Abstract
The oncogenic Epstein-Barr virus (EBV)-encoded latent infection membrane protein 1 (LMP1) mimics a constitutive active tumor necrosis factor (TNF) family receptor in its ability to recruit TNF receptor-associated factors (TRAFs) and TNF receptor-associated death domain protein (TRADD) in a ligand-independent manner. As a result, LMP1 constitutively engages signaling pathways, such as the JNK and p38 mitogen-activated protein kinases (MAPK), the transcription factor NF-κB, and the JAK/STAT cascade, and these activities may explain many of its pleiotropic effects on cell phenotype, growth, and transformation. In this study we demonstrate the ability of the TRAF-binding domain of LMP1 to signal on the JNK/AP-1 axis in a cell type- dependent manner that critically involves TRAF1 and TRAF2. Thus, expression of this LMP1 domain in TRAF1-positive lymphoma cells promotes significant JNK activation, which is blocked by dominant-negative TRAF2 but not TRAF5. However, TRAF1 is absent in many established epithelial cell lines and primary nasopharyngeal carcinoma (NPC) biopsy specimens. In these cells, JNK activation by the TRAF-binding domain of LMP1 depends on the reconstitution of TRAF1 expression. The critical role of TRAF1 in the regulation of TRAF2-dependent JNK signaling is particular to the TRAF-binding domain of LMP1, since a homologous region in the cytoplasmic tail of CD40 or the TRADD-interacting domain of LMP1 signal on the JNK axis independently of TRAF1 status. These data further dissect the signaling components used by LMP1 and identify a novel role for TRAF1 as a modulator of oncogenic signals.
The Epstein-Barr virus (EBV)-encoded latent infection membrane a protein 1 (LMP1) resembles a classical oncogene in its ability to transform rodent fibroblast cell lines and drive the immortalization of primary human B lymphocytes in vitro. Thus, recombinant EBV lacking LMP1 is unable to transform resting B cells into permanently growing lymphoblastoid cell lines (31). This oncogenic potential of LMP1, which is unique among the various EBV- encoded proteins (17, 49), is supported by in vivo findings demonstrating that targeted expression of LMP1 in the B-cell compartment of transgenic mice results in lymphomagenesis (34). These experimental data, coupled with clinical evidence demonstrating LMP1 expression in a number of EBV-associated malignancies, such as nasopharyngeal carcinoma (NPC) and Hodgkin's disease, suggest that LMP1 may function as a viral oncogene.
The oncogenic properties of LMP1 could be attributed to its combined effects on proliferation, survival, differentiation, and metastasis. Thus, LMP1 expression promotes DNA synthesis in resting normal B cells (47) and inhibits cell death through the up-regulation of various antiapoptotic proteins, such as Bcl-2, Mcl-1, and A20 (20, 24, 60). Furthermore, retrovirus-mediated expression of LMP1 in mouse embryonic fibroblasts suppresses senescence and prolongs the lifespan of these primary cultures (62). In epithelial cells LMP1 blocks the normal process of differentiation, a property which may be important in the pathogenesis of NPC (6), and induces the production of the angiogenic factors interleukin-8, prostaglandin E2, and vascular endothelial growth factor (13, 42, 66), suggesting that LMP1 may directly influence the metastasis of EBV-associated tumors. Consistent with this notion, LMP1 expression in MDCK cells results in increased cell motility and invasive growth (33). Finally, LMP1 may indirectly affect oncogenesis through the inhibition of transforming growth factor β-mediated signaling and function (1, 48) and/or the up-regulation of growth factor receptors, such as epidermal growth factor receptor (38).
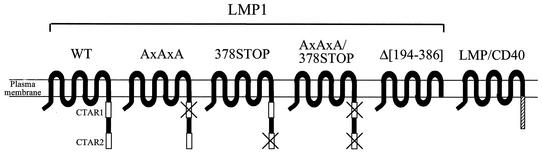
Structurally, LMP1 is a 386-amino-acid (aa) transmembrane protein comprising a 24-aa N-terminal cytoplasmic tail, six hydrophobic membrane-spanning domains, and a 200-aa cytoplasmic C terminus (Fig. 1). The short N-terminal cytoplasmic tail is responsible for the correct orientation of LMP1 in the plasma membrane but is dispensable for B-cell transformation. The six membrane-spanning domains promote the oligomerization of LMP1 molecules, a function necessary for the transduction of oncogenic signals from the C-terminal cytoplasmic portion of the protein. Two domains have been identified within the C-terminal cytoplasmic sequences of LMP1 as being important for B-lymphocyte growth transformation and phenotypic changes in a variety of cell types, CTAR1/TES1 and CTAR2/TES2 (26-28). CTAR1 (C-terminus activating region 1) comprises the membrane-most proximal 34 aa (aa 196 to 231) and contains a P204xQ206xT208D209 motif which serves as a docking site for adapter proteins of the tumor necrosis factor (TNF) receptor (TNFR)- associated factor (TRAF) family, such as TRAF1, TRAF2, TRAF3, and TRAF5 (3, 7, 8, 41). With the exception of TRAF1, which has rather restricted expression, TRAF proteins are widely expressed and regulate the function and signal transduction of a number of TNF receptor (TNFR) family members, such as CD40, CD30, TNFRI, and TNFRII (for reviews, see references 5 and 68). The CTAR2 domain of LMP1 comprises the extreme C-terminal 54 residues of the protein (aa 332 to 386) and recruits TNFR-associated death domain protein (TRADD) and receptor-interacting protein (RIP) (28, 29), two proteins initially identified by their ability to bind TNFRI. TRADD, in turn, may function as a platform for the recruitment of other molecules, such as TRAF2 and RIP. A putative CTAR3 region located between CTAR1 and CTAR2 has been described as a JAK3-interacting and STAT-activating domain (21), but these findings have been recently challenged by other investigators (2, 25).
FIG. 1.
Schematic representation of LMP1-based constructs used in this study. Two domains in the cytoplasmic C terminus of LMP1 are important for cell growth transformation: CTAR1/TES1 (aa 196 to 231), which contains a PxQxT motif and directly binds TRAF1, TRAF2, TRAF3, and TRAF5, and CTAR2/TES2 (aa 332 to 386), which interacts with TRADD and RIP. LMP1AxAxA has a triple PxQxT→AxAxA point mutation which abrogates TRAF binding to CTAR1 domain, and the LMP1/378STOP construct contains a STOP codon at aa 378 which abolishes the interaction of TRADD and RIP with CTAR2. LMP1AxAxA/378STOP contains a combined PxQxT→AxAxA and 378STOP mutation, while LMP1Δ[194-386] has the entire cytoplasmic tail of LMP1 deleted. LMP/CD40 is a hybrid molecule generated by the fusion of the N-terminal cytoplasmic tail and transmembrane domains of LMP1 with the cytoplasmic C- terminus of CD40 and functions as a constitutively active CD40 receptor (15, 23).
Many of the oncogenic effects of LMP1 can be explained by its ability to constitutively engage signaling pathways, such as the Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) and the transcription factor NF-κB, among others (for a review, see reference 16). As a result, LMP1 has been proposed to function as a constitutively active TNF family receptor (4, 18, 22, 23, 58). Activation of the NF-κB and p38 signaling pathways is mediated through both the CTAR1 and CTAR2 domains, with CTAR2 being the major effector, accounting for 60 to 80% of the total effects of LMP1 on NF-κB (13, 26, 39). TRAF2 modulates these signals by virtue of its ability to bind directly to CTAR1 and indirectly to CTAR2 via TRADD (13, 30). In contrast to NF-κB and p38, LMP1-mediated JNK/AP-1 activation in human embryonic kidney (HEK) 293 cells occurs solely through the CTAR2 domain of LMP1 (15, 32). The inability of CTAR1 to engage JNK signaling in these cells is intriguing and prompted us to investigate this phenomenon in more detail. We have found that expression of the TRAF-binding CTAR1 domain of LMP1 may result in JNK activation depending on the presence of TRAF1. Thus, CTAR1 engages JNK in TRAF1-positive cells but not in 293 cultures, where TRAF1 is not detectable. However, TRAF1 alone is unable to activate JNK, and its critical role in LMP1 signaling may involve modulation of TRAF2 function. These data further dissect the signaling components used by LMP1 and highlight the critical role of TRAF1 in the modulation of oncogenic signals engaged by this viral protein.
MATERIALS AND METHODS
Plasmids, cell lines, and culture media.
The pSG5-based LMP1 (B95.8 strain) expression constructs have been previously described (13). FLAG-tagged TRAF1Δ (TRAF1Δ[2-183]) (7) expressed from a pSG5 vector was a kind gift from G. Mosialos, A. Fleming Institute of Immunology, Athens, Greece. FLAG-tagged N-terminally deleted TRAF5 expression vector (57) was kindly provided by J. Inoue (Department of Oncology, University of Tokyo, Tokyo, Japan), and dominant negative TRAF2 (30) was kindly provided by E. Kieff (Harvard Medical School, Boston, Mass.). The absence of the N-terminal sequences in these TRAF constructs was confirmed by DNA sequencing in our laboratory. The hemagglutinin (HA)-tagged JNK1 (HA-p46SAPKγ-pcDNA3) expression vector was a gift from James Woodgett (The Ontario Cancer Institute, Ontario, Canada), and the AP-1 reporter construct pRTU14 was provided by Eike Floettmann (AstraZeneca R&D, Leicestershire, United Kingdom).
The human bladder carcinoma cell line EJ and the human embryonic kidney (HEK) 293 cell line were cultured in Dulbecco minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 2 mM glutamine. SVK is an immortalized but nontumorigenic human epithelial line which is maintained under conditions described previously (12). HEK 293 cells stably expressing ecdysone-regulatable LMP1 (293EcR/LMP1) and EJ cells carrying the tetracycline transactivator (EJ/Tet) have been previously described (12, 13). BJAB cells stably expressing tetracycline-regulatable LMP1 were kindly provided by Martin Rowe, University of Cardiff, Cardiff, United Kingdom (19). These cells, as well as the EBV-negative lymphoma cell lines DG75 and Ramos, were maintained in RPMI supplemented with 10% FCS (SBS Lty Ltd.) and 2 mM glutamine. COS-1 cells were cultured in DMEM supplemented with 5% FCS and 2 mM glutamine. All basic cell culture media were purchased from Gibco unless otherwise indicated.
Immunoblotting.
LMP1 expression was detected in 40 to 75 μg of total-cell lysates analyzed on a 10% gel by using the anti-LMP1 monoclonal antibodies CS1 to CS4 (51) and enhanced chemiluminescence (ECL; Amersham). For TRAF detection, the C20 anti-TRAF2 rabbit polyclonal and the H3 anti- TRAF1 mouse monoclonal antibodies from Santa-Cruz Biotechnology were used at 1:500 and 1:250 dilutions, respectively. Expression of FLAG-TRAF5 was assessed using an anti-FLAG monoclonal antibody. TRAF2 and TRAF5 were detected in 25 to 50 μg of total-protein extracts by using ECL (Amersham), and TRAF1 reactivity was analyzed in 75-μg protein extracts by using a sensitive Supersignal West Pico chemiluminescent substrate (Pierce). N-terminally deleted TRAF1Δ[1-185] was detected in 50 μg of total-cell lysate by using the S19 anti- TRAF1 rabbit polyclonal antibody (1:250 dilution), which is raised against an epitope at the C terminus of the protein (Santa Cruz Biotechnology). JNK phosphorylation was examined in 25-μg lysates, using the phospho-specific JNK (Thr183/Tyr185) antibody from Cell Signaling Technology. An antibody against β-actin was purchased from Sigma and used at a 1:1,000 dilution.
Transfections, reporter assays, and JNK in vitro kinase assays.
For transient transfections, 7 × 105 HEK 293 cells were plated out on a 60-mm dish and the following day were transfected using either Lipofectamine Plus (Invitrogen), as specified by the manufacturer, or calcium phosphate. COS-1 and SVK cells were transfected using a dextran method as previously described (12). BJAB and DG75 cultures (approximately 3 × 106 to 4 × 106 cells per transfection) were electroporated at 280 V and 950 μF. c-Jun reporter activity was measured using a two-vector system (Stratagene, La Jolla, Calif.) comprising a GAL4 DNA binding domain/c-Jun(1-233) chimaera (pFA2-cJun) which, upon JNK-mediated c-Jun phosphorylation, transactivates a luciferase reporter under the control of GAL4 binding elements (pFR-Luc). To determine c-Jun reporter activity, HEK 293 cells were transfected with 50 ng of pFA-cJun and 50 ng of pFR-Luc in the presence of 200 ng of Rous sarcoma virus-driven β-galactosidase-expressing plasmid and luciferase and β-gal values were measured 36 h posttransfection. For AP-1 reporter assays, 100 ng of the AP-1 reporter vector pRTU14 were transfected in 106 HEK 293 cells together with various effector plasmids and the activity was determined 36 h later in 50-μg lysates with parallel evaluation of the LMP1 and TRAF1 levels by immunoblotting. To evaluate the effects of LMP1 and LMP1 mutants on JNK activation, cell lines were transfected with optimized amounts of HA-JNK1 unless otherwise indicated: HEK 293 cells were transfected with 0.5 μg HA-JNK1, COS-1 and SVK cells were transfected with 1 μg of HA-JNK1, and BJAB and DG75 cells were electroporated with 8 μg plasmid DNA. The amounts of LMP1 vectors used (1 μg for HEK 293, SVK, and COS-1 cells and 8 μg for BJAB and Ramos cells) yielded similar levels of LMP1 expression across the cell lines used, with the exception of SVK, where higher levels of LMP1 were obtained. In all experiments, the total amount of DNA used was normalized to the larger amount by the addition of empty vector(s). In vitro kinase assays were carried out as previously described (13). Anti-HA immunoprecipitatates were analyzed for kinase activity by using glutathione S-transferase (GST)-c-Jun (aa 1 to 89) as a substrate and 100 μM cold ATP. Half of the kinase reaction was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide), blotted with an antibody which recognizes the phosphorylated c-Jun protein at Ser63 (Cell Signalling Technology), and reprobed for HA-JNK1 levels by using a goat polyclonal anti-JNK1 serum (Santa Cruz Biotechnology).
RNase protection assays.
RNA isolated from various cell lines was treated with RNase-free DNase for 30 min at 37°C and quantified by UV spectroscopy. Then 2 μg of total RNA was hybridised to33P- labeled antisense probes in vitro transcribed from 10 DNA templates containing human TRAF1, TRAF2, CART, I-TRAF, TRAF5, TRAF6, TRAF3, TRIP, and the L32 and glyceraldehyde-3-phosphate dehydrogerase (GAPDH) housekeeping genes, as specified by the manufacturer (Pharmingen; hAPO-5b multiprobe template set). Following treatment with RNase, protected fragments were analyzed by denaturing polyacrylamide gel electrophoresis in 0.5× Tris-borate-EDTA (TBE) and visualized on a phosphorimager.
LCM of NPC biopsy specimens and reverse Transcriptase PCR.
For laser capture microdissection (LCM) frozen NPC biopsy specimens were cut to uncoated RNase-free slides. After brief fixation in water-free alcohol and hematoxylin counterstaining, sections were thoroughly dehydrated and left to stand in xylene for 10 min. The slides were then air dried for a minimum of 10 min. Pure populations of NPC cells were isolated by microdissection using the PixCell II LCM system (Arcturus Engineering Inc.). Caps were screened microscopically after capture to ensure that nontumor cells had not been collected. RNA extraction was performed using the NucleoSpin RNA II kit (Macherey-Nagel) as specified by the manufacturer. RNA was subjected to cDNA synthesis using avian myeloblastosis virus reverse transcriptase (Roche) at 42°C for 1 h followed by PCR using avian myeloblastrosis virus buffer (Roche) and Red Hot Taq polymerase (Abgene). Synthesized cDNAs were PCR amplified for TRAF1 by using forward primer 5′-GCCACCTCTATCCACCAGA-3′ and reverse primer 5′- CTGGCCACGTTGGTTTCAC-3′ and 35 cycles of amplification steps comprising denaturation at 94°C for 45 s, annealing at 61°C for 30 s, and extension at 72°C for 50 s. For LMP1, 30 cycles of amplification steps comprising denaturation at 94°C for 30 s, annealing at 45°C for 1 min, and extension at 72°C for 1 min were performed using the forward primer 5′ CTTCAGAAGAGACCTTCTCT-3′ and the reverse primer 5′-ACAATGCCTGTCCGTGCAAA-3′. The LMP2a primers used were 5′-ATGACTCATCTCAACACATA-3′ and 5′- CATGTTAGGCAAATTGCAAA-3′. PCR products were analyzed on a 1.5% agarose gel, and following Southern transfer to Hybond N+ membranes (Amersham), they were hybridized using the32P-labeled oligonucleotide probes 5′-CTACTGATGATCACCCTCCT-3′ for LMP1 and 5′- CATGTTAGGCAAATTGCAAA-3′ for LMP2a. Primers and conditions for TRAF3 and GAPDH amplification have been previously described (12).
Immunohistochemical detection of TRAF1 and LMP1 in primary NPC biopsy specimens.
Expression of TRAF1 or LMP1 was determined by immunohistochemistry using the monoclonal antibodies H3 and CS1-4 respectively. Paraffin wax sections were deparaffinized and transferred to Tris-buffered saline (pH 7.6) (TBS). The demonstration of both antigens required microwave pretreatment in 0.01 M citrate buffer (pH 6.0) for 15 min. Following pretreatment, endogenous peroxidase activity was blocked in 0.3% hydrogen peroxide in methanol and sections were transferred to TBS. The sections were then incubated in the appropriate primary antibody. Bound primary antibodies were visualized using the peroxidase-based avidin biotin Duet system (Dako Corp.) followed by the demonstration of peroxidase activity using the standard diaminobenzidine (DAB) reaction. Sections of normal tonsil and samples from EBV-positive Hodgkin's disease patients were used as positive controls. Negative controls consisted of the replacement of primary antibodies by appropriate nonimmune serum.
RESULTS
The TRAF binding domain of LMP1 transduces JNK signals in a cell line-dependent manner.
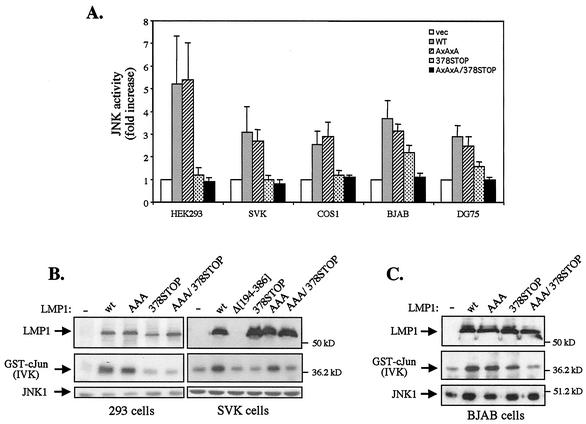
Previous studies have demonstrated that LMP1-induced JNK/AP-1 signaling occurs through the TRADD-interacting CTAR2 but not the TRAF binding CTAR1 region of LMP1 (11, 15, 32). Since these experiments were performed only with HEK 293 cells, we analyzed the relative contribution of these LMP1 domains to JNK activation in a broader panel of cell lines. To this end, LMP1 or mutated LMP1 sequences which lack functional CTAR1 (LMP1AxAxA), CTAR2 (LMP1/378STOP) or both regions (LMP1AxAxA/378STOP and LMP1Δ[194-386] [Fig. 1] were cotransfected with an HA-tagged JNK1 expression vector into epithelial (HEK 293 and SVK), lymphoid (DG75 and BJAB), or fibroblast (COS-1) cell lines. JNK activity was assessed in HA immunoprecipitates by in vitro kinase assays using GST-c-Jun (aa 1 to 89) as the substrate. The results of three independent experiments are summarized in Fig. 2A and confirm the inability of the TRAF binding domain of LMP1 to promote JNK-dependent c-Jun phosphorylation in the epithelial and fibroblast cell lines under study. Interestingly, however, expression of this LMP1 domain in the EBV-negative BJAB and DG75 lymphoma cell lines consistently induced JNK activation (Fig. 2A). The levels of CTAR1-mediated JNK activation in these cells were approximately equivalent to 40 and 30% of those induced by wild-type LMP1, respectively.
FIG. 2.
The TRAF binding domain of LMP1 activates the JNK pathway in a cell type- dependent manner. (A) Summary of data (means from triplicate determinations, with error bars indicating standard deviation) showing the effects of wild- type (WT) and mutated LMP1 sequences on JNK activation in transiently transfected HEK 293, SVK, COS-1, BJAB, and DG75 cells. JNK kinase assays were performed as described in Materials and Methods. vec, vector. (B) Representative in vitro kinase assays showing the effects of wild-type (wt) and mutated LMP1 sequences on JNK activation in human epithelial cells. HEK 293 and SVK cells were transiently transfected with HA-JNK1 and 1 μg of wild-type or mutated LMP1 constructs, and JNK activity was examined 30 h later by in vitro kinase assays (IVK) using GST-c-Jun (aa 1 to 89) as a substrate (middle panel). Anti-HA immunoprecipitates were blotted with an anti-JNK1 polyclonal antibody (bottom panel), and total-cell lysates were analyzed for LMP1 expression using the CS1 to CS3 monoclonal antibodies (top panel). (C) Representative in vitro kinase assay showing the effects of wild-type and mutated LMP1 sequences on JNK activation in EBV-negative BJAB human lymphoma cells. BJAB cells were electroporated with 8 μg of HA-JNK1 and 8 μg of wild-type or mutated LMP1 constructs, and JNK activity was examined 30 h later by in vitro kinase assays (IVK) using GST-c-Jun (aa 1 to 89) as a substrate (middle panel). JNK1 and LMP1 levels were monitored as in panel B.
Results of representative in vitro kinase assays from these experiments with epithelial and lymphoid cells are shown in Fig. 2B and C, respectively. Transfection of wild-type LMP1 or a mutated LMP1 carrying only CTAR2 (LMP1AxAxA) induced robust JNK activation in HEK 293, SVK, and BJAB cells. Expression of mutated LMP1 containing functional CTAR1 but not CTAR2 sequences (LMP1/378STOP) failed to promote c-Jun phosphorylation above background levels in HEK 293 and SVK cells but significantly induced JNK activation in BJAB cells (Fig. 2B and C). Taken together, these data demonstrate that expression of the TRAF binding domain of LMP1 engages the JNK pathway in a cell type-dependent manner.
CTAR1-mediated JNK activation occurs in cell lines that express TRAF1.
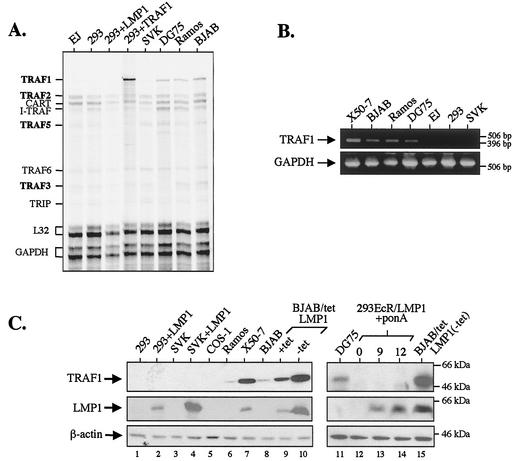
The ability of CTAR1 to induce JNK activation in some but not all cell lines tested prompted us to analyze them for the expression of molecules which are known to directly interact with this LMP1 domain, namely, TRAF1, TRAF2, TRAF3, and TRAF5. For this purpose, a multiprobe RNase protection assay was used to detect TRAF transcripts in total RNA isolated from the epithelial cell lines EJ, HEK 293, and SVK and the lymphoma cell lines Ramos, DG75, and BJAB. This analysis revealed that TRAF1 is barely detectable in cell lines that fail to activate the JNK pathway in response to LMP1 CTAR1 expression (Fig. 3A). However, TRAF1 was readily detected in RNA isolated from B-cell cultures as well as HEK 293 cells transfected with a TRAF1 expression vector which serves as a positive control. The RNA levels of other TRAF molecules which directly bind LMP1 CTAR1, such as TRAF2, TRAF3, and TRAF5, were similar across the panel of cell lines examined (Fig. 3A). The results of this RNase protection assay were confirmed by reverse transcriptase PCR using primers specific for TRAF1 (Fig. 3B, upper panel) or GAPDH as an internal control (lower panel). After 30 cycles of PCR amplification, TRAF1 mRNA was readily detected in RNA isolated from lymphoid cultures but was barely detectable in RNA isolated from the epithelial cell lines SVK, HEK 293, and EJ.
FIG. 3.
CTAR1-mediated JNK activation occurs in cell lines that express TRAF1. (A) RNase protection assay demonstrates similar basal levels of TRAF2, TRAF3, and TRAF5 but not TRAF1 RNA expression across a panel of lymphoid and epithelial cell lines. HEK 293 cells transfected with 1 μg TRAF1 were used as a positive control for TRAF1 expression; note that transfection of TRAF1 in these cells does not affect the endogenous levels of other TRAF molecules. (B) Reverse transcriptase PCR was performed to confirm the differential expression of TRAF1 RNA mRNA in a panel of lymphoid and epithelial cell lines. Primers specific for TRAF1 (top panel) or GAPDH (bottom panel) were used. Following 30 cycles of amplification, PCR products were electrophoresed on a 1.5% agarose gel, which was then stained with ethidium bromide and photographed. The molecular size markers are also shown. (C) Immunoblot analysis of total-cell lysates demonstrating levels of TRAF1 protein expressed in various cell lines in the presence or absence of LMP1 expression, as indicated.
Immunoblot analysis using a monoclonal antibody against TRAF1 was performed to evaluate the levels of TRAF1 protein expressed in lymphoid and epithelial cell lines and COS-1 fibroblasts (Fig. 3C, top panel). In these experiments, TRAF1 was detected in cell extracts isolated from the EBV-negative B-cell lines Ramos, DG75, and BJAB and the EBV-positive lymphoblastoid cell line X50-7 but was absent in lysates from the epithelial cell lines SVK and HEK 293 as well as in lysates from COS-1 fibroblasts. In addition, LMP1 expression promoted the induction of TRAF1 in some but not all cell lines tested. Thus, TRAF1 levels were markedly increased in BJAB cells carrying a tetracycline-regulatable LMP1 (BJAB/TetLMP1). These cultures were maintained in the presence of tetracycline to suppress LMP1 expression or in the absence of the antibiotic to induce LMP1. Since this inducible system is not always tightly regulated, some LMP1 was seen in the presence of tetracycline but expression was further induced on removal of the antibiotic (Fig. 3C, middle panel, lanes 8 to 10). Evaluation of the TRAF1 protein levels expressed in identical lysates demonstrated increased expression in the presence of tetracycline compared to that in control parental BJAB cells and a further induction when tetracycline was removed (upper panel, lanes 8 to 10). Unlike BJAB cells, transient transfection of HEK 293 or SVK cultures with LMP1 is unable to induce TRAF1 (Fig. 3C) but is known to promote the production of IL-6 and IL-8 (13, 14). This inability of LMP1 to up-regulate TRAF1 in epithelial cells was confirmed in experiments with HEK 293 clones stably expressing an ecdysone-inducible LMP1 (293EcR/LMP1 [13]). In these cells, a significant induction of LMP1 expression was seen at 8 and 12 h following addition of the ecdysone analogue ponasterone A, which promoted the up-regulation of COX-2, an LMP1-inducible gene (42), but not of TRAF1 (Fig. 3C, lanes 12 to 14, and data not shown). The same immunoblots were probed for β-actin, which serves as a loading control (Fig. 3C, bottom panel).
TRAF1 is not expressed in primary epithelial NPCs.
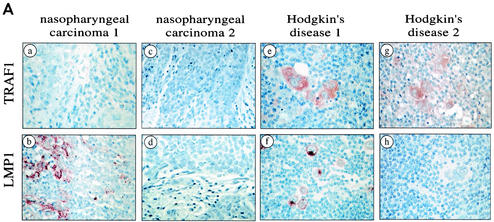
The absence of detectable basal TRAF1 levels in the established epithelial cell lines described above only may represent an in vitro phenomenon. To address this issue, the expression of TRAF1 was examined in primary biopsy specimens from patients with NPC, an EBV-associated epithelial malignancy, using immunohistochemistry. We analyzed 10 paraffin-embedded and 5 frozen NPC tumors. One of the paraffin-embedded and one of the frozen specimens stained positive for LMP1 using the monoclonal antibodies CS1 to CS4. As a positive control for TRAF1 immunoreactivity, representative biopsies from patients with Hodgkin's disease, a lymphoid malignancy where TRAF1 is commonly found (10, 43), were analyzed in parallel with the NPC specimens. This immunohistochemical analysis readily detected TRAF1 in the HD biopsy specimens but failed to detect the protein in any of the NPC specimens examined, irrespective of LMP1 status (Fig. 4A).
FIG. 4.
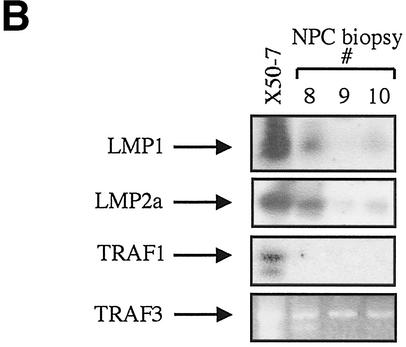
TRAF1 is not detectable in primary NPC biopsy specimens. (A) Representative immunohistochemical staining of biopsy specimens taken from patients with NPC (a to d) or Hodgkin's disease (e and h). TRAF1 was detected in the Hodgkin's disease specimens (e and g, red staining) but not in the NPC biopsy specimens (a and c). Note that normal lymphocytes also stain weakly for TRAF1 (e and g). One representative Hodgkin's disease specimen and one of the NPC specimens shown were also positive for LMP1 (b and f). (B) TRAF1 is not detected in RNA from NPC cells. Tumor cells were selected from laser capture microdissected NPC biopsy specimens, and RNA was isolated and reverse transcribed. The cDNAs were then subjected to PCR using primers specific for LMP1, LMP2a, TRAF1 and TRAF3, as indicated. RNA extracted from the lymphoblastoid cell line X50-7 was used as a positive control. Representative PCR amplifications from three biopsy specimens (specimens 8, 9, and 10) are shown.
The absence of detectable TRAF1 protein in NPC biopsy specimens was confirmed at the RNA level. Malignant cells were selectively retrieved from the tumor mass of the frozen NPC biopsy specimens by LCM. The quality of the isolated RNA was confirmed by cDNA synthesis and subsequent PCR amplification of the GAPDH housekeeping gene (data not shown). Equal amounts of synthesized cDNAs were then analyzed for expression of TRAF1, TRAF3, and the EBV-encoded LMP1 and LMP2a by PCR (Fig. 4B and data not shown). To increase the sensitivity of the TRAF1, LMP1, and LMP2a detection, the amplified samples were Southern blotted and probed with32P-labeled TRAF1-, LMP1- or LMP2a-specific oligonucleotides respectively. LMP2a amplification was observed to different degrees in all RNAs from NPC patients. LMP1 mRNA was strongly detected in the frozen NPC biopsy which stained positive for the protein, and weak LMP1 RNA expression was seen in one additional specimen (specimens 8 and 10, respectively, in Fig. 4B). TRAF1 was absent in LCM-isolated NPC cells but was detected in the EBV-positive lymphoma line X50-7 which serves as a positive control (Fig. 4B). Unlike TRAF1, TRAF3 was strongly expressed in all RNAs from NPC patients. These findings strengthen the results of the immunohistochemical examination of a larger number of specimens and demonstrate the absence of TRAF1 in NPC. Thus, the established epithelial cell lines under study mirror primary tumors with respect to the absence of TRAF1 expression.
TRAF1 is a critical modulator of JNK signaling by the TRAF binding domain of LMP1.
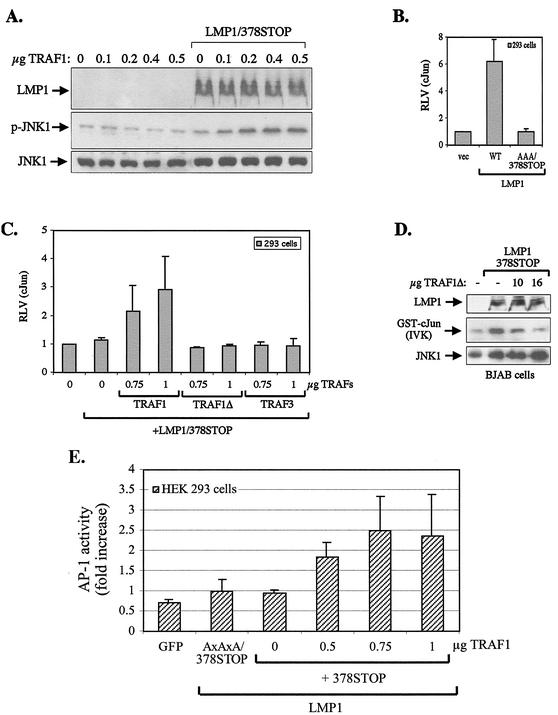
The results described above reveal a correlation between the expression of TRAF1 and the ability of the TRAF binding domain of LMP1 to engage signaling on the JNK axis; they indicate that TRAF1 may be required for CTAR1-mediated JNK activation. To verify or refute this hypothesis, we first examined whether reconstitution of TRAF1 in HEK 293 cells which do not express detectable TRAF1 (Fig. 3) enables CTAR1 to signal for JNK activation. To this end, HEK 293 cultures were transiently transfected with a CTAR1 effector (LMP1/378STOP) plasmid and HA-JNK1 in the presence or absence of a wild-type TRAF1-expression vector, and JNK activity was assessed by immunoblotting using an antibody specific for the phosphorylated, active form of JNK. As shown in Fig. 5A, transfection of TRAF1 or LMP1/378STOP alone had essentially no effect on JNK activation. However, coexpression of these molecules promoted a significant increase in JNK phosphorylation above background levels. In these experiments, the levels of LMP1 and anti-HA-immunoprecipitated JNK1 were also monitored by immunoblot analysis (Fig. 5A).
FIG. 5.
TRAF1 plays a critical role in LMP1 CTAR1-induced JNK signaling. (A) Reconstitution of TRAF1 in HEK 293 cells promotes CTAR1-mediated JNK activation. HEK 293 cells were transfected with 1 μg of HA-JNK1 and 0.4 μg of pSG5-LMP1/378STOP plasmid in the presence or absence of increasing amounts of pSG5-TRAF1, as indicated. At 16 h posttransfection, lysates were analyzed for JNK phosphorylation and LMP1 and HA-JNK expression by immunoblot assay. (B) Evaluation of the ability of LMP1 to promote JNK-dependent transactivation of a c-Jun reporter system. HEK 293 cells were transiently transfected with 50 ng each of a GAL4 DNA binding domain c-Jun 1 (aa 1 to 233) chimaera (pFA2-cJun) and a luciferase reporter under the control of GAL4 binding elements (pFR-Luc), 200 ng of Rous sarcoma virus-driven β-galactosidase plasmid, and 1 μg of pSG5-LMP1, pSG5-LMP1AxAxA/378STOP, or control vector. At 36 h later, luciferase and β-gal activities were measured, and the results (mean and standard deviation from three independent experiments) are depicted as the ratio of the two measurements (relative luciferase values [RLV]). (C) CTAR1 activates the JNK pathway in vivo only in the presence of TRAF1. HEK 293 cells were transfected with reporter constructs as described in panel B and 1 μg of pSG5-LMP1/378STOP or control vector, in the presence or absence of increasing amounts (0, 0.75, or 1 μg) of pSG5-TRAF1, pSG5TRAF1Δ (TRAF1 with aa 1 to 185 deleted), or pSG5-TRAF3. The relative luciferase values (RLV) were calculated 36 hs later, as described for panel B. Data (mean and standard deviation from six independent experiments on the effects of wild-type TRAF1 and from three independent experiments on the effects of TRAF1Δ and TRAF3 on CTAR1-induced c-Jun transactivation) are presented. (D) N-terminally deleted TRAF1 (TRAF1Δ) functions as a dominant negative inhibitor of CTAR1-induced JNK signaling in BJAB cells. BJAB B-cell lymphoma cultures were electroporated with 8 μg of HA-JNK1, 8 μg of pSG5-LMP1/378STOP, and increasing amounts (10 or 16 μg) of pSG5-TRAF1Δ, and JNK activity was assessed in anti-HA immunoprecipitates by in vitro kinase (IVK) assays using GST-c-Jun (aa 1 to 89) as a substrate (middle panel). The levels of LMP1 and anti-HA immunoprecipitated JNK1 were monitored by immunoblot analysis (top and bottom panels). The data shown are representative of three independent experiments. (E) Reporter assays showing the effects of various amounts of TRAF1 (0, 0.5, 0.75, or 1 μg) on CTAR1-induced AP-1 activity.
To confirm that TRAF1 plays a critical role in CTAR1-mediated JNK signaling in vivo, a c-Jun reporter system (PathDetect; Stratagene) was used to transfect HEK 293 cells. This system comprises a c-Jun (aa 1 to 233)/GAL4 DNA binding domain chimera which, when phosphorylated by endogenous JNK, transactivates a multiple GAL4 binding element-containing luciferase reporter plasmid. Using this system, transfection of 1 μg of LMP1 was found to induce a 6.2- ± 1.6-fold induction of endogenous JNK activity compared to vector-transfected HEK 293 cells, an effect which was abolished when the TRADD -binding region of CTAR2 was rendered non-functional (LMP1/378STOP and LMP1AxAxA/378STOP mutants (Fig. 5B and C). This finding confirms the reported inability of CTAR1 to activate JNK in HEK 293 cells (11, 15, 32). Interestingly, however, a significant 2- to 2.8-fold increase in c-Jun transactivation was observed when CTAR1 was coexpressed with TRAF1 (Fig. 5C). This synergistic effect was not seen when a mutated TRAF1 molecule with aa 2 to 183 deleted (TRAF1Δ) was cotransfected with LMP1/378STOP, suggesting that N-terminal TRAF1 sequences play a critical role in its ability to modulate CTAR1-induced JNK activation (Fig. 5C). This deleted TRAF1 molecule is similar to wild-type TRAF1 in binding to LMP1 CTAR1 (7). The specificity of the TRAF1 effect is further underlined by the inability of TRAF3 to promote the induction of JNK when coexpressed with LMP1/378STOP (Fig. 5C). Like TRAF1, TRAF3 directly binds LMP1 CTAR1 and lacks NF-κB- and MAPK-inducing capacity when overexpressed. As an additional control, TRAF1 expression in HEK 293 cells did not affect c-Jun transactivation by LMP1AxAxA or LMP1AxAxA/378STOP, the LMP1 mutants with a deficient CTAR1 domain (data not shown).
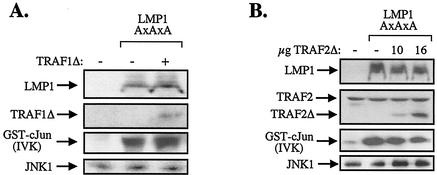
On the basis of the data described in Fig. 5C, we examined the possibility that the N-terminally deleted TRAF1 mutant (TRAF1Δ) may function as a dominant negative inhibitor of CTAR1-induced JNK signaling in BJAB cells, which naturally express TRAF1. To this end, exponentially growing BJAB cultures were cotransfected with a CTAR1-expressing plasmid (LMP1/378STOP) in the presence or absence of N-terminally deleted TRAF1 (TRAF1Δ) and HA-JNK1. At 36 h later, the cells were lysed and JNK activity was assessed in anti-HA immunoprecipitates by in vitro kinase assays using GST-c-Jun (aa 1 to 89) as a substrate. TRAF1Δ was found to exert a dramatic inhibitory effect on CTAR1-induced JNK activation in a concentration-dependent manner (Fig. 5D).
JNK activation is an essential step for the induction of the transcription factor AP-1. To confirm that the effects of TRAF1 on LMP1 CTAR1-induced JNK signaling translate to AP-1 activation, reporter assays were performed with HEK 293 cells transfected with the CTAR1-expressing plasmid LMP1/378STOP in the presence or absence of TRAF1. CTAR1 alone did not activate AP-1 above background levels, as determined by transfection of a green fluorescent protein or an LMP1AxAxA/378STOP expression vector. However, a significant increase in AP-1 activity was noted when CTAR1 was coexpressed with TRAF1 (Fig. 5E), consistent with the findings obtained using c-Jun reporter assays. Taken together, these data suggest that TRAF1 is required for JNK signaling by the TRAF binding CTAR1 domain of LMP1.
TRAF1 is not critical for CD40-induced JNK activation.
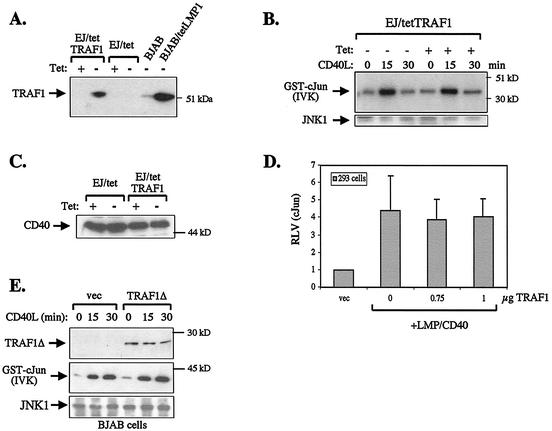
Since the cytoplasmic tail of CD40 contains a PxQxT motif similar to that found in the CTAR1 domain of LMP1, we examined the possibility that TRAF1 may also influence CD40-induced JNK signaling. To this end, EJ carcinoma cells stably expressing a tetracycline-regulatable TRAF1 (EJ/tetTRAF1, clone 5) were cultured in the presence of tetracycline to suppress TRAF1 expression or in the absence of the antibiotic to induce the protein. The levels of TRAF1 expressed in these cultures were evaluated by immunoblot analysis using EJ cells that carry only the tetracycline transactivator (EJ/tet) as a negative control (Fig. 6A). As a positive control for TRAF1 expression, cell lysates from BJAB and BJAB/tet LMP1 cultures were used.
FIG. 6.
TRAF1 does not play a critical role in CD40-induced JNK signaling. (A) Characterization of a tetracycline-regulatable TRAF1 in EJ bladder carcinoma cells (EJ/tetTRAF1). In the absence of tetracycline (tet), TRAF1 is induced. Lysates from BJAB cultures serve as a positive control for the detection of TRAF1 by immunoblot analysis. (B) Tetracycline-regulated induction of TRAF1 does not modify CD40-transduced JNK signals. EJ/tetTRAF1 cells were incubated for 36 h in the presence of tetracycline to suppress TRAF1 expression or in the absence of the antibiotic to induce TRAF1 and then stimulated with 0.5 μg of recombinant CD40 ligand (CD40L) per ml for 0, 15, or 30 min. Endogenous JNK1 was immunoprecipitated from cell lysates and assessed for kinase activity. Data are representative of two independent experiments. (C) TRAF1 induction does not alter the levels of CD40 expressed in EJ cells, as determined by immunoblot analysis using a polyclonal anti-CD40 antibody (C20; Santa Cruz Biotechnology). (D) Expression of TRAF1 in HEK 293 cells does not modify the ability of an LMP1/CD40 chimera to induce JNK-dependent c-Jun transactivation. Cells were cotransfected with 1 μg of pSG5-LMP1/CD40 in the presence of increasing amounts (0, 0.75, or 1 μg) of TRAF1 and reporter constructs as described in the legend to Fig. 5B. The relative levels of c-Jun transactivation from three independent experiments (± standard deviation) are shown. (E) TRAF1Δ does not inhibit CD40-mediated JNK activation in TRAF1-positive cells. BJAB cells were transfected with 8 μg of HA-JNK1 in the presence or absence of 13 μg of TRAF1Δ and 30 h later were either left untreated or stimulated with 0.5 μg of recombinant soluble CD40L per ml for 15 or 30 min. Lysates from these cultures were analyzed for TRAF1Δ expression (top panel) or JNK activity (middle panel). Following analysis of c-Jun phosphorylation by in vitro kinase assays (IVK), the blot was probed for immunoprecipitated HA-JNK by using a goat polyclonal anti- JNK1 antibody (bottom panel).
Having established that EJ/tetTRAF1 cells express TRAF1 only following tetracycline removal, we proceeded to determine whether the TRAF1 status may influence CD40-mediated JNK activation. To this end, EJ/tetTRAF1 cells were either incubated for 36 h with tetracycline or left untreated and then stimulated with recombinant soluble CD40 ligand (CD40L) for various time intervals (0, 15, or 30 min) before being analyzed for endogenous JNK activity by in vitro kinase assays. CD40 engagement in these cells was found to induce JNK activation independently of TRAF1 status and with identical kinetics (Fig. 6B). The expression levels of CD40 in EJ/tet and EJ/tetTRAF1 cells cultured with or without tetracycline were assessed by immunoblotting using a rabbit polyclonal antibody raised against the cytoplasmic C terminus of CD40. This assay confirmed that induction of TRAF1 does not affect CD40 expression and that the inability of TRAF1 to modulate CD40-induced JNK signaling is not due to altered CD40 levels (Fig. 6C).
In a second set of experiments, TRAF1 was expressed in CD40- and TRAF1-negative HEK 293 cells together with an LMP1/CD40 hybrid molecule (15) (Fig. 1). Endogenous JNK activity was evaluated by the c-Jun reporter system. By virtue of its LMP1 transmembrane domains, the LMP/CD40 chimera constitutively activates the JNK pathway when expressed in HEK 293 cells, and this effect was not influenced by the presence of coexpressed TRAF1 (Fig. 6D).
The preceding data demonstrate that overexpression of TRAF1 in TRAF1-negative cell lines does not influence CD40L-induced JNK activation, suggesting a nonessential role for TRAF1 in this CD40-transduced signaling pathway. To determine whether TRAF1 may still contribute to these signals, we examined the effects of transiently transfected TRAF1Δ on CD40L-induced JNK activation in BJAB cells which naturally express TRAF1. Lysates from transfected cells were harvested before and 15 or 30 min after stimulation with 0.5 μg of rsCD40L per ml. Immunoprecipitated HA-JNK1 that was coexpressed in these cultures was analyzed for activity using GST-c-Jun (aa 1 to 89) as a substrate. CD40 ligation promoted the activation of JNK in TRAF1Δ-transfected cells with kinetics and potency similar to those observed in vector-transfected cultures. Taken together, the above data demonstrate that TRAF1 does not influence CD40-transduced signals leading to JNK activation.
LMP1 CTAR1-induced JNK activation occurs via a TRAF2-dependent pathway.
TRAF1 does not possess catalytic activity and, when overexpressed, does not activate the JNK pathway (44) (Fig. 5A). It is therefore likely that the observed contribution of TRAF1 to CTAR1-induced JNK signaling is indirect and occurs through modulation of the function of another TRAF protein. TRAF2 is a primary candidate since its overexpression is known to result in JNK activation and dominant-negative or knockout approaches have demonstrated its essential role in CD40 and TNF-induced JNK signaling (35, 44, 65).
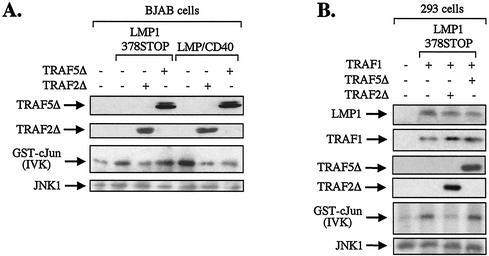
To determine whether TRAF2 is involved in CTAR1-induced JNK activation, BJAB cells were cotransfected with HA-JNK1 and LMP1/378STOP in the presence or absence of a dominant negative N-terminally deleted TRAF2 mutant (TRAF2Δ). Total cell lysates were isolated 36 h posttransfection, and JNK activity was assessed in anti-HA immunoprecipitates by using GST-c-Jun (aa 1 to 89) as a substrate. These experiments consistently showed that the ability of CTAR1 to promote JNK activation is abolished in the presence of dominant negative TRAF2 and demonstrate the critical role of this TRAF molecule in CTAR1-mediated signals (Fig. 7A). This effect was not the nonspecific result of ectopic expression of dominant negative TRAF mutants, since N-terminally deleted TRAF5 (TRAF5Δ) had no effect on CTAR1-induced JNK activation (Fig. 7A, Fourth lane from left). However, both TRAF2Δ and TRAF5Δ negatively influenced CD40-mediated JNK activation. Thus, expression of LMP/CD40 chimera in BJAB cells was found to induce robust JNK activity, which was suppressed by TRAF2Δ and, to a lesser extent, by TRAF5Δ (Fig. 7A, three right lanes), consistent with the established role for TRAF2 and TRAF5 in CD40-mediated JNK signaling.
FIG. 7.
N-terminally deleted TRAF2 (TRAF2Δ) functions as a dominant negative inhibitor of JNK activation by the CTAR1 domain of LMP1. (A) TRAF2Δ abolishes CTAR1-induced JNK signaling in BJAB cells. BJAB B-cell lymphoma cultures were electroporated with 8 μg of HA-JNK1, 8 μg of pSG5-LMP1/378STOP, and 10 μg of either TRAF2Δ or TRAF5Δ. JNK activity was assessed in anti-HA immunoprecipitates by in vitro kinase (IVK) assays using GST-c-Jun (aa 1 to 89) as a substrate. As a control, BJAB cells were transfected with 8 μg of pSG5-LMP/CD40 chimera instead of LMP1/378STOP, to confirm that both TRAF2Δ and TRAF5Δ are functional. The levels of TRAF2Δ, TRAF5Δ, and anti-HA- immunoprecipitated JNK1 were monitored by immunoblot analysis as indicated. Data shown are representative of three independent experiments. In these experiments, TRAF2Δ consistently induced more than 85% inhibition in CTAR1-mediated JNK activation. (B) TRAF2Δ abolishes CTAR1-induced JNK signaling in HEK 293 cells transfected with TRAF1. HEK 293 cultures were cotransfected with 0.5 μg of HA-JNK1, 1 μg of pSG5-LMP1/378STOP, and 1 μg of pSG5-TRAF1, in the presence or absence of either TRAF2Δ or TRAF5Δ. JNK activity was assessed in anti-HA immunoprecipitates by in vitro kinase (IVK) assays using GST-c-Jun (aa 1 to 89) as a substrate. The levels of LMP1, TRAF1, TRAF2Δ, TRAF5Δ, and anti-HA-immunoprecipitated JNK1 were monitored by immunoblot analysis as indicated. Data shown are representative of two independent experiments.
The effects of mutated TRAF2 and TRAF5 on CTAR1-induced JNK activation were also analyzed by using HEK 293 cells reconstituted for TRAF1 expression. In these experiments, HEK 293 cultures were transfected with TRAF1 and the CTAR1 effector LMP1/378STOP in the absence or presence of equivalent amounts of TRAF2Δ or TRAF5Δ expression vectors. The levels of LMP1, TRAF1, TRAF2Δ, and TRAF5Δ were monitored by immunoblot analysis (Fig. 7B). Biochemical evaluation of JNK activity in lysates isolated from these cultures confirmed that TRAF2 but not TRAF5 is a component of CTAR1-induced JNK signaling, inasmuch as cotransfected TRAF2Δ but not TRAF5Δ abolished the ability of CTAR1 to transduce JNK signals in the presence of TRAF1 (Fig. 7B).
LMP1 CTAR2 activates JNK in lymphoid cells via a pathway that is independent of TRAF1 and partially dependent on TRAF2.
Our previous data suggest that TRAF2 contributes to JNK signals emanating from the CTAR2 domain of LMP1 in HEK 293 cells (11). To examine whether CTAR2-induced JNK is influenced by TRAF1, BJAB cells were cotransfected with HA-JNK1 and the CTAR2 effector LMP1AxAxA in the presence or absence of TRAF1Δ. In vitro JNK assays were performed and demonstrated that TRAF1Δ concentrations that abolish CTAR1-mediated signaling (Fig. 5D) did not affect CTAR2-induced JNK activation (Fig. 8A).
FIG. 8.
The TRADD-interacting CTAR2 domain of LMP1 activates JNK in a TRAF2- dependent but TRAF1-independent manner. (A) N-terminally deleted TRAF1 (TRAF1Δ) does not inhibit CTAR2-induced JNK signaling in BJAB cells. BJAB B-cell lymphoma cultures were electroporated with 8 μg of HA-JNK1 and 8 μg of pSG5-LMP1(AxAxA) in the presence or absence of 16 μg of pSG5-TRAF1Δ. JNK activity was assessed in anti-HA immunoprecipitates by in vitro kinase (IVK) assays using GST-c-Jun (aa 1 to 89) as a substrate. The levels of TRAF1Δ, LMP1, and anti-HA immunoprecipitated JNK1 were monitored by immunoblot analysis. Data shown are representative of two independent experiments. (B) TRAF2Δ partially suppresses CTAR2-induced JNK signaling in BJAB cells. BJAB B-cell lymphoma cultures were cotransfected with 8 μg of HA-JNK1, 8 μg of pSG5-LMP1AxAxA, and increasing amounts (10 or 16 μg) of pcDNA3-TRAF2Δ, and JNK activity was assessed as for panel A. The levels of LMP1 and anti-HA-immunoprecipitated JNK1 were monitored by immunoblot analysis as indicated. Expression of transfected TRAF2Δ and of endogenous TRAF2 was determined by immunoblot analysis using the C20 polyclonal antibody from Santa-Cruz Biotechnology. Data are representative of three independent experiments.
To determine whether CTAR2-induced JNK is influenced by TRAF2 in TRAF1-positive cells, BJAB cultures were transfected with HA-JNK1 and LMP1AxAxA in the presence or absence of increasing amounts of dominant negative TRAF2. Expression of LMP1AxAxA alone induced robust JNK activity as measured by in vitro phosphorylation of GST-c-Jun (aa 1 to 89), and this effect was reduced but not abolished in the presence of cotransfected TRAF2Δ (Fig. 8B). Expression of anti-HA-immunoprecipitated JNK1, LMP1, and TRAF2Δ was determined by immunoblot analysis (Fig. 8B). Taken together, these data suggest that CTAR2 activates JNK via a pathway that is independent of TRAF1 and partially dependent on TRAF2.
DISCUSSION
The EBV-encoded LMP1 has structural features and functions reminiscent of a constitutively active TNF family receptor. LMP1 recruits TRAFs and TRADD through two distinct domains in its cytoplasmic C terminus, namely, CTAR1/TES1 and CTAR2/TES2 (Fig. 1), and engages downstream signaling in a ligand-independent manner. The constitutive engagement of NF-κB and JNK/AP-1 signals is of particular interest, taking into account the contribution of these pathways to oncogenesis. A number of published reports support the notion that signals transduced by CTAR1 and CTAR2 cooperate to promote the transformed phenotype and indicate that the relative input of these domains to LMP1-induced transformation may be influenced by the intracellular milieu (8, 31, 40, 61).
In this study, we have confirmed and extended previous observations concerning the cell type-specific expression of TRAF1, an adapter protein which interacts directly with the CTAR1 domain of LMP1. Using RNase protection assays and reverse transcriptase PCR, we have found that TRAF1 is barely detectable in the HEK 293, EJ, and SVK epithelial cell lines but is readily detected in RNA isolated from B-cell lymphoma cultures. Immunoblot analysis using the H3 anti-TRAF1 monoclonal antibody confirmed these observations at the protein level. Other investigators have reached similar conclusions by using different anti-TRAF1 antibodies (8, 67). An extensive analysis of TRAF expression in a large number of tumor cell lines demonstrated that TRAF1 is absent from most cells of epithelial origin but is widely expressed in B-cell lymphomas (67). In addition, we have demonstrated that TRAF1 is undetected in biopsy specimens from patients with NPC, an EBV-associated malignancy (Fig. 4). The mechanism of TRAF1 regulation is still unclear but has been proposed to involve NF-κB-dependent transcriptional activation. Thus, TRAF1 is up-regulated following long-term exposure to mitogenic stimuli which activate the NF-κB pathway (53). However, the inability of LMP1 to induce TRAF1 in certain epithelial cell lines points to the requirement of other, as yet unidentified factors which may be critical for its regulation at the transcriptional and/or posttranscriptional level. Alternatively, it is possible that a particular subset of NF-κB subunits is necessary for the transactivation of TRAF1. In support of these possibilities, Devergne et al. have demonstrated that CTAR1 is solely responsible for the ability of LMP1 to up-regulate TRAF1 in BJAB lymphoma cells, despite the more potent effects of CTAR2 on NF-κB activation (8). Taken together, these data suggest that there are considerable differences in the expression of TRAF1 and its inducibility in established cell lines. What could be the significance of this observation for the signaling potential and function of proteins that recruit TRAF1?
While the functional significance of constitutively elevated levels of TRAF1 remains to be fully evaluated, recent studies support a role for this protein in lymphomagenesis. Thus, targeted expression of TRAF1 in the T-cell compartment of transgenic mice promotes resistance to antigen-induced apoptosis in vitro and in vivo (54). The antiapoptotic nature of TRAF1 is further emphasized by the observation that TNFRI-transduced death signals are suppressed in HT1080 fibrosarcoma cells transfected with TRAF1 (59). The physiological role of TRAF1 has been recently addressed in TRAF1 knockout mice. T cells isolated from these mice respond to TNFRII stimulation by enhanced proliferation and signaling (56). However, unlike the negative effects of TRAF1 on TNFRII function, signaling from the Hodgkin's lymphoma cell surface marker CD30 is positively regulated by TRAF1 (9). Taken together, these data suggest that TRAF1 differentially affects receptor-transduced signals and controls critical proliferative and antiapoptotic functions that may contribute to lymphomagenesis. Consistent with this notion, elevated levels of TRAF1 have been observed in many lymphoid malignancies, including Hodgkin's disease, where the transforming EBV-encoded LMP1 is frequently expressed (10, 37, 43).
In vitro evidence suggests that TRAF1 can affect LMP1 signaling by augmenting CTAR1-induced NF-κB activation (7). In the present study, we have extended this finding and described a novel role for TRAF1 as a critical modulator of LMP1-induced JNK activation, an essential step for the induction of the transcription factor AP-1. Thus, we have shown that the ability of the TRAF binding CTAR1 domain of LMP1 to signal on the JNK axis correlates with the TRAF1 status of the cell line under consideration. Indeed, expression of a CTAR1-effector construct activates JNK only in cells that express TRAF1. Importantly, reconstitution of TRAF1 in cell lines lacking detectable levels of this adapter protein enables CTAR1 to engage the JNK pathway, resulting in AP-1 transactivation (Fig. 5). The synergistic effect of TRAF1 on CTAR1-induced JNK activation is specific for TRAF1, since coexpression of an N-terminally deleted TRAF1 mutant or of wild-type TRAF3 is unable to promote CTAR1-mediated JNK activation in 293 cells. Furthermore, it is specific for the CTAR1 domain of LMP1, since TRAF1 fails to augment LMP1 CTAR2 or CD40-mediated JNK activation. The latter finding is in agreement with recently published data demonstrating similar kinetics and potency of AP-1 activation in CD40-stimulated B cells isolated from TRAF1+/+ and TRAF1−/− mice (56). Taken together, these data point to a specific critical role for TRAF1 in JNK signals transduced by the major transformation effector site of LMP1.
TRAF1 physically interacts with various MAPK kinase kinases (MAPKKK), such as NIK, Ask1 and MEKK1 but fails to activate them. However, TRAF1 may function as a modulator of signals transduced by other TRAFs, such as TRAF2, TRAF5, and TRAF6, which not only interact with these MAPKKKs but also promote their activation. The involvement of TRAF2 in LMP1-induced JNK signaling is highlighted by the specific inhibitory effects of dominant-negative TRAF2 on CTAR1- and CTAR2-induced JNK activation (Fig. 7 and 8). However, whilst dominant-negative TRAF2 completely inhibited CTAR1-induced JNK, it had only a partial effect on CTAR2-mediated JNK activation, suggesting a complementary role for other molecules in the regulation of CTAR2 signaling.
The ability of both CD40 and LMP1 CTAR2 to activate JNK in cell lines lacking detectable TRAF1 indicates that TRAF1 is not critical for these activities. Furthermore, we have demonstrated that exogenous expression of TRAF1 or TRAF1Δ does not influence CD40 and LMP1 CTAR2-mediated JNK signaling. Given the role of TRAF2 in CD40, LMP1 CTAR1- and LMP1 CTAR2-induced JNK activation, it is intriguing that TRAF1 is exclusively required by CTAR1 to transduce TRAF2-dependent JNK signals. However, significant differences exist in the primary structure and molecular components used by these molecules. Thus, while TRAF1 directly binds CTAR1 (7), it associates indirectly with CD40 and, presumably, with the CTAR2-TRADD complex via TRAF2 (36, 50). Furthermore, TRAF2 strongly binds the N terminus of TRADD (46, 55) and a PxQxT motif in the CD40 cytoplasmic tail (50) but only weakly interacts with a PxQxxD motif in LMP1 CTAR1 (7, 52, 63, 64). It is therefore possible that TRAF1 may influence the affinity or avidity of TRAF2 for CTAR1. Alternatively, TRAF1 may promote an LMP1 CTAR1 conformational change necessary to recruit appropriate MAPKKKs to the TRAF2 “signalosome” or to modify the structure of this multiprotein complex, allowing recruitment and activation of SEK, the JNK kinase. Consistent with these possibilities, the conformation of LMP1 has been proposed to regulate its association with TRAF2 (52). Interestingly, recent crystallographic data demonstrate that the TRAF-binding domain of CD40 assumes different conformations when bound to different TRAF family members (45). Such “molecular adaptations,” influenced by the relative levels of TRAFs in a cell type-dependent manner, may in turn affect TRAF-dependent signaling and function. This may be particularly important for the oncogenic effects of LMP1 in different tissues and the relative input of CTAR1-transduced signals to these effects.
Acknowledgments
We are grateful to G. Mosialos, M. Rowe, E. Kieff, J. Woodgett, and J. Inoue for providing reagents and to Liz Hodgkins for expert technical assistance. We also thank Louise Laverick for help in developing EJ cells expressing the tetracycline-inducible TRAF1.
This work was supported by a Medical Research Council (MRC, UK) Career Development Award to A.G.E. and by a Cancer Research UK grant (SP2584) to A.G.E. and L.S.Y.
REFERENCES
- 1.Arvanitakis, L., N. Yaseen, and S. Sharma. 1995. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-beta 1-mediated growth inhibition in EBV-positive B cells. J. Immunol. 155:1047-1056. [PubMed] [Google Scholar]
- 2.Brennan, P., J. E. Floettmann, A. Mehl, M. Jones, and M. Rowe. 2001. Mechanism of action of a novel latent membrane protein-1 dominant negative. J. Biol. Chem. 276:1195-1203. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur, S. R., G. Cheng, D. Baltimore, and D. A. Thorley-Lawson. 1997. Localization of the major NF-κB-activating site and the sole TRAF3 binding site of LMP1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777-19784. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. D., B. S. Hostager, and G. A. Bishop. 2001. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med. 193:943-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, C. W., A. B. Rickinson, and L.S. Young. 1990. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature (London) 344:777-780. [DOI] [PubMed] [Google Scholar]
- 7.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. Kleijner, E. Kieff, and G. Mosialos. 1996. TRAF1, TRAF2 and TRAF3 effect NF-κB activation by an Epstein- Barr virus LMP1 domain important for B lymphocyte transformation. Mol. Cell. Biol. 16:7098-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devergne, O., E. C. McFarland, G. Mosialos, K. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duckett, C. S., R. W. Gedrich, M. C. Gilfillan, and C. B. Thompson. 1997. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol. Cell. Biol. 17:1535-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durkop, H., H. D. Foss, G. Demel, H. Klotzbach, C. Hahn, and H. Stein. 1999. Tumor necrosis factor receptor-associated factor 1 is overexpressed in Reed-Sternberg cells of Hodgkin's disease and Epstein-Barr virus-transformed lymphoid cells. Blood 93:617-623. [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., S. M. S. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos, A. G., C. W. Dawson, G. Mosialos, J. E. Floettmann, M. Rowe, R. J. Armitage, J. Dawson, J. M. Zapata, D. J. Kerr, M. J. O. Wakelam, J. C. Reed, E. Kieff, and L. S. Young. 1996. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 13:2243-2254. [PubMed] [Google Scholar]
- 13.Eliopoulos, A. G., N. J. Gallagher, S. M. S. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 MAPK pathway by Epstein-Barr virus encoded latent membrane protein 1 (LMP1) co-regulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene 14:2899-2916. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., and L. S. Young. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731-1742. [DOI] [PubMed] [Google Scholar]
- 16.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435-444. [DOI] [PubMed] [Google Scholar]
- 17.Farrell, P. J. 1995. Epstein-Barr virus immortalizing genes. Trends Microbiol. 3:105-109. [DOI] [PubMed] [Google Scholar]
- 18.Floettmann, J. E., A. G. Eliopoulos, M. Jones, L. S. Young, and M. Rowe. 1998. Epstein-Barr virus latent membrane protein-1 (LMP1) signalling is distinct from CD40 and involves physical cooperation of its two C-terminus functional regions. Oncogene 17:2383-2392. [DOI] [PubMed] [Google Scholar]
- 19.Floettmann, J. E., K. Ward, A. B. Rickinson, and M. Rowe. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 (LMP1) analysed using tetracycline- regulated expression in B cell lines. Virology 223:29-40. [DOI] [PubMed] [Google Scholar]
- 20.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gires, O., F. Kohlhuber, E. Kilger, B. M., A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gires, O., U. Zimber-Strobl, R. Gonella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzivassilliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the Epstein-Barr virus latent membrane protein 1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor expression, NF-κB and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 24.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. B. Rickinson. 1991. Induction of bcl-2 expression by the Epstein-Barr virus latent membrane protein-1 protects infected B-cells from programmed cell death. Cell 65:1107-1115. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi, M., E. Kieff, and K. M. Izumi. 2002. The Epstein-Barr virus latent membrane protein 1 putative Janus kinase 3 (JAK3) binding domain does not mediate JAK3 association or activation in B-lymphoma or lymphoblastoid cell lines. J. Virol. 76:455-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein 1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 27.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 94:1447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumour necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumi, K. M., E. C. McFarland, A. T. Ting, E. Riley, B. Seed, and E. D. Kieff. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaye, K. M., O. Devergne, J. N. Harada, K. M. Izumi, R. Yalamanchili, E. Kieff, and G. Mosialos. 1996. Tumour necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA 93:11085-11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N- terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, K.-R., T. Yoshizaki, H. Miyamori, K. Hasegawa, T. Horikawa, M. Furukawa, S. Harada, M. Seiki, and H. Sato. 2000. Transformation of Madin-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 19:1764-1771. [DOI] [PubMed] [Google Scholar]
- 34.Kulwichit, W., R. H. Edwards, E. M. Davenport, J. F. Baskar, V. Godfrey, and N. Raab-Traub. 1998. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc. Natl. Acad. Sci. USA 95:11963-11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, S. Y., A. Reichlin, A. Santana, K. A. Sokol, M. C. Nussenzweig, and Y. Choi. 1997. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity 7:703-713. [DOI] [PubMed] [Google Scholar]
- 36.Leo, E., K. Welsh, S.-I. Matsuzawa, J. M. Zapata, S. Kitada, R. S. Mitchell, K. R. Ely, and J. C. Reed. 1999. Differential requirements for tumor necrosis factor receptor- associated factor family proteins in CD40-mediated induction of NF-κB and Jun N- terminal kinase activation. J. Biol. Chem. 274:22414-22422. [DOI] [PubMed] [Google Scholar]
- 37.Messineo, C., M. H. Jamerson, E. Hunter, R. Braziel, A. Bagg, S. G. Irving, and J. Cossman. 1998. Gene expression by single Reed-Sternberg cells: pathways of apoptosis and activation. Blood 91:2443-2451. [PubMed] [Google Scholar]
- 38.Miller, W. E., H. S. Earp, and N. Raab-Traub. 1995. The Epstein-Barr virus latent membrane protein-1 induces expression of the epidermal growth factor receptor. J. Virol. 69:4390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell, T., and B. Sudgen. 1995. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moorthy, R. K., and D. A. Thorley-Lawson. 1993. All three domains of the Epstein- Barr virus-encoded latent membrane protein LMP1 are required for transformation of Rat-1 fibroblasts. J. Virol. 67:1638-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP-1 engages signaling proteins for the tumour necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 42.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray, P. G., J. R. Flavell, K. R. Baumforth, S. M. Toomey, D. Lowe, J. Crocker, R. F. Ambinder, and L. S. Young. 2001. Expression of the tumour necrosis factor receptor-associated factors 1 and 2 in Hodgkin's disease. J. Pathol. 194:158-164. [DOI] [PubMed] [Google Scholar]
- 44.Natoli, G., A. Costanzo, A. Ianni, D. J. Templeton, J. R. Woodgett, C. Balsano, and M. Levrerop. 1997. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200-203. [DOI] [PubMed] [Google Scholar]
- 45.Ni, C. Z., K. Welsh, E. Leo, C. K. Chiou, H. Wu, J. C. Reed, and K. R. Ely. 2000. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. USA 97:10395-10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, Y. C., H. Ye, C. Hsia, D. Segal, R. L. Rich, H. C. Liou, D. G. Myszka, and H. Wu. 2000. A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell 101:777-787. [DOI] [PubMed] [Google Scholar]
- 47.Peng, M., and E. Lundgren. 1992. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene 7:1775-1782. [PubMed] [Google Scholar]
- 48.Prokova, V., G. Mosialos, and D. Kardassis. 2002. Inhibition of transforming growth factor beta signaling and Smad-dependent activation of transcription by the latent membrane protein 1 of Epstein-Barr virus. J. Biol. Chem. 277:9342-9350. [DOI] [PubMed] [Google Scholar]
- 49.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Raven Press, New York, N.Y.
- 50.Rothe, M., V. Sarma, V. M. Dixit, and D. V. Goeddel. 1995. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269:1424-1427. [DOI] [PubMed] [Google Scholar]
- 51.Rowe, M., H. S. Evans, L. S. Young, K. Hennessy, E. Kieff, and A. B. Rickinson. 1987. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J. Gen. Virol. 68:1575-1586. [DOI] [PubMed] [Google Scholar]
- 52.Sandberg, M., W. Hammerschmidt, and B. Sudgen. 1997. Characterization of LMP1's association with TRAF1, TRAF2 and TRAF3. J. Virol. 71:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwenzer, R., K. Siemienski, S. Liptay, G. Schubert, N. Peters, P. Scheurich, R. M. Schmid, and H. Wajant. 1999. The human tumor necrosis factor (TNF) receptor- associated factor 1 gene (TRAF1) is up-regulated by cytokines of the TNF ligand family and modulates TNF-induced activation of NF-κB and cJun N-terminal kinase. J. Biol. Chem. 274:19368-19374. [DOI] [PubMed] [Google Scholar]
- 54.Speiser, D. E., S. Y. Lee, B. Wong, J. Arron, A. Santana, Y. Y. Kong, P. S. Ohashi, and Y. Choi. 1997. A regulatory role for TRAF1 in antigen-induced apoptosis of T cells. J. Exp. Med. 185:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsao, D. H., T. McDonagh, J. B. Telliez, S. Hsu, K. Malakian, G. Y. Xu, and L. L. Lin. 2000. Solution structure of N-TRADD and characterization of the interaction of N- TRADD and C-TRAF2, a key step in the TNFR1 signaling pathway. Mol. Cell 5:1051-1057. [DOI] [PubMed] [Google Scholar]
- 56.Tsitsikov, E. N., D. Laouini, I. F. Dunn, T. Y. Sannikova, L. Davidson, F. W. Alt, and R. S. Geha. 2001. Traf1 is a negative regulator of TNF signaling: enhanced TNF signaling in Traf1-deficient mice. Immunity 15:647-657. [DOI] [PubMed] [Google Scholar]
- 57.Tsukamoto, N., N. Kobayashi, S. Azuma, T. Yamamoto, and J.-I. Inoue. 1999. Two differently regulated nuclear factor κB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA 96:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida, J., T. Yasui, Y, Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300-303. [DOI] [PubMed] [Google Scholar]
- 59.Wang, C.-Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin. 1998. NF-κB anti-apoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP1 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 60.Wang, S., M. Rowe, and E. Lundgren. 1996. Expression of the Epstein-Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl-2 homologue Mcl-1 levels in B-cell lines. Cancer Res. 56:4610-4613. [PubMed] [Google Scholar]
- 61.Xin, B., Z. He, X. Yang, C. P. Chan, M. H. Ng, and L. Cao. 2001. TRADD domain of Epstein-Barr virus transforming protein LMP1 is essential for inducing immortalization and suppressing senescence of primary rodent fibroblasts. J. Virol. 75:3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, X., Z. He, B. Xin, and L. Cao. 2000. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16(INK4a) expression. Oncogene 19:2002-2013. [DOI] [PubMed] [Google Scholar]
- 63.Ye, H., Y. C. Park, M. Kreishman, E. Kieff, and H. Wu. 1999. The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol. Cell 4:321-330. [DOI] [PubMed] [Google Scholar]
- 64.Ye, H., and H. Wu. 2000. Thermodynamic characterization of the interaction between TRAF2 and tumor necrosis factor receptor peptides by isothermal titration calorimetry. Proc. Natl. Acad. Sci. USA 97:8961-8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh, W.-C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 66.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zapata, J. M., M. Krajewska, S. Krajewski, S. Kitada, K. Welsh, A. Monks, N. McCloskey, J. Gordon, T. J. Kipps, R. D. Gascoyne, A. Shabaik, and J. C. Reed. 2000. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J. Immunol. 165:5084-5096. [DOI] [PubMed] [Google Scholar]
- 68.Zapata, J. M., and J. C. Reed. 2002. TRAF1: lord without a RING. Sci. STKE. http://www.stke.org/cgi/content/full/oc_sigtrans; 2002/133/pe27. [DOI] [PubMed]