Abstract
The lung is the primary target of infection with Mycobacterium tuberculosis. It is well established that, in mouse lung, expression of adaptive, Th1-mediated host immunity inhibits further multiplication of M. tuberculosis. Here, real-time RT-PCR was used to define the pattern of expression against time of lung infection of key genes involved in Th1-mediated immunity and of selected genes of M. tuberculosis. Inhibition of bacterial multiplication was preceded by increased mRNA synthesis for IFN-γ and inducible NO synthase (NOS2) and by NOS2 protein synthesis in infected macrophages. Concurrently, the pattern of transcription of bacterial genes underwent dramatic changes. mRNA synthesis increased for α-crystallin (acr), rv2626c, and rv2623 and decreased for superoxide dismutase C (sodC), sodA, and fibronectin-binding protein B (fbpB). This pattern of M. tuberculosis transcription is characteristic of the nonreplicating persistence [Wayne, L. G. & Sohaskey, C. D. (2001) Annu. Rev. Microbiol. 55, 139–163] associated with adaptation of tubercle bacilli to hypoxia in vitro. Based on this similarity, we infer that host immunity induces bacterial growth arrest. In IFN-γ gene-deleted mice, bacterial growth was not controlled; NOS2 protein was not detected in macrophages; sodC, sodA, and fbpB transcription showed no decrease; and acr, rv2626c, and rv2623 transcription increased only at the terminal stages of lung pathology. These findings define the transcription signature of M. tuberculosis as it transitions from growth to persistence in the mouse lung. The bacterial transcription changes measured at onset of Th1-mediated immunity are likely induced, directly or indirectly, by nitric oxide generated by infected macrophages.
Bacterial infection elicits an interplay between host immune defenses (reviewed in refs. 1 and 2) and mechanisms possessed by the bacterium that enable it to evade or protect itself from host immunity (3). These host–pathogen interactions are very intricate in infections caused by intracellular pathogens, such as Mycobacterium tuberculosis, where the connection between host cells and bacteria is most intimate (4). A large body of literature provides a definition of the key events in the generation of host immunity to M. tuberculosis infection. In mice, airborne infection with this pathogen is characterized by exponential bacterial growth in the lung for ≈20 days followed by stable bacterial cell numbers for the rest of the infection (discussed in ref. 5). Control of infection is mediated by M. tuberculosis-specific CD4+ and CD8+ T cells via the secretion of IFN-γ and other Th1 cytokines that activate antimycobacterial mechanisms of infected macrophages (1, 2, 6). Activated macrophages perform their effector function by synthesizing inducible NO synthase (NOS2), which catalyzes high-output generation of NO and NO intermediates from L-arginine. Thus, mice incapable of making IFN-γ (7, 8) or NOS2 (9, 10) or of generating NO (9, 11) cannot control growth of tubercle bacilli in the lung. On the pathogen's side, much is known about the modulation of transcription in response to environmental changes during M. tuberculosis growth in vitro (12, 13–15) or when tubercle bacilli are ingested by macrophages in culture (16–18). However, there is little, if any, knowledge concerning shifts in transcription patterns of tubercle bacilli responding to defense mechanisms in a host animal.
In the present study, we developed methods based on real-time RT-PCR to measure changes in the transcription pattern of M. tuberculosis in response to adaptive, Th1-mediated host immunity. Because the expression of Th1-mediated immunity causes bacterial numbers to stabilize in the mouse lung, we reasoned that perturbations of M. tuberculosis transcription associated with host immunity could be similar to those known to be associated with arrest of bacterial division in vitro. In the present study, six genes of M. tuberculosis were selected for analysis from among those known to be modulated in vitro when aerated cultures of M. tuberculosis transition from exponential growth to stationary phase, or when cultures undergo growth arrest in response to hypoxia. The latter state has been defined as nonreplicating persistence by L. G. Wayne in his classic model of adaptation to hypoxia of M. tuberculosis in vitro (reviewed in ref. 12).
This study shows that, coincident with the onset of Th1-mediated immunity in the lungs of WT mice, the transcription pattern of the selected M. tuberculosis gene set undergoes dramatic changes consistent with those associated with the Wayne model of nonreplicating persistence. Furthermore, these changes in bacterial transcription measured in WT mice did not occur in the lungs of IFN-γ−/− mice, in which Th1-mediated immunity is not expressed and, hence, growth of M. tuberculosis is not controlled. These findings provide evidence that control of M. tuberculosis lung infection by adaptive, Th1-mediated immunity correlates with a transcription pattern of M. tuberculosis that is characteristic of nonreplicating persistence.
Materials and Methods
Detailed protocols for all methods used are published online (www.phri.org/gennaro/gennarobio.htm). Also, Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Mice.
WT mice and IFN-γ−/− mice on a C57BL/6 background were purchased from the Trudeau Institute. Mice were used in experiments at 8–10 weeks of age.
Bacteria and Infection.
M. tuberculosis strain H37Rv (Trudeau Mycobacterial Culture Collection no. 102) was grown as a suspension culture as described (19). Infection was carried out in an aerosol infection apparatus (Tri Instruments, Jamaica, NY). Mice were exposed to an aerosol generated by nebulizing 10 ml of a suspension of 106 bacilli per ml for 30 min.
Histology and Immunocytochemistry.
Protocols for preparation of paraffin lung sections and immunocytochemistry are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Quantitation of Host Gene Expression in Infected Lungs by Real-Time RT-PCR.
Lungs were harvested at the times indicated in Results and snap frozen in liquid nitrogen. Total RNA was extracted from lungs homogenized in Trizol (Life Sciences, St. Petersburg, FL), according to the manufacturer's instructions. Primers and probe for IFN-γ were designed with PRIMER EXPRESS (Applied Biosystems), whereas those for NOS2 were designed according to a published procedure (20) (oligonucleotide sequences are shown in Table 1, which is published as supporting information on the PNAS web site). Quantitation of IFN-γ and NOS2 mRNAs in mouse lungs involved reverse transcription with a TaqMan Gold RT-PCR kit (Applied Biosystems) and real-time PCR performed in the ABI Prism 7700 sequence detector. Reaction conditions, methods for standard curve determinations, and copy number calculations are found in the supporting information on the PNAS web site.
Quantitation of Bacterial Gene Expression.
Total RNA extraction from lungs was based on the combination of a guanidinium thiocyanate-based buffer and rapid mechanical lysis of M. tuberculosis (21). Reverse transcription primers, PCR primers, and molecular beacons for genes of M. tuberculosis are listed in Table 2, which is published as supporting information on the PNAS web site. Molecular beacons, which are hairpin-shaped oligonucleotide probes that become fluorescent upon hybridization to their target sequence (22–24), were synthesized at Biosearch. Reverse transcription was performed with gene-specific primers and ThermoScript transcriptase (Invitrogen) according to the manufacturer's recommendations. Quantitation of M. tuberculosis mRNAs was carried out by real-time PCR using gene-specific primers, molecular beacons, and AmpliTaq Gold polymerase (Applied Biosystems) in a Prism 7700 spectrofluorometric thermal cycler (Applied Biosystems). Reaction conditions, methods for standard curve determinations, controls of DNA carryover, and copy number calculations are found in the supporting information on the PNAS web site.
Results
IFN-γ and NOS2 mRNA Synthesis Precedes Control of M. tuberculosis Infection in the Lungs.
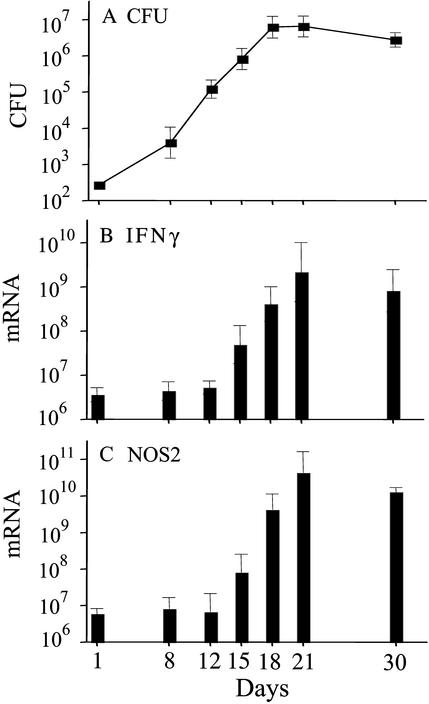
The expression of host immunity against M. tuberculosis lung infection is evidenced by inhibition of further bacterial multiplication at ≈3 weeks of infection (discussed in ref. 5). With a view to determining whether bacterial growth inhibition is preceded by or associated with the generation and expression of Th1-mediated immunity, copy numbers of mRNAs of a key Th1 cytokine, IFN-γ, and a key macrophage enzyme, NOS2, required for inhibition of M. tuberculosis growth (1, 2, 6) were monitored against time of infection. As seen in Fig. 1A, M. tuberculosis grew exponentially for ≈18 days in the lungs of WT mice, after which bacterial growth ceased and infection entered a stationary phase. Real-time RT-PCR measurements showed that copy numbers of mRNA for IFN-γ and for NOS2 increased 10-fold in the lungs between days 12 and 15 of infection, and ≥1,000-fold by day 21 (Fig. 1 B and C). Thus, increase of IFN-γ and NOS2 mRNA synthesis preceded control of M. tuberculosis growth and onset of stationary-phase infection.
Figure 1.
IFN-γ and NOS2 mRNA synthesis during the course of M. tuberculosis lung infection. WT C57BL/6 mice were infected with 2 × 102 CFU of M. tuberculosis H37Rv via the respiratory route. At the times indicated, bacterial CFU in the mouse lung were determined. Total RNA was isolated from lung, and the copy numbers per lung of IFN-γ and NOS2 mRNA were measured by real-time RT-PCR, as described in Materials and Methods. (A) Course of M. tuberculosis infection in mouse lung. Each data point represents the mean (±SD) of M. tuberculosis CFU (in log units) obtained from five mice per time point. (B and C) Measurements of copy numbers of mRNA of IFN-γ (B) and NOS2 (C). mRNA copy number (log units per lung) were determined in triplicate by using pooled RNA from the lungs of three mice killed at the times indicated. Shown are the means (±SD) of three separate determinations of the pooled RNA. In separate, ongoing experiments, we have determined that the number of IFN-γ and NOS2 mRNA copies is essentially the same immediately before (day −1) and immediately after (day +1) aerosol infection with M. tuberculosis (data not shown). These basal levels are explained by the continued exposure of the lung to environmental agents, including living microorganisms.
Increased IFN-γ and NOS2 mRNA Synthesis Is Associated with Accumulation of NOS2-Positive Macrophages at Sites of Infection in WT Mice.
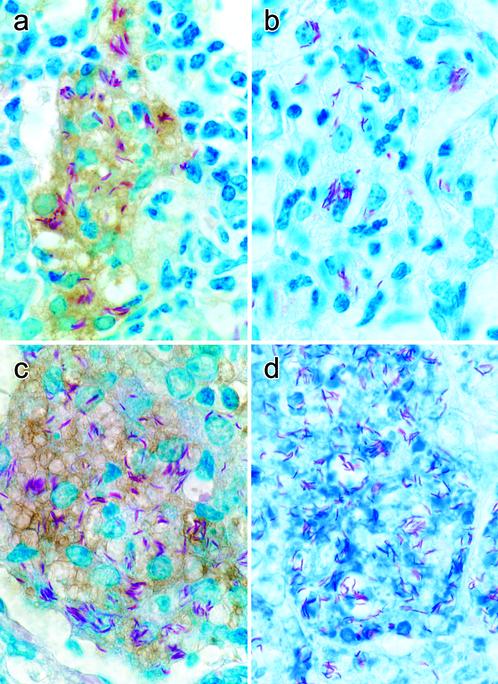
To determine whether increased NOS2 mRNA synthesis was associated with the synthesis of NOS2 protein in infected macrophages, lung sections were examined by immunocytochemistry using a NOS2-specific antibody as the primary reagent. Examination of lungs harvested on days 10, 20, and 35 of infection showed that sites of infection in WT mouse lungs were characterized by accumulation of macrophages and other mononuclear cells, with many of the macrophages being infected with M. tuberculosis (Fig. 2). The number of macrophages in 10-day lesions was relatively small, and most of the macrophages contained acid-fast bacilli. None of the macrophages in day-10 lesions stained positively for NOS2 (data not shown). At day 20 of infection (Fig. 2a), lung lesions were larger and remained populated by macrophages and other mononuclear cells. Moreover, on day 20, lesion-infected macrophages stained strongly positively for NOS2. By day 35 (Fig. 2c), WT lung lesions were larger still and contained cells similar to those seen in day-20 lesions, except that the infected macrophages appeared more vacuolated. Most infected macrophages stained positively for NOS2.
Figure 2.
Sections of lungs of M. tuberculosis-infected WT and IFN-γ−/− mice stained for acid-fast bacteria and for NOS2 by immunocytochemistry. On day 20 of infection, lung lesions in WT mice (a) consisted of accumulation of macrophages that stained positively for NOS2 (brown color) and contained acid-fast bacteria (red rods). In contrast, macrophages containing acid-fast bacilli in lesions of IFN-γ−/− mice (b) failed to stain for NOS2. On day 35 of infection, lung lesions in WT mice (c) were of similar appearance to those on day 20. However, day-35 lung lesions in IFN-γ−/− mice (d) were more numerous and much larger than those in WT mice (not shown) and were clearly of a different cellular composition, being populated predominantly by neutrophils, most of which were dead and degenerating, and many of which contained acid-fast bacilli. (Magnification, ×650.)
Lung lesions in IFN-γ−/− mice on days 10 and 20 of infection were similar histologically to those in WT mice on the same days. Lung lesions on day 20 (Fig. 2b) were dominated by macrophages and other mononuclear cells, and many of the macrophages were infected. None of the macrophages, however, stained positively for NOS2 (Fig. 2b). By day 35, lung lesions in IFN-γ−/− mice were much larger than those in WT mice, and they had become populated almost exclusively by neutrophils (Fig. 2d). Most of the neutrophils were dead and degenerating, and they contained acid-fast bacilli. Moreover, all of these lesions showed extensive areas of necrosis. Almost none of the cells in day-35 lesions stained positively for NOS2.
Analysis of M. tuberculosis Gene Expression in Mouse Lung.
Evidence presented in Fig. 1 establishes that onset of expression of host-adaptive immunity in mouse lung leads to control of M. tuberculosis growth around day 20. The question thus arises as to the nature of changes in bacterial gene transcription that are associated with Th1-mediated control of M. tuberculosis growth in the lung. Real-time RT-PCR makes it possible to address this question. Thus, we developed methods based on real-time RT-PCR to enumerate copies of specific bacterial mRNAs in the presence of dominant amounts of lung RNA (detailed protocols are found in the supporting information on the PNAS web site). We then selected six genes of M. tuberculosis for mRNA measurements. We next identified 16S rRNA as normalization factor for measurement of bacterial mRNA copies. Finally, we defined patterns of transcription of selected genes in response to adaptive, Th1-mediated immunity by measuring copy numbers of bacterial mRNAs during the course of infection of WT and IFN-γ−/− mice. Results of each step of our experimental strategy are presented in the sections below.
Selection of Six Genes of M. tuberculosis for Expression Analysis in the Mouse Lung.
Five genes (acr, rv2626c, rv2623, sodC, and sodA) were selected because their expression is known to be modulated when tubercle bacilli transition from exponential growth to either stationary phase or hypoxia-induced nonreplicating persistence, or to both. acr was chosen because it encodes a 16-kDa chaperonin that is up-regulated in vitro by hypoxia (13, 15, 25, 26), during stationary phase of growth of aerated M. tuberculosis cultures (27), by addition of NO donors (14), and on ingestion of M. tuberculosis by cultured macrophages (28). Likewise, rv2626c, a gene of unknown function, was chosen because, like acr, it is up-regulated in vitro during stationary phase of growth of aerated cultures (29) and under hypoxic conditions (15, 29, 30). Selection of rv2623 was based on the knowledge that its expression is increased in vitro by low O2 tension (15, 29, 30) but is independent of phase of growth (ref. 29 and K. McDonough, personal communication). rv2623, which is also up-regulated on ingestion of Mycobacterium bovis bacillus Calmette–Guérin by cultured macrophages (18), encodes a protein of unknown function that contains an ATP-binding motif and shares homology with the family of prokaryotic universal stress proteins (29, 30). sodA and sodC, which encode superoxide dismutases, were selected because superoxide dismutase activity decreases during nonreplicating persistence in the Wayne model (31).
Finally, we included in the analysis fbpB, the gene encoding antigen 85B, one of three homologous mycolyl transferases required for cell envelope biogenesis (reviewed in ref. 32). This sixth gene was selected because its expression was expected to be associated with actively multiplying, rather than nonreplicating, bacilli.
Changes in M. tuberculosis Colony-Forming Units (CFU) During the Course of Infection Correlate with Changes in the Copy Number of M. tuberculosis 16S Ribosomal RNA.
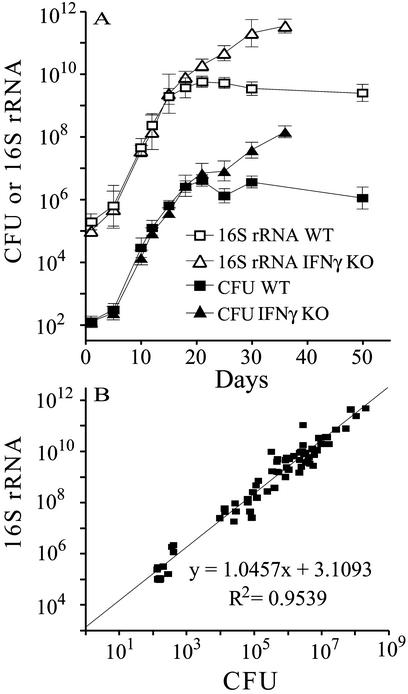
Interpretation of measurements of copies of M. tuberculosis mRNA requires normalization to bacterial CFU. The copy number of 16S rRNA per genome equivalent was shown to remain stable throughout different phases of M. tuberculosis growth in vitro (25). Thus, we set out to assess the correlation between CFU and 16S rRNA copy numbers for M. tuberculosis in mouse lung. In these experiments, half of the lung (attached to the left bronchus) was used to enumerate CFU, and the other half (attached to the right bronchus) was used to measure 16S rRNA and mRNAs. The copy number of M. tuberculosis 16S rRNA, as measured by real-time RT-PCR, increased exponentially between days 1 and 21 of infection and then leveled off in lungs of WT mice. In contrast, 16S rRNA copy number continued to increase beyond day 21 in the lungs of IFN-γ−/− mice (Fig. 3A). CFU determinations (Fig. 3A) closely paralleled 16S rRNA copy numbers. The correlation between 16S rRNA copy number and M. tuberculosis CFU was high (R2 = 0.9539) throughout the course of infection (Fig. 3B). Our data suggest that the rate of M. tuberculosis rRNA synthesis is so low during exponential growth (because of the very slow growth rate in the lungs, ≈23 h doubling time) that any decrease in rRNA transcription during stationary infection is small relative to exponential growth. Thus, the overall ribosome content is not detectably altered throughout the course of infection.
Figure 3.
Correlation of CFU with the copy number of 16S rRNA from M. tuberculosis growing in the lungs of WT and IFN-γ−/− mice. Lungs were harvested from M. tuberculosis-infected mice at selected times during infection. (A) Half the lung (attached to the right bronchus) was used to quantitate the number of copies of 16S rRNA by molecular-beacon real-time RT-PCR, and the other half (attached to the left bronchus) was used to determine the number of CFU. The data points shown in the figure represent mean (±SD) of data (expressed in log units) per lung obtained from four animals per time point per mouse strain. (B) Correlation curve for CFU and 16S rRNA copies (in log units) obtained with lungs from WT and IFN-γ−/− mice pooled together. Correlation coefficients obtained separately with WT mice (R2 = 0.9536) and with IFN-γ−/− mice (R2 = 0.9570) were similar to that shown in B.
The high correlation between 16S rRNA and M. tuberculosis CFU indicates that copy numbers of a given mRNA species of M. tuberculosis can be normalized to M. tuberculosis CFU by dividing the copy number of that mRNA by the copy number of 16S rRNA in the same lung sample.
Changes in Transcription of M. tuberculosis Genes Associated with Expression of Host Immunity.
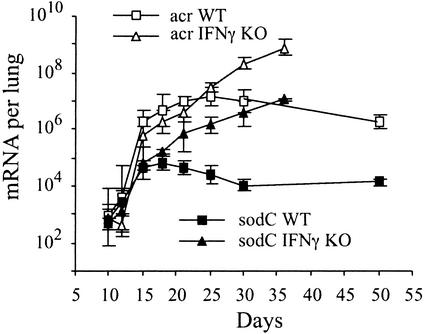
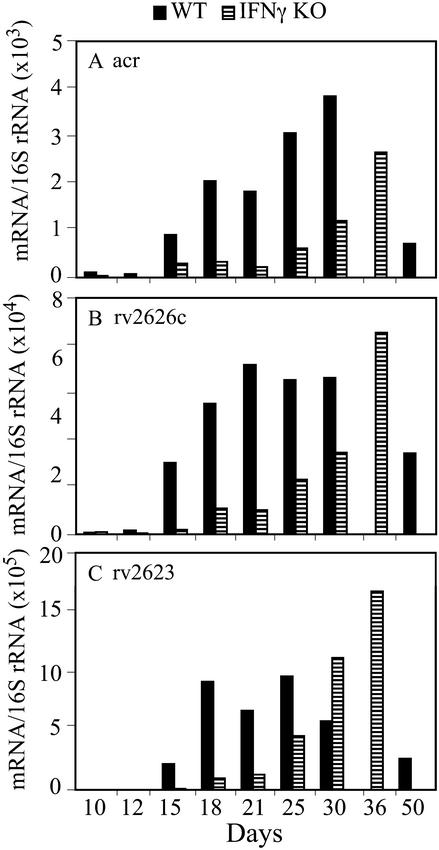
RNA isolated from the half lung used for quantitation of 16S rRNA was also used to measure copy numbers of mRNAs of M. tuberculosis. Raw data (mRNA copy numbers per lung) are shown for two genes, acr and sodC, in Fig. 4, and for all six selected genes in Table 3, which is published as supporting information on the PNAS web site. Copy numbers of acr, rv2626c, and rv2623 mRNA, normalized to 16S rRNA, are shown in Fig. 5. In WT mice, normalized mRNA copy numbers were very low on days 10 and 12 of infection and sharply increased on day 15, coincident with expression of host-adaptive immunity (refer to Fig. 1). The increase of mRNA copy numbers in WT mice on day 15 over day 12 was 15-fold for acr, 21-fold for rv2626c, and >20-fold for rv2623. mRNA copies remained high until day 30. By day 50 of infection, mRNA synthesis had declined relative to day 30 (≈4-fold for acr, 2-fold for rv2626c, and 2-fold for rv2623). (An explanation for the observed decrease by day 50 will need to be sought by extending the analyses performed in this study to later stages of infection). In IFN-γ−/− mice, expression of the three M. tuberculosis genes showed a different pattern relative to that in WT mice. Normalized mRNA copy numbers showed a very slow increase until day 25 of infection (the increase between days 21 and 25 was ≈3-fold for acr, 2-fold for rv2626c, and ≈4-fold for rv2623). From day 25 on, mRNA copy numbers steadily increased until day 36, just before these mice were expected to die. Therefore, the expression of acr, rv2626c, and rv2623 increased with at least a 10-day delay in IFN-γ−/− mice.
Figure 4.
Quantitation by molecular-beacon real-time RT-PCR of selected mRNAs of M. tuberculosis in the lungs of WT and IFN-γ−/− mice during the course of infection. Lungs were harvested from M. tuberculosis-infected mice at selected times during infection. RNA isolated from the half lung used for 16S rRNA measurements (see Fig. 3) was also used to determine the number of copies of selected mRNAs by molecular-beacon real-time RT-PCR. Shown are the means ± SD (in log units) of data obtained for two bacterial genes, acr and sodC, by using lungs from four mice per time point per mouse strain. Raw data for four additional M. tuberculosis genes are presented in the supporting information on the PNAS web site.
Figure 5.
Normalized copy numbers of mRNA of M. tuberculosis acr (A), rv2626 (B), and rv2623c (C) in the lungs of WT and IFN-γ−/− mice during the course of infection. The number of copies of M. tuberculosis mRNA per lung was measured by molecular-beacon real-time RT-PCR as described in Materials and Methods and in the legend to Fig. 4. At each time point and for each mouse strain, normalized mRNA values were obtained by dividing the mean of mRNA copy numbers per lung (shown for acr in Fig. 4 and for the remaining genes in the supporting information on the PNAS web site) by the corresponding mean of 16S RNA copy numbers per lung (Fig. 3A). Shown are the ratios of mRNA and 16S rRNA. Each panel presents results obtained with one gene, as indicated.
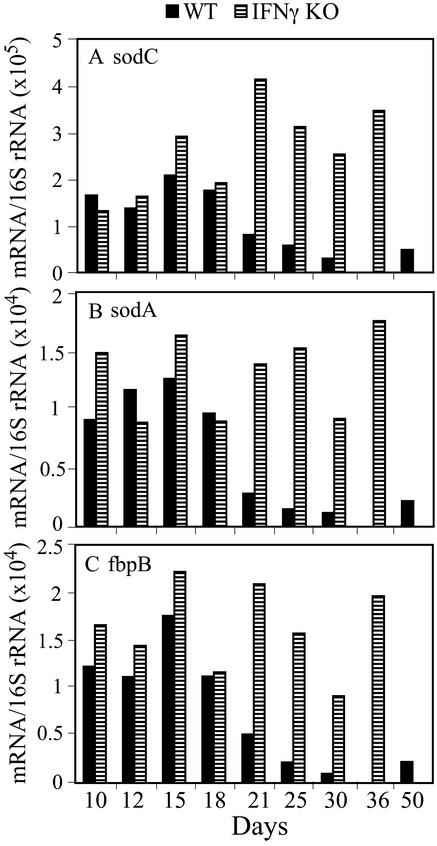
A different pattern of transcription was obtained with sodC, sodA, and fbpB (Fig. 6). In WT mouse lung, normalized mRNA copy numbers were approximately stable during the logarithmic phase of infection, started to decline on day 18, and continued to do so through day 30 of infection. On day 30, the decrease relative to day 15 was 7-fold for sodC, 11-fold for sodA, and 18-fold for fbpB. In contrast, in IFN-γ−/− mice, all three mRNAs remained elevated throughout the course of infection.
Figure 6.
Normalized copy numbers of sodC, sodA, and fbpB mRNA of M. tuberculosis in the lungs of WT and IFN-γ−/− mice during the course of infection. Normalized mRNA values for sodC (A), sodA (B), and fbpB (C) were calculated as described in the legend to Fig. 5.
Discussion
Adaptive host immunity inhibits further multiplication of M. tuberculosis in the mouse lung at ≈3 weeks of infection by the respiratory route (discussed in ref. 5). This paper shows that the transition of M. tuberculosis from logarithmic to stationary phase of infection in mouse lung is marked by changes in the expression of selected bacterial genes. The pattern of bacterial transcription that we found associated with inhibition of M. tuberculosis growth in the lung of WT mice is that characteristic of the nonreplicating persistence described by L.G. Wayne in his classic model of adaptation to low O2 tension of M. tuberculosis cultures in vitro (12). In the Wayne model, nonreplicating persistence is characterized by the abrupt cessation of DNA synthesis. Albeit drastically reduced, RNA and protein synthesis are still operative, as they support synthesis of new enzymes needed for utilization of alternative energy sources and of new, chaperonin-like proteins that protect bacterial cells against stress associated with low O2 tension (12). Based on the parallel with nonreplicating persistence in vitro, we propose that stable bacterial numbers induced by expression of Th1-mediated immunity in mouse lung are best explained as growth arrest. (Given the dynamic nature of events at foci of lung infection, it is possible, however, that at any given time of stable infection, a very small proportion of the bacterial population is in a replicating state.) Thus, the positive and negative shifts in mRNA synthesis described here for six genes of M. tuberculosis represent the first molecular signature of tubercle bacilli during growth and persistence in the mouse lung. A complete description of the transcription program of M. tuberculosis in the lung awaits improved DNA microarray techniques applicable to studying microorganisms in infected organs.
It is our view that growth arrest induced by Th1-mediated immunity is not the same as stationary phase of bacterial growth in vitro. Stationary phase is induced by, among other things, depletion of nutrients, accumulation of toxic by-products, and the activation of quorum-sensing mechanisms. None of these conditions could be expected to develop at an early stage (≈18 days) of lung infection, i.e., at onset of adaptive, Th1-mediated immunity. In immunocompromised mice, the lung supports exponential growth of tubercle bacilli until the animal succumbs to infection. Growth arrest, rather than stationary phase of growth, is also indicated by the up-regulation we observe at onset of host immunity of rv2623, a gene encoding a putative stress protein. In vitro studies have shown that rv2623 undergoes a pronounced increase of expression under low O2 tension but that the increase is independent of phase of bacterial growth (ref. 29 and K. McDonough, personal communication).
The present study does not address the chemical nature of the signal(s) that induces the M. tuberculosis transcription pattern measured at onset of adaptive, Th1 immunity in the lung of WT mice. An obvious candidate is NO produced by activated macrophages. NO may regulate M. tuberculosis transcription either directly, i.e., NO itself or one of its derivatives, or indirectly, by contributing to the creation of a low O2 environment. There is no information available concerning the effect of activation on O2 tension in macrophages. There is, however, evidence supporting the idea that exposure to NO, produced by activated macrophages in the lung of WT mice, might mimic low O2 tension, as experienced by tubercle bacilli in the Wayne model. NO has been shown to induce genes required for adaptation of Neisseria gonorrhoeae to an anaerobic environment (33) and to contribute to the ability of Drosophila to respond to O2 deprivation (34). In mammalian cells, NO can activate the hypoxia-inducible factor-1 (35), thus mimicking a situation of reduced O2 availability. Furthermore, acr, one of the M. tuberculosis genes that is up-regulated at onset of Th1-mediated immunity (Fig. 5A), is also induced by addition of NO donors to in vitro cultures of tubercle bacilli (14).
Down-regulation of fbpB, sodA, and sodC (Fig. 6) is best explained in the context of the metabolic slowdown associated with cessation of bacterial multiplication induced by host immunity. This interpretation easily accommodates the down-regulation of fbpB, which encodes an enzyme involved in cell wall biosynthesis. On the other hand, down-regulation of the genes encoding superoxide dismutases is counterintuitive, given the fact that these enzymes can participate in the detoxification of reactive O2 (RO) and its intermediates (ROI) generated inside the activated macrophages. However, mutant mice incapable of generating RO and ROI have been found to be only transiently more susceptible to M. tuberculosis infection than WT mice (36), or not more susceptible at all (R.J.N., unpublished results). In addition, the knowledge that sodC null mutants are not defective in the ability to grow in guinea pigs (37) or in mice (38) suggests the possibility that superoxide dismutases may not play an essential role in the protection of M. tuberculosis against host immunity.
The up-regulation of acr, rv2623, and rv2626c (Fig. 5) is more difficult to explain because no known function has yet been attributed to these genes. acr, a small-molecular-weight chaperonin capable of protecting proteins from heat-induced denaturation (39, 40) and known to be associated with cell wall thickening (13), may protect essential M. tuberculosis functions from host insult. The product of rv2623 contains universal stress protein domains (29, 30), as does the Escherichia coli UspA, a protein involved in survival of growth-arrested cultures (41). Thus, a possible role in stress response could be suggested for this gene product. Finally, the product of rv2626c is predicted to contain two cystathionine β-synthase domains involved in protein–protein interactions (29). Gene disruption and biochemical studies are needed to address the role of the genes induced at onset of adaptive, Th1-mediated immunity. It is intriguing that the expression levels of all these three genes gradually increased in IFN-γ−/− mice and peaked at the terminal stages of infection. Because acr is known to respond to numerous environmental cues (13, 25, 27, 39), it could be inferred that the signal(s) responsible for the delayed induction of these genes in IFN-γ−/− mice may be different from those generated in the WT animals at onset of adaptive, Th1-mediated immunity. The fulminating acute pathology seen in IFN-γ−/− mice (characterized by large lung lesions containing large numbers of dead and degenerating neutrophils, as well as extensive areas of necrosis, Fig. 2d) is very likely to generate conditions stressful to M. tuberculosis, including low O2 tension.
In conclusion, the evidence presented in this study establishes that onset of adaptive, Th1-mediated immunity in the mouse lung induces a transcription pattern of M. tuberculosis that is characteristic of nonreplicating persistence. An understanding of this metabolic state opens the way to the identification of mechanisms developed by this pathogen to escape the immune defenses of the host. Hence, this line of investigation is highly relevant to the design of antituberculosis drugs and of an efficacious vaccine.
Supplementary Material
Acknowledgments
We thank Ben Gold and Issar Smith for advice in preparation of RNA from cultured mycobacteria, Salvatore Marras and Hiyam El-Hajj for technical assistance with real-time PCR, Eddie Beck and Lynn Ryan for assistance in figure preparation, and Karl Drlica for critical reading of the manuscript. We are very grateful to Rick Gourse for elucidating critical aspects of growth rate regulation of bacterial ribosome synthesis and to Kathleen McDonough for sharing results on regulation of rv2623 expression before publication. We give special thanks to Boris Magasanik, whose questions broadened our perspective on the nature of the stationary phase of lung infection, and to Carol J. Lusty for generously devoting time to critically reading this manuscript. This work was supported by National Institutes of Health Grants AI-36989 (to M.L.G.) and AI-37844 and HL-64565 (to R.J.N.).
Abbreviations
- NOS2
inducible NO synthase
- CFU
colony-forming units
References
- 1.Flynn J L, Chan J. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Raupach B, Kaufmann S H. Curr Opin Immunol. 2001;13:417–428. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 3.Guiney D G. J Clin Invest. 1997;99:565–569. doi: 10.1172/JCI119196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theriot J A. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- 5.Mogues T, Goodrich M E, Ryan L, LaCourse R, North R J. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom W H. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 7.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scanga C A, Mohan V P, Tanaka K, Alland D, Flynn J L, Chan J. Infect Immun. 2001;69:7711–7717. doi: 10.1128/IAI.69.12.7711-7717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn J L, Scanga C A, Tanaka K E, Chan J. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 12.Wayne L G, Sohaskey C D. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham A F, Spreadbury C L. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garbe T R, Hibler N S, Deretic V. Infect Immun. 1999;67:460–465. doi: 10.1128/iai.67.1.460-465.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman D R, Voskuil M, Schnappinger D, Liao R, Harrell M I, Schoolnik G K. Proc Natl Acad Sci USA. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham J E, Clark-Curtiss J E. Proc Natl Acad Sci USA. 1999;96:11554–11559. doi: 10.1073/pnas.96.20.11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani F, Cappelli G, Riccardi G, Colizzi V. Gene. 2000;253:281–291. doi: 10.1016/s0378-1119(00)00249-3. [DOI] [PubMed] [Google Scholar]
- 18.Monahan I M, Betts J, Banerjee D K, Butcher P D. Microbiology. 2001;147:459–471. doi: 10.1099/00221287-147-2-459. [DOI] [PubMed] [Google Scholar]
- 19.Dunn P L, North R J. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overbergh L, Valckx D, Waer M, Mathieu C. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 21.Mahenthiralingam E. Methods Mol Biol. 1998;101:65–75. doi: 10.1385/0-89603-471-2:65. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi S, Kramer F R. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi S, Bratu D P, Kramer F R. Nat Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 24.Tyagi S, Marras S A, Kramer F R. Nat Biotechnol. 2000;18:1191–1196. doi: 10.1038/81192. [DOI] [PubMed] [Google Scholar]
- 25.Desjardin L E, Hayes L G, Sohaskey C D, Wayne L G, Eisenach K D. J Bacteriol. 2001;183:5311–5316. doi: 10.1128/JB.183.18.5311-5316.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkrands I, Slayden R A, Crawford J, Aagaard C, Barry C E, III, Andersen P. J Bacteriol. 2002;184:3485–3491. doi: 10.1128/JB.184.13.3485-3491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Y, Crane D D, Barry C E., III J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Y, Crane D D, Simpson R M, Zhu Y Q, Hickey M J, Sherman D R, Barry C E., III Proc Natl Acad Sci USA. 1998;95:9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon C, Li R, Qi R, Dick T. J Bacteriol. 2001;183:2672–2676. doi: 10.1128/JB.183.8.2672-2676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Florczyk M A, McCue L A, Stack R F, Hauer C R, McDonough K A. Infect Immun. 2001;69:5777–5785. doi: 10.1128/IAI.69.9.5777-5785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wayne L G, Lin K Y. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daffe M. Trends Microbiol. 2000;8:438–440. doi: 10.1016/s0966-842x(00)01844-8. [DOI] [PubMed] [Google Scholar]
- 33.Householder T C, Fozo E M, Cardinale J A, Clark V L. Infect Immun. 2000;68:5241–5246. doi: 10.1128/iai.68.9.5241-5246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingrove J A, O'Farrell P H. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brune B, von Knethen A, Sandau K B. Cell Signalling. 2001;13:525–533. doi: 10.1016/s0898-6568(01)00175-9. [DOI] [PubMed] [Google Scholar]
- 36.Cooper A M, Segal B H, Frank A A, Holland S M, Orme I M. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dussurget O, Stewart G, Neyrolles O, Pescher P, Young D, Marchal G. Infect Immun. 2001;69:529–533. doi: 10.1128/IAI.69.1.529-533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piddington D L, Fang F C, Laessig T, Cooper A M, Orme I M, Buchmeier N A. Infect Immun. 2001;69:4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbert H F, Quiocho F A. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 40.Yang H, Huang S, Dai H, Gong Y, Zheng C, Chang Z. Protein Sci. 1999;8:174–179. doi: 10.1110/ps.8.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diez A, Gustavsson N, Nystrom T. Mol Microbiol. 2000;36:1494–1503. doi: 10.1046/j.1365-2958.2000.01979.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.