependent transcriptional activation at tcpA. Using a promoter deletion analysis along with PCR-based mutagenesis of the promoter region, we found that ToxT interacts with two A-rich regions centered at −72 and −51. The mutations that prevent ToxT-mediated activation of tcpA expression are clustered within these two regions, which we have designated site I (−72) and site II (−51). Using gel shift analysis, we determined that a His-tagged version of ToxT binds to a probe that includes both site I and site II. ToxT does not detectably bind to either site I or site II alone. ToxT also does not detectably bind to probes carrying various point mutations in site I, but it does bind weakly to a probe carrying a point mutation in site II. The region proposed for ToxT binding at the tcp operon promoter does not contain any obvious dyad symmetrical sequences. Upon binding to these sites ToxT appears to activate transcription of the tcp operon via a mechanism that involves the alpha C-terminal domain (αCTD) of RNA polymerase.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics.
The strains and plasmids used in this study are listed in Table 1. V. cholerae and E. coli strains were maintained at −70°C in Luria-Burtani (LB) medium (40) containing 30% (vol/vol) glycerol. E. coli was grown in LB medium with a starting pH of either 7.0 or 6.5 at 37 or 30°C. V. cholerae was grown at 30°C in LB medium with a starting pH of 6.5. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml; and streptomycin, 100 μg/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was added to LB agar at a concentration of 40 μg/ml. Arabinose was added to the growth media at a final concentration of 0.02%.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| E. coli strains | ||
| CC118 | araD139 Δ(ara leu)7697 ΔlacX74 ΔphoA20 galE galK thi rpsE rpoB argE(Am) recA1 | 37 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | 58 |
| SM10λpir | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu (λpir) | 43 |
| AA60 | CC118, pTSS-5 | This study |
| AJA1 | CC118, pAJA1 | This study |
| AJA2 | CC118, pAJA2 | This study |
| AJA3 | CC118, pAJA3 | This study |
| RRH26 | CC118, pRH8 | This study |
| RRH72 | CC118, pRH72 | This study |
| RRH81 | CC118, pRH81 | This study |
| RRH82 | CC118, pRH82 | This study |
| RRH98 | CC118, pRH98 | This study |
| RRH100 | MC4100 (λtcpA-lacZYA) (−458/+2) | This study |
| RRH102 | MC4100 (λtcpA-lacZYA) (−162/+2), T to A at −38 | This study |
| RRH103 | MC4100 (λtcpA-lacZYA) (−162/+2), A to T at −50 | This study |
| RRH113 | MC4100 (λtcpA-lacZYA) (−95/+2) | This study |
| RRH114 | MC4100 (λtcpA-lacZYA) (−63/+72) | This study |
| RRH115 | MC4100 (λtcpA-lacZYA) (−162/+2) | This study |
| RRH131 | MC4100 (λtcpA-lacZYA) (−118/+2) | This study |
| V. cholerae strains | ||
| O395 Sm | Classical Ogawa, Smr | 63 |
| CG842 | O395 Sm ΔlacZ | 8 |
| MBN135 | CG842 tcpA-lacZ | M. Nye |
| MBN142 | MBN135 ΔtoxT | M. Nye |
| MBN168 | MBN135 ΔtoxT Δhns | M. Nye |
| RRH175 | MBN135 (pRH170) | This study |
| RRH176 | MBN135 (pRH171) | This study |
| RRH178 | MBN142 (pRH170) | This study |
| RRH179 | MBN142 (pRH171) | This study |
| RRH181 | MBN168 (pRH170) | This study |
| RRH182 | MBN168 (pRH171) | This study |
| MBN032 | CG842 toxT-lacZ | 49 |
| RRH197 | MBN032 (pRH170) | This study |
| RRH201 | MBN032 (pRH171) | This study |
| Plasmids | ||
| pTSS-5 | pACYC184 toxT+, Cmr | 3 |
| pRS415 | lacZYA transcriptional fusion vector, Apr | 59 |
| pAJA1 | pRS415::(tcpA-lacZYA) (−118/+2) | This study |
| pAJA2 | pRS415::(tcpA-lacZYA) (−162/+2) | This study |
| pAJA3 | pRS415::(tcpA-lacZYA) (−458/+2) | This study |
| pRH8 | pRS415::(tcpA-lacZYA) (−95/+2) | This study |
| pRH72 | pRS415::(tcpA-lacZYA) (−230/+2) | This study |
| pRH82 | pRS415::(tcpA-lacZYA) (−310/+2) | This study |
| pRH98 | pRS415::(tcpA-lacZYA) (−63/+72) | This study |
| pCJ1.3 | pRS415::(tcpA-lacZYA) (−162/+2), T to A at −38 | This study |
| pCJ10.4 | pRS415::(tcpA-lacZYA) (−162/+2), A to T at −50 | This study |
| pBAD22 | Expression plasmid, Apr | 21 |
| pRH81 | pBAD22 six-His toxT | This study |
| pMMB66EH | Expression plasmid, Apr | 17 |
| pRH170 | Full-length rpoA in pMMB66EH | This study |
| pRH171 | rpoAΔ235 in pMMB66EH | This study |
Plasmid and strain construction.
The tcpA-lacZ deletion series in pRS415 was constructed as follows. The tcpA promoter fragments for pAJA3, pRH82, pRH72, pAJA2, pAJA1, and pRH8 were amplified by PCR from V. cholerae O395 genomic DNA by using 5′ primers TcpHAA1 (5′-AAAAGAATTCCGCCTAGATAGTGTGTGA-CG-3′), RH11 (5′-GATCGGAATTCGCACGAGACGAACACTGTC-3′), RH04 (5′-GATCGGAATTCAATTTCGATCTCCACTCCGG-3′), RH03 (5′-GACTGAATTCTTGAATTGAATAAGTTGGCC-3′), RT35 (5′-AAAACTCGAGGTGCGTGAATGTTACTCGTG-3′), and RH01 (5′-ATGCGAATTCCTTTCAATGCAAGTGTG-3′). The 3′ primer used for all these constructs was RTAA36 (5′-AAAAGGATCCACACGCACATTTAACCACAC-3′). The tcpA promoter fragment for pRH98 was amplified from O395 chromosomal DNA by using primers RH12 (5′-GATCGAATTCAAAAAACACAGCAAAAAATG-3′) and RH13 (5′-GACTGGATCCATGTAACTCCACCATTTTAC-3′). The products were cloned into the EcoRI-BamHI site of pRS415 and verified by PCR. toxT was provided on plasmid pTSS-5.
tcpA-lacZ fusions in pAJA3, pAJA2, pAJA1, pRH8, pRH98, pRH80, pCJ1.3, and pCJ10.4 were recombined onto λRS45 (59) and lysogenized into MC4100 to create strains RRH100, RRH115, RRH131, RRH113, RRH114, RRH101, RRH102, and RRH103, respectively. Single-copy lysogens were confirmed by PCR (53). pTSS-5 was introduced into each strain by calcium chloride transformation.
Plasmid pRH81 was constructed in the following manner. The toxT gene was amplified from O395 chromosomal DNA by using primers RH07 (5′ AGCCATGGCACACCACCACCACCACCACATGATTGGGAAAAAATCTTTTC-3′), which adds a six-His tag on the N-terminal end, and RH08 (5′-AGCTTCTAGACCCAAAATCAGTGATACAATCG-3′). The product was cloned into the NcoI-XbaI site of pBAD22 and confirmed by PCR and sequencing.
Plasmids pRH170 and pRH171 were constructed in the following manner. For pRH170, the entire rpoA gene was amplified from O395 chromosomal DNA by using primers RH17 (5′-GATCCTGCAGTTATCTTCAGCGATTGACG-3′) and RH19 (5′-GATCGGATCCCGAACAATTGATCGTCGAGC-3′). The product was cloned into the PstI-BamHI site of pMMB66EH and confirmed by both PCR and sequencing. For pRH171, primers RH17 and RH18 (5′-GATCCTGCAGTTATTACTCGTGCTGGTTCTTAAGATCTACGAACGCATCC-3′) were used to amplify the truncated rpoA gene (amino acids 1 to 235) from O395 chromosomal DNA. Primer RH18 encodes a five-amino-acid polar linker (KNQHE), followed by two ochre stops at the 5′ end. The linker was added for stabilization purposes (39, 52). The product was also cloned into the PstI-BamHI site of pMMB66EH and confirmed by PCR and sequencing. Both pRH170 and pRH171 were introduced into V. cholerae strains by mating (64) from strain SM10λpir.
β-Galactosidase assays.
All β-galactosidase assays (40) were carried out after growth in LB medium (pH 6.5) at 30°C. Strains carrying tcpA-lacZ fusions were assayed following overnight growth with aeration, while assays with toxT-lacZ fusion strains were performed by using mid-log-phase cultures. β-Galactosidase activity was expressed in Miller units (40).
Random PCR mutagenesis.
Random PCR mutagenesis was carried out as previously described by Cadwell and Joyce (5) and Fromant et al. (16). Specifically, for the method of Cadwell and Joyce, the PCR mixtures contained 7 mM MgCl2, 0.2 mM dATP, 0.2 mM dGTP, 1 mM dTTP, 1 mM dCTP, 0.5 mM MnCl2, and 5 U of Taq polymerase. For the method of Fromant et al., the PCR mixtures contained 4.2 mM MgCl2, 0.2 mM dGTP, 0.2 mM dCTP, 0.2 mM dTTP, 3.4 mM dATP, and 5 U of Taq polymerase. Each PCR consisted of 30 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min with primers RH03 and RTAA36 (see above). pAJA2 plasmid DNA was used as a template. Five separate pools were generated with each method. Pooled PCR products were purified on spin columns (Qiagen), digested with EcoRI and BamHI, gel purified, and ligated into pRS415 to create lacZ fusions. The resulting pools of mutant constructs were electroporated into E. coli strain AA60, which carries toxT on plasmid pTSS-5. Colonies that appeared pink and shiny on 1% lactose-MacConkey agar plates were chosen for further study. Plasmid DNA was isolated from mutants by using Qiagen kits and was backcrossed into AA60 to confirm the phenotype. PCR was used to verify the presence of the desired insert. Inserts were sequenced by using automated fluorescence dye sequencing (Molecular Core Facility, Dartmouth Medical School).
Purification of ToxT, SDS-PAGE, and immunoblotting.
The toxT gene was inserted into pBAD22 under control of the arabinose-inducible promoter paraBAD, and this construct was transformed into E. coli CC118. The resulting strain, RRH81, was grown to the mid-log phase at 30°C, and then arabinose was added to a final concentration of 0.02% and the strain was induced for 4 h at 30°C. Cell pellets were resuspended in extraction buffer (50 mM sodium phosphate, 300 mM NaCl; pH 7.0) and lysed by passage through a French pressure cell two or three times. Following a clarifying spin at 10,000 rpm in a Sorvall SS34 rotor for 20 min, supernatant and pellet fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Supernatants containing His-tagged ToxT were passed over Talon metal affinity resin (Clontech), and after extensive washing, ToxT was eluted with elution buffer (50 mM sodium phosphate, 300 mM NaCl, 150 mM imidazole). Column fractions were analyzed by SDS-PAGE, and fractions containing ToxT were pooled and dialyzed overnight against TEN buffer (0.1 M NaCl, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA [pH 8]) containing 1 mM dithiothreitol. Protein concentrations were determined by the bicinchoninic acid procedure (Pierce). His-tagged ToxT in crude extracts and as pure protein was visualized by immunoblotting as follows. Proteins were transferred to nitrocellulose membranes, probed with anti-TetraHis antibody (Qiagen), and visualized by using ECL detection reagents (Amersham Pharmacia). Protein was stored at −70°C in 10% glycerol.
Gel mobility shift assays.
PCR products of tcpA promoter regions were end labeled by using digoxigenin (DIG) (Gel Shift kit; Roche). The −95/+2, CJ10.4, CJ3.3, CJ2.6, and F4.2 probes were amplified from V. cholerae O395 chromosomal DNA or plasmids by using primers RH01 and RTAA36, the −63/+2 probe was amplified by using primers RH12 and RTAA36, and the −43/+2 probe was amplified by using primers RH16 (5′-GATCGAATTCGACATCTGTCAATTGTAGGT-3′) and RTAA36. The site I probe (probe −162/−51) was amplified from chromosomal DNA by using primers RH24 (5′-GTACTGCTGTGTTTTTTTATTTTTTTAATAAC-3′) and RH03. Probes were purified on agarose gels and extracted by using Gel Extraction kits (Qiagen). One microgram of probe was used for each labeling reaction. Labeled probe was resuspended in TEN buffer to a final concentration of 5 to 10 ng/μl. Five nanograms of labeled probe plus various amounts of pure His-tagged ToxT were used for each binding reaction. Also, 500 ng of calf thymus nonspecific competitor DNA was added to each reaction mixture. Binding reactions were carried out in binding buffer (10 mM Tris-HCl [pH 7.5], 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 200 μg of bovine serum albumin per ml, 10% glycerol), and the reaction mixtures were incubated at 30°C for 30 min. Loading buffer without bromphenol blue was added, and samples were loaded onto a preelectrophoresed 6% native polyacrylamide gel. Gels were electrophoresed in 1× Tris-borate-EDTA at 120 V and 4°C. DNA was transferred to a positively charged nylon membrane (Roche) by electroblotting, cross-linked by using a UV Stratalinker (Stratagene), and visualized with an anti-DIG antibody by chemiluminescent detection by using reagents provided in the DIG kit (Roche).
RESULTS
Deletion series of the tcpA promoter region defines a minimal region required for ToxT-dependent activation.
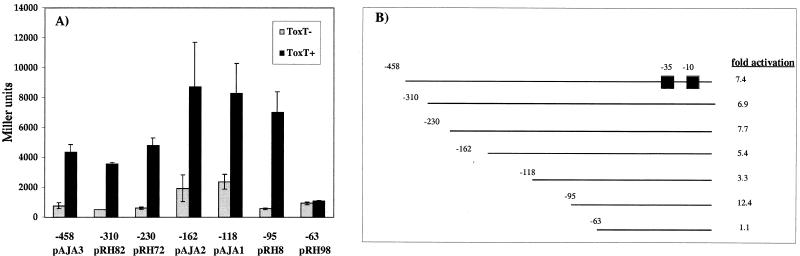
ToxT has previously been shown to directly activate tcpA expression with an E. coli host (3, 12). In order to delineate the cis requirements for activation by ToxT, a deletion series encompassing a region from −458 to −63 of the tcpA promoter was constructed in the cloning vector pRS415, which carries a promoterless lacZ gene downstream of the cloning site. The resulting tcpA-lacZ fusion constructs were transformed into E. coli lacZ deletion strain CC118, and toxT was provided on the pACYC184 derivative pTSS-5. The ability of ToxT to activate each tcpA promoter deletion was examined by determining the induction value for activated levels (ToxT+) compared with basal levels (ToxT−) for each deletion construct. As shown in Fig. 1, ToxT was able to activate transcription for the entire deletion series except pRH98, which is deleted to position −63 relative to the transcriptional start site of tcpA. This suggests that ToxT binds to a region of the tcpA promoter between positions −95 and +2, perhaps either upstream of or encompassing position −63. This region is extremely A rich. The promoter fusions upstream of −162 showed decreased levels of basal transcription compared to the levels of shorter fusions, indicating that there was a possible repressor binding site upstream of this position. The histone-like nuclear structuring protein H-NS has been shown to repress transcription of tcpA (49), and it has been proposed that this protein binds to one or more sites in this region of the promoter. ToxT partially overcomes the negative effect of H-NS at tcpA and ctxA (49).
FIG. 1.
ToxT-dependent activation of tcpA-lacZ promoter deletions in E. coli. (A) Strains with no ToxT (shaded bars) or strains with the ToxT-expressing plasmid pTSS-5 (solid bars) were grown overnight in LB medium (pH 6.5) at 30°C. Promoter deletions are carried on plasmid pRS415. The values are averages for at least two independent experiments. (B) tcpA-lacZ deletion series. Most promoter deletions are within a promoter fragment that extends to position +2 relative to the start of transcription; the only exception is the −63 deletion, which extends to +72. The level of activation in the presence of ToxT is indicated for each deletion.
Point mutations in the tcpA promoter that affect activation by ToxT cluster in three regions.
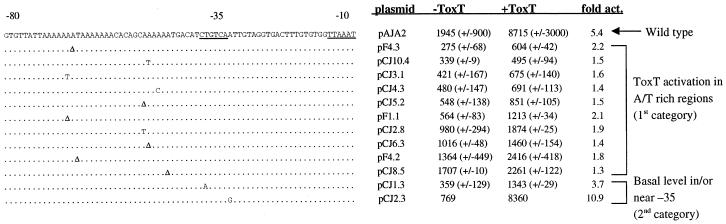
To further define the location of the ToxT binding site at the tcpA promoter, PCR mutagenesis was carried out to create random point mutations in the promoter region. The region from −162 to +2 of the tcpA promoter was chosen as the template for mutagenic PCR since it included the region from −95 to −63, as well as flanking regions that might influence activation. Five separate pools of mutant PCR products were generated by the two procedures described in Materials and Methods. These products were cloned into pRS415 to create lacZ fusion constructs that were then transformed into AA60, an E. coli strain harboring toxT on pTSS-5. The transformants were plated on 1% lactose-MacConkey agar plates and grown at 30°C. Wild-type tcpA-lacZ fusion strains were dark red on these plates due to full activation by ToxT. White colonies lacked the promoter insert. Colonies harboring plasmids that were potentially defective for activation had a shiny pink appearance and white edges. Transformants that displayed this phenotype were restreaked on 1% lactose-MacConkey agar plates, and the presence of the tcpA promoter insert was confirmed by colony PCR. Plasmid DNA was isolated and backcrossed into AA60 to confirm that the activation defect was linked to the tcpA promoter-containing plasmid. β-Galactosidase assays were performed on the mutant strains, and the level of activation by ToxT for each mutant was compared to that of the wild-type strain. The mutants could be separated into two categories. The mutants in the first category showed an overall decreased level of transcription, particularly decreased activation by ToxT, which is the result expected for a mutation that disrupts activator function. The mutants in the second category showed a decreased level of basal transcription but retained wild-type levels of activation by ToxT, as expected for a mutation which disrupts RNA polymerase-DNA interactions. The majority of the mutants fell into the first category, but two mutants that are discussed below fell into the second category. It was found that for all of the mutants in the first category, ToxT was able to activate only one- to twofold compared with basal levels of transcription, compared to the fivefold activation observed for the wild type and for mutants in the second category.
Twenty representative mutants were sequenced, and their sequences were aligned with the wild-type sequence for the region. Seven of the mutants had a single base pair substitution or deletion, while the other 13 mutants had two or more base pair substitutions or deletions. The point mutations clustered in three distinct regions: the −35 region, a region centered at −60, and an upstream region centered at −117. No single mutations in the −117 region that did not have an accompanying mutation around −60 or −35 were isolated in our mutagenesis experiments, suggesting that the region centered at −117 is not in and of itself critical for activation by ToxT. This hypothesis is consistent with the results of the deletion analysis. Figure 2 shows the sequences of 12 of the mutants that have single mutations in either the −60 or −35 region. Ten of the mutants shown in Fig. 2 fall into the first category of mutants with decreased levels of activation by ToxT. All 10 of these mutants have single base pair substitutions or deletions in the region centered at −60, and ToxT is able to activate only one- to twofold compared with the basal levels, compared to the fivefold activation by the wild type. The mutations around −60 cluster in two regions separated by more than 10 bp, suggesting that they may define two distinct sites required for ToxT function. The single base pair deletions may also affect the spacing and rotation of these sites with respect to each other or the RNA polymerase binding site. Two of the mutants shown in Fig. 2 belong in the second category of mutants with decreased levels of basal transcription and wild-type levels of activation by ToxT. One of these mutants, CJ1.3, was found to have a single point mutation within the putative −35 region. The base pair change from T to A alters the sequence of the putative −35 hexamer further from the consensus TTGACA sequence. This mutant showed decreased levels of both basal and activated transcription compared to the wild-type levels, but the level of activation by ToxT was comparable to that of the wild type (3.7-fold versus 5-fold) (Fig. 2). Because the mutation falls in the putative −35 region and has only a minor effect on ToxT-mediated activation, we hypothesized that this point mutation affects RNA polymerase binding and does not greatly affect ToxT activity. The other mutant in the second category, CJ2.3, has only a defect in the basal level of transcription. ToxT is able to activate to wild-type levels (8,360 U) (Fig. 2). This mutant has a single base pair change, A to G at −33, within the putative −35 consensus region, which alters the sequence further from the consensus sequence. Since the only defect is a defect in basal transcription, we hypothesized that the point mutation of this mutant, like the CJ1.3 mutation, also affects the RNA polymerase binding site and the presence of ToxT is able to overcome this defect and provide full levels of activated transcription.
FIG. 2.
Sequences of single-point mutants with mutations in the region from −80 to −30, aligned with the sequence of the wild-type tcpA promoter. Base pair changes or deletions are shown; wild-type bases are indicated by dots. Strains carrying the tcpA-lacZ fusions on plasmid pRS415 with no ToxT or with the ToxT-expressing plasmid pTSS-5 were grown overnight in LB medium (pH 6.5) at 30°C. The β-galactosidase activity (in Miller units) and the level of activation (act.) in the presence of ToxT are shown for each strain. The values are averages for at least two independent experiments.
Analysis of a single T-to-C point mutation at −117 and creation of an internal deletion of the region from −140 to −95 allowed us to determine that the upstream region centered at −117 is not required for activation by ToxT (data not shown). The region closer to the transcriptional start site, centered at −60, is essential for activation by ToxT, as shown by the promoter deletion series and the random point mutations.
ToxT activation of single-copy chromosomal tcpA promoter fusions in E. coli.
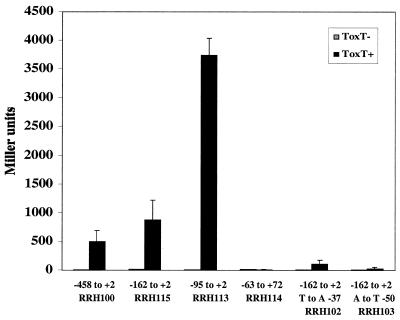
All of the experiments described above were carried out with multicopy plasmids in E. coli. In order to confirm that the results obtained were not due to alterations in plasmid supercoiling or multicopy effects, several representative tcpA-lacZ fusions were integrated into the E. coli chromosome as λ lysogens for further analysis. These included the construct from −458 to +2 (pAJA3), the construct from −162 to +2 (pAJA2), the construct from −95 to +2 (pRH8), and the construct from −63 to +72 (pRH98). A single point mutation in the −35 region (CJ1.3) and a single point mutation at −50 in the putative ToxT binding site (CJ10.4) were also used. Single copies of these six constructs were integrated into the chromosome of E. coli MC4100 as λRS45 derivatives (59). ToxT was provided from plasmid pTSS-5, and β-galactosidase assays were performed. The overall trends followed what was observed with multicopy plasmids (Fig. 3). ToxT-dependent activation was lost with the fusion at −63 to +72. ToxT was not able to activate at levels significantly above basal levels when the A-to-T substitution at −50 was present but was able to activate in the strain containing the T-to-A substitution at −38.
FIG. 3.
ToxT-dependent activation of representative tcpA-lacZ promoter constructs as λ lysogens integrated into the E. coli chromosome. Strains with no ToxT or strains with the ToxT-expressing plasmid pTSS-5 were grown overnight in LB medium (pH 6.5) at 30°C. The values are averages for at least two independent experiments.
Similar motifs at other ToxT-regulated promoters in V. cholerae.
Data obtained with the deletion series and random point mutations suggest that the ToxT binding site at the tcpA promoter is located in an A-rich region from −82 to −45 relative to the transcriptional start site. Other ToxT-activated promoters were examined for similar motifs. An alignment of the putative −10 regions of the tcpA, tcpI, tagA, and ctxA promoters is shown in Fig. 4. The first three promoters, tcpA, tcpI, and tagA, are all very A rich. The tcpI promoter closely resembles tcpA in that there is a long A tract located around −65 relative to the transcriptional start site and a shorter A tract located closer to the −35 region. The tagA promoter has only one A tract, and it is located proximal to the −35 region. The similarity among these three ToxT-regulated promoters, together with the results of the genetic analysis of the tcpA promoter, suggests that ToxT may interact with these A-rich regions. The sequence upstream of ctx does not resemble the sequence upstream of tcpA, tcpI, or tagA; however, regulation at the ctxA promoter is much different from regulation at the other three promoters in that ctxA is regulated by both ToxT and the membrane-bound activator ToxR (12, 42, 49).
FIG. 4.
Alignment of the tcpA promoter with other V. cholerae promoters regulated by ToxT. Sequences were aligned by using the −10 consensus sequence. The tcpI start site has been described previously (47), as have the tcpA start site (3) and the ctxA start site (51). The tagA start site is hypothetical.
His-tagged ToxT binds directly to the tcpA promoter in vitro.
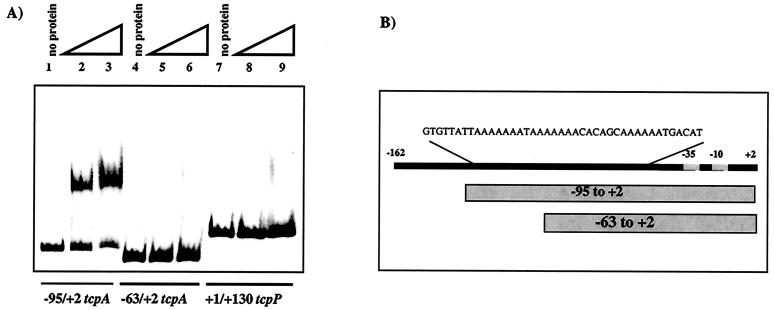
The ability of ToxT to interact with the region of the tcpA promoter determined by genetic means was confirmed by gel shift analysis by using six-His-tagged ToxT. Purified His-tagged ToxT was used in gel mobility shift assays with DIG-labeled fragments of the tcpA promoter, as described in Materials and Methods. As shown in Fig. 5, ToxT was able to bind to and shift the region of the tcpA promoter from −95 to +2, which contains both of the A-rich regions identified by the deletion series and point mutations. ToxT was not able to shift the region of the tcpA promoter from −63 to +2, in which one-half of the promoter-distal A tract is removed. This is consistent with the data described above that show that this region is unresponsive to transcriptional activation by ToxT (Fig. 1). These results suggest that ToxT requires the promoter-distal A tract to bind and activate and cannot bind to the promoter-proximal A tract alone. ToxT also does not bind to a nonspecific probe encompassing the region from +1 to +130 of the tcpP promoter (Fig. 5), further demonstrating that the promoter-distal site-dependent binding is specific.
FIG. 5.
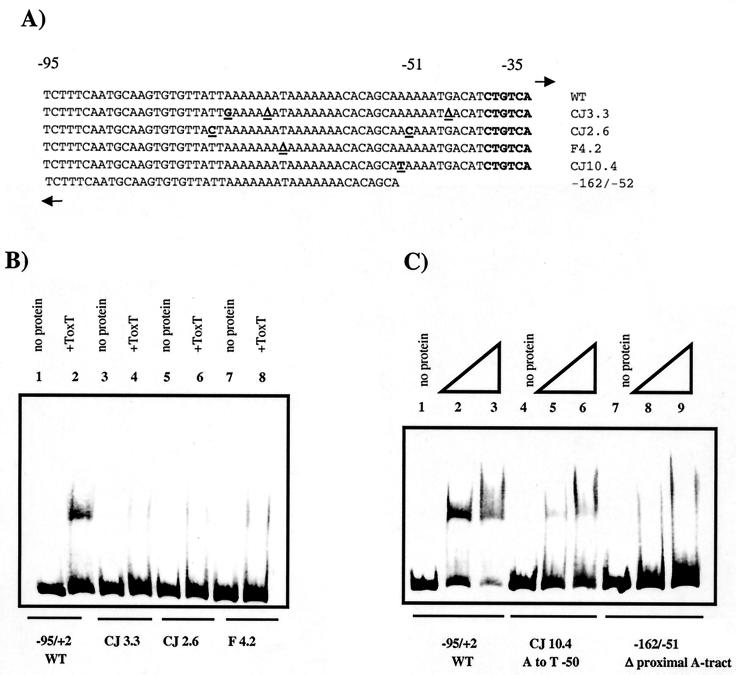
Six-His-tagged ToxT binds to tcpA promoter fragments in gel mobility shift assays. (A) Lanes 1 to 3, −95/+2 tcpA probe (lane 1, no protein; lane 2, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 3, 300 ng [9.3 pmol] of six-His-tagged ToxT); lanes 4 to 6, −63/+2 tcpA probe (lane 4, no protein; lane 5, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 6, 300 ng [9.3 pmol] of six-His-tagged ToxT); lanes 7 to 9, +1/+130 tcpP probe (lane 7, no protein; lane 8, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 9, 300 ng [9.3 pmol] of six-His-tagged ToxT). (B) Diagram showing the positions of tcpA promoter fragments used in the gel shift analysis. The −95/+2 probe includes both A tracts, while the −63/+2 probe includes one-half of the distal A tract and the entire proximal A tract.
Next we wished to examine whether the lack of activation resulting from various point mutations in the tcpA promoter was due to a defect in ToxT binding. CJ3.3 has two mutations in the promoter-distal site and one mutation in the promoter-proximal site, CJ2.6 has one mutation in the promoter-distal site and one mutation in the promoter-proximal site, and F4.2 has one mutation in the promoter-distal site (Fig. 6A). ToxT was not able to detectably bind to a probe containing the mutations (Fig. 6B), suggesting that these point mutations are activation defective because they are binding defective. To specifically assess the role of the promoter-proximal site in binding, we used CJ10.4, which has an A-to-T mutation at −50 in this A tract. ToxT was able to bind to this probe, but with less avidity than to a wild-type probe (Fig. 6C), which is consistent with the decreased activation of this mutation (Fig. 2). To further investigate the relative roles of the two A tracts in ToxT binding, a probe was constructed from −162 to −51 containing the entire promoter-distal site but only one-half of the promoter-proximal site. The inability of ToxT to produce a detectable shift of this probe suggests that ToxT is not able to bind to the distal site in the absence of the proximal site (Fig. 6C). This, coupled with the results of the analysis of the distal site presented above, suggests that ToxT requires the presence of both A tracts for binding.
FIG. 6.
Gel mobility shift assays with various tcpA promoter mutants. (A) Sequences of mutant probes used in binding studies. Base pair changes are underlined. Most of the probes are from −95 to +2; the only exception is the probe from −162 to −51. WT, wild type. (B) Lanes 1 and 2, −95/+2 tcpA probe (lane 1, no protein; lane 2, 150 ng [4.6 pmol] of six-His-tagged ToxT); lanes 3 and 4, CJ3.3 probe (lane 3, no protein; lane 4, 150 ng [4.6 pmol] of six-His-tagged ToxT); lanes 5 and 6, CJ2.6 probe (lane 5, no protein; lane 6, 150 ng [4.6 pmol] of six-His-tagged ToxT); lanes 7 and 8, F4.2 probe (lane 7, no protein; lane 8, 150 ng [4.6 pmol] of six-His-tagged ToxT). (C) Lanes 1 to 3, −95/+2 tcpA probe (lane 1, no protein; lane 2, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 3, 300 ng [9.3 pmol] of six-His-tagged ToxT); lanes 4 to 6, CJ10.4 probe (lane 4, no protein; lane 5, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 6, 300 ng [9.3 pmol] of six-His-tagged ToxT); lanes 7 to 9, site I probe (lane 7, no protein; lane 8, 150 ng [4.6 pmol] of six-His-tagged ToxT; lane 9, 300 ng [9.3 pmol] of six-His-tagged ToxT).
ToxT requires αCTD of RNA polymerase to activate transcription at tcpA.
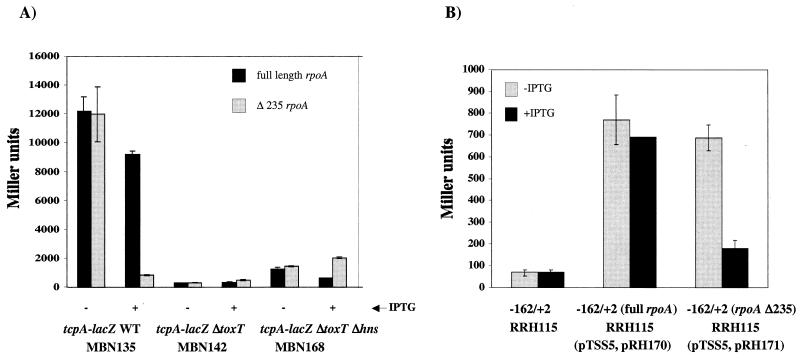
It has been demonstrated that many AraC family members require the αCTD of RNA polymerase to activate transcription at their respective promoters (27, 30, 32, 38, 56). We tested the requirement for αCTD for ToxT-dependent transcriptional activation at tcpA by performing a genetic analysis similar to that described by Holcroft and Egan (27). The region of the V. cholerae rpoA gene corresponding to residues 1 to 235 was amplified from O395 chromosomal DNA and cloned under control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. This resulted in a truncated α subunit that was missing the C-terminal domain. We hypothesized that when the truncated α subunit is overexpressed in V. cholerae, it assembles with RNA polymerase subunits expressed from the chromosome and acts as a dominant negative allele with respect to promoters at which αCTD has a role in expression. This hypothesis is based on previous experiments performed with E. coli (23). A full-length rpoA construct was also constructed as a control. Each of the rpoA constructs was introduced into V. cholerae strains carrying a chromosomal tcpA-lacZ fusion. In a wild-type background, overexpression of the truncated α subunit resulted in an 11-fold decrease in tcpA-lacZ expression compared to the expression when a full-length α subunit was overexpressed (Fig. 7A). This effect was not seen in a ΔtoxT background or a ΔtoxT Δhns background, which was used to elevate the level of tcpA expression in the ΔtoxT background (Fig. 7A). These results suggest that only ToxT-dependent transcription and not basal transcription of the tcpA promoter depends upon the α subunit of RNA polymerase.
FIG. 7.
Effects of dominant negative RNA polymerase alpha subunit on tcpA-lacZ expression in V. cholerae (A) and E. coli (B). (A) Cultures of MBN135 (tcpA-lacZ), MBN142 (tcpA-lacZ ΔtoxT), or MBN168 (tcpA-lacZ ΔtoxT Δhns) carrying either pRH170 (full-length rpoA) or pRH171 (rpoA Δ235) were grown overnight in LB medium (pH 6.5) at 30°C with or without 0.04 mM IPTG. The values are averages for at least two independent experiments. WT, wild type. (B) Cultures of RRH115 (λtcpA-lacZ −162/+2) carrying no plasmid or pTSS-5 and either pRH170 or pRH171 were grown overnight in LB medium (pH 6.5) at 30°C with or without 0.04 mM IPTG. The values are averages for at least two independent experiments.
To determine whether the 11-fold defect in transcription of tcpA-lacZ was due to a direct effect of the truncated αCTD at the tcpA promoter and not to the effects of other promoters upstream in the regulatory cascade, we expressed ToxT from the αCTD-independent tetR promoter using plasmid pTSS-5. These experiments were carried out with E. coli since the tetR promoter is not expressed well in V. cholerae. E. coli λtcpA-lacZ strain RRH115 (−162/+2) carrying either full-length rpoA or Δ235 rpoA on pMMB66EH and pTSS-5 as a source of ToxT was assayed by performing β-galactosidase assays. We found that when ToxT was expressed from an αCTD-independent promoter, there was an approximately fourfold defect in tcpA-lacZ transcription due to overexpression of Δ235 rpoA (Fig. 7B). These results also suggest that ToxT interacts with αCTD to activate transcription at tcpA. Due to the difference between the 11-fold defect in V. cholerae when ToxT was expressed from its own promoter and the 4-fold defect in E. coli when ToxT was expressed from the tetR promoter, we introduced the rpoA constructs into a V. cholerae strain carrying a chromosomal toxT-lacZ fusion. We observed a twofold defect when the dominant negative allele was overexpressed (data not shown). These results suggest that αCTD has a role at the toxT promoter, perhaps through an interaction with ToxR or TcpP.
DISCUSSION
AraC family members can be divided into different categories based on the types of genes that they regulate, such as the genes involved in sugar catabolism, stress response, and bacterial pathogenesis. It has been shown that family members involved in the regulation of sugar catabolism, such as AraC itself, function as dimers and have both a C-terminal DNA binding domain and an N-terminal dimerization domain. The N-terminal domain also functions to bind regulatory cofactors, such as sugar molecules. These proteins have binding sites that overlap the −35 position (class II promoters) (38). Family members such as Rob, MarA, and SoxS regulate genes involved in stress response and function as monomers. These proteins can have binding sites that are upstream of the −35 position (class I promoters) or overlap the −35 position (class II promoters) (30, 38). ToxT belongs to the subgroup of AraC homologs that regulate virulence gene expression. Less is known about this subgroup than about any other subgroup, but the members have been shown to activate transcription at both class I and class II promoters. The tcpA promoter appears to be a class I promoter since ToxT binds upstream of the −35 position. We propose that the primary binding site does not overlap the −35 position for the following reasons. A point mutation at −38 (CJ1.3) affects overall transcription but does not affect the level of activation by ToxT (Fig. 2), suggesting that this mutation interferes only with RNA polymerase binding. It has been shown that SoxS requires only the αCTD of RNA polymerase to activate transcription at promoters where the binding site does not overlap the −35 position (i.e., class I promoters) (30). We have shown that ToxT requires the RNA polymerase αCTD to activate transcription at tcpA (Fig. 7). Furthermore, a recent paper by Yu et al. showed that ToxT protects the region from −84 to −41 of tcpA in DNase I protection assays (69). This footprint does not overlap the putative −35 site and is consistent with the data presented here. Munson and Scott have recently demonstrated that the AraC homolog Rns from enterotoxigenic E. coli requires a binding site downstream of the transcriptional start site to activate transcription at its own promoter (46). We have no indication that ToxT binds downstream of tcpA; in fact, our tcpA-lacZ fusions ended at +2, which suggests that ToxT does not require downstream sequences to activate expression from the tcpA promoter.
The binding site of ToxT at tcpA consists of two A-rich regions on the sense strand. Deletions of a single A residue or base pair substitutions in these A-rich regions and the surrounding DNA interfere with ToxT-dependent transcriptional activation and binding. There is no obvious dyad symmetry in the binding site, but AraC homologs tend to recognize asymmetric binding sites (18, 45). Two AraC homologs in particular have been shown to bind to A-rich DNA sequences. ExsA from P. aeruginosa binds to a consensus sequence (TXAAAAXA) located around −50 relative to the transcriptional start site (29). BfpT (PerA) from enteropathogenic E. coli requires an A-rich region from −85 to −46 of the bfpA promoter to activate transcription, and deletions of As in this region were found to negatively affect BfpT-dependent transcriptional activation (4). BfpT is particularly interesting because like ToxT, this protein regulates genes encoding a type IV pilus (bundle-forming pilus) (4).
Many transcriptional activators have been shown to interact with different subunits of RNA polymerase to activate transcription (67). There are data which suggest that various AraC homologs require the αCTD of RNA polymerase (1, 27, 30, 32, 38, 56) at class I promoters. In this paper we present data obtained by using a truncated alpha subunit which suggest that ToxT requires αCTD to activate transcription at tcpA. Our experiments also indicated that the αCTD does not seem to be required for basal transcription at tcpA. This implies that the A-rich sequence upstream of tcpA does not act as an UP element. UP elements are A-T-rich sequences that can bind αCTD and stimulate transcription independent of activator proteins (14, 19). UP elements consist of two subsites, a promoter-proximal subsite and a promoter-distal subsite. The proximal subsite is typically centered at −40 and has a consensus 5′-AAAAAARNR-3′ sequence, while the distal subsite is centered at −50 and has a consensus 5′-AWWWWWTTTTT-3′ sequence (14). The sequence of the promoter-proximal A tract loosely resembles that of a consensus UP element proximal site, but the tract is centered at −47, not at −40, and is therefore rotated around the DNA compared to a typical UP element. There is no distal site at tcpA. These observations, along with our dominant negative data, strongly suggest that there is no functional UP element at the tcpA promoter.
There is genetic evidence that there is an interaction between the AraC homolog RhaS and the sigma 70 subunit of RNA polymerase (1). Bhende and Egan (1) found a specific residue within RhaS, D241, that may be important for contacting sigma. This residue is conserved in many AraC family members (1, 18) but not in ToxT. Because ToxT does not have an Asp at position 241 and because its binding site does not overlap the −35 position (69), we propose that the mechanism of ToxT-dependent activation at tcpA does not involve an interaction with sigma. This remains to be determined experimentally. Some activators are known to contact the N-terminal domain of alpha, but an extensive genetic screening analysis performed by Egan et al. (13) suggested that AraC family members do not use this mechanism to activate transcription (13).
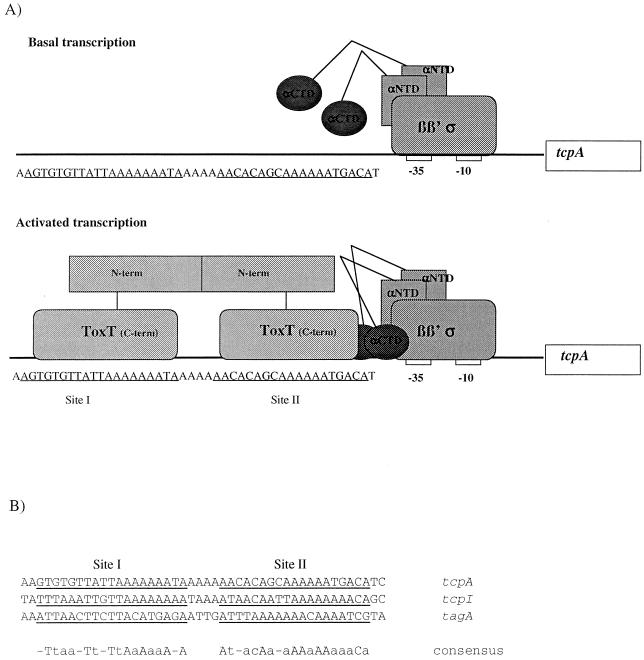
Our data begin to elucidate the mechanism of ToxT-dependent transcriptional activation at the tcpA promoter, a model of which is depicted in Fig. 8A. In our model, basal transcription at tcpA is independent of αCTD since there does not appear to be a functional UP element. In order to activate transcription, ToxT binds to an A-rich region from −84 to −41, the region that ToxT protected in a DNase I footprint published recently by Yu and DiRita (69). While the footprint defines the general region where ToxT binds, in our study we used a mutational analysis to further investigate individual nucleotides important for ToxT binding and activation. The region bound by ToxT is approximately 40 bp long, which could encompass four major grooves. Based on the AraC model (24, 48), this provides sufficient space for one dimer of ToxT to bind. It is not known whether ToxT is able to dimerize, but we have found that mutations that delete either one or both of the C-terminal HTH domains have a dominant negative phenotype with respect to tcpA expression, indicating that there is functional multimerization of ToxT (unpublished data). These preliminary results suggest that ToxT can dimerize prior to binding DNA. The activation-defective point mutations define two small regions from −65 to −67 and from −46 to −51, and we propose that these regions represent two of the major grooves contacted by ToxT. These major grooves are not adjacent, further supporting the hypothesis that ToxT binds as a dimer. Our mutational analysis did not identify the other two major grooves contacted by ToxT, but the locations of the two major grooves that we did identify are consistent with two ∼20-bp half-sites from −82 to −64 (site I) and from −59 to −41 (site II). This is similar to the AraC model (48). The ToxT molecule centered at site II, closer to the −35 region, should be in position to interact with RNA polymerase. We have demonstrated that ToxT requires the αCTD of RNA polymerase to activate transcription at tcpA (Fig. 7). It remains to be determined experimentally whether αCTD contacts DNA and/or ToxT. Figure 8B shows an alignment of the tcpA promoter with tcpI and tagA, two other V. cholerae promoters that are regulated by ToxT. Similarly positioned A-rich motifs suggest that ToxT may bind to these promoters in a similar manner.
FIG. 8.
Model of transcriptional activation at tcpA. (A) During basal transcription, αCTD does not contact DNA. During activated transcription, a dimer of ToxT binds to site I (−82 to −64) and site II (−59 to −41). The ToxT molecule at site II recruits RNA polymerase through a mechanism involving the αCTD. See text for details. αNTD, alpha N-terminal domain. (B) Alignment of site I and site II at tcpA, tcpI, and tagA. Site I and site II at each promoter are underlined. The consensus sequence is shown at the bottom. An uppercase letter indicates that a base pair is present in all three promoters, and a lowercase letter indicates that base pair is conserved in two of the three promoters.
Acknowledgments
We thank Anthony Accurso for plasmids, Mindy Nye for strains, and Karen Skorupski for many helpful discussions and a critical review of the manuscript. We also thank an anonymous reviewer for insightful comments.
This work was supported by NIH grant AI39654 to R.K.T.
Footnotes
For a commentary on this article, see p. 5529 in this issue.
REFERENCES
- 1.Bhende, P. M., and S. M. Egan. 2000. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J. Bacteriol. 182:4959-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgerie, S. J., C. M. Michan, M. S. Thomas, S. J. W. Busby, and E. I. Hyde. 1997. DNA binding and DNA bending by the MelR transcription activator protein from Escherichia coli. Nucleic Acids Res. 25:1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, R. C., and R. K. Taylor. 1995. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol. Microbiol. 16:425-439. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante, V. H., E. Calva, and J. L. Puente. 1998. Analysis of cis-acting elements required for bfpA expression in enteropathogenic Escherichia coli. J. Bacteriol. 180:3013-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadwell, R. C., and G. Joyce. 1992. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 2:26-33. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. [DOI] [PubMed] [Google Scholar]
- 7.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, S. L., R. K. Taylor, M. Koomey, and J. J. Mekalanos. 1995. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination and serum resistance. Mol. Microbiol. 17:1133-1142. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, J. A., J. B. Kaper, and V. J. DiRita. 1998. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol. Microbiol. 29:235-246. [DOI] [PubMed] [Google Scholar]
- 11.DiRita, V. J., and J. J. Mekalanos. 1991. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell 64:29-37. [DOI] [PubMed] [Google Scholar]
- 12.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, S. M., A. J. Pease, J. Lang, X. Li, V. Rao, W. K. Gillette, R. Ruiz, J. L. Ramos, and R. E. Wolf, Jr. 2000. Transcription activation by a variety of AraC/XylS family activators does not depend on the class II-specific activation determinant in the N-terminal domain of the RNA polymerase alpha subunit. J. Bacteriol. 182:7075-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrem, S. T., W. Ross, T. Gaal, Z. W. Chen, W. Niu, R. H. Ebright, and R. L. Gourse. 1999. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase α subunit. Genes Dev. 13:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everiss, K. D., K. J. Hughes, and K. M. Peterson. 1994. The accessory colonization factor and toxin-coregulated pilus gene clusters are physically linked on the Vibrio cholerae O395 chromosome. DNA Seq. 5:51-55. [DOI] [PubMed] [Google Scholar]
- 16.Fromant, M., S. Blanquet, and P. Plateau. 1995. Direct random mutagenesis of gene-sized DNA fragments using polymerase chain reaction. Anal. Biochem. 224:347-353. [DOI] [PubMed] [Google Scholar]
- 17.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos, M., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourse, R. L., W. Ross, and T. Gaal. 2000. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37:687-695. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 65:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Häse, C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward, R. S., K. Igarashi, and A. Ishihama. 1991. Functional specialization within the α-subunit of Escherichia coli RNA polymerase. J. Mol. Biol. 221:23-29. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickson, W., and R. Schleif. 1985. A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc. Natl. Acad. Sci. USA 82:3129-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-co-regulated pili, and the toxR regulon are essential for Vibrio cholerae colonization in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins, D., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holcroft, C. C., and S. M. Egan. 2000. Roles of cyclic AMP receptor protein and the carboxyl-terminal domain of the α subunit in transcription activation of the Escherichia coli rhaBAD operon. J. Bacteriol. 182:3529-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holcroft, C. C., and S. M. Egan. 2000. Interdependence of activation at rhaSR by cyclic AMP receptor protein, the RNA polymerase alpha subunit C-terminal domain, and RhaR. J. Bacteriol. 182:6774-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovey, A., and D. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jair, K., W. Fawcett, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Ambidextrous transcriptional activation by SoxS: requirement for the C-terminal domain of the RNA polymerase alpha subunit in a subset of Escherichia coli superoxide-inducible genes. Mol. Microbiol. 19:307-317. [DOI] [PubMed] [Google Scholar]
- 31.Jair, K., X. Yu, K. Skarstad, B. Thony, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J. Bacteriol. 178:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jair, K., R. G. Martin, J. L. Rosner, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1995. Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J. Bacteriol. 177:7100-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karaolis, D. K. R., J. A. Johnson, C. C. Bailey, E. C. Boedeker, J. B. Kaper, and P. R. Reeves. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc. Natl. Acad. Sci. USA 95:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon, H., M. Bennik, B. Demple, and T. Ellenberger. 2000. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat. Struct. Biol. 7:424-430. [DOI] [PubMed] [Google Scholar]
- 37.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 39.Milla, M. E., B. M. Brown, and R. T. Sauer. 1993. P22 Arc repressor: enhanced expression of unstable mutants by addition of polar C-terminal sequences. Protein Sci. 2:2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Miller, V. L., V. J. DiRita, and J. J. Mekalanos. 1989. Identification of toxS, a regulatory gene whose product enhances ToxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, V. L., and J. J. Mekalanos. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by ToxR. Proc. Natl. Acad. Sci. USA 81:3471-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 45.Munson, G., and J. R. Scott. 1999. Binding site recognition by Rns, a virulence regulator in the AraC family. J. Bacteriol. 181:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munson, G., and J. R. Scott. 2000. Rns, a virulence regulator within the AraC family, requires binding sites upstream and downstream of its own promoter to function as an activator. Mol. Microbiol. 36:1391-1402. [DOI] [PubMed] [Google Scholar]
- 47.Murley, Y., J. Behari, R. Griffin, and S. B. Calderwood. 2000. Classical and El Tor biotypes of Vibrio cholerae differ in timing of transcription of tcpPH during growth in inducing conditions. Infect. Immun. 68:3010-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niland, P., R. Huhne, and B. Muller-Hill. 1996. How AraC interacts specifically with its target DNAs. J. Mol. Biol. 264:667-674. [DOI] [PubMed] [Google Scholar]
- 49.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogierman, M., and P. Manning. 1992. Homology of TcpN, a putative regulatory protein of Vibrio cholerae, to the AraC family of transcriptional activators. Gene 116:93-97. [DOI] [PubMed] [Google Scholar]
- 51.Pfau, J. D., and R. K. Taylor. 1996. Genetic footprint of the ToxR-binding site in the promoter for cholera toxin. Mol. Microbiol. 20:213-222. [DOI] [PubMed] [Google Scholar]
- 52.Pfau, J. D., and R. K. Taylor. 1998. Mutations in toxR and toxS that separate transcriptional activation from DNA binding at the cholera toxin gene promoter. J. Bacteriol. 180:4724-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell, B. S., D. L. Court, V. Nakamura, M. P. Rivas, and C. L. Turnbough. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 97:10220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhee, S., R. Martin, J. Rosner, and D. Davies. 1998. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. USA 95:10413-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz, R., J. L. Ramos, and S. M. Egan. 2001. Interactions of the XylS regulators with the C-terminal domain of the RNA polymerase alpha subunit influence the expression level from the cognate Pm promoter. FEBS Lett. 491:207-211. [DOI] [PubMed] [Google Scholar]
- 57.Schuhmacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 59.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 60.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 61.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 63.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 171:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas, V. J., and C. M. Collins. 1999. Identification of UreR binding sites in the Enterobacteriaceae plasmid-encoded and Proteus mirabilis urease gene operons. Mol. Microbiol. 31:1417-1428. [DOI] [PubMed] [Google Scholar]
- 66.Tobe, T., G. K. Schoolnik, I. Sohel, V. H. Bustamante, and J. L. Puente. 1996. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol. Microbiol. 21:963-975. [DOI] [PubMed] [Google Scholar]
- 67.Vicente, M., K. Chater, and V. de Lorenzo. 1999. Bacterial transcription factors involved in global regulation. Mol. Microbiol. 33:8-17. [DOI] [PubMed] [Google Scholar]
- 68.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 69.Yu, R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]
- 70.Yu, R., and V. J. DiRita. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J. Bacteriol. 181:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]