Abstract
Fas (Tnfrsf6, Apo-1, CD95) is a death receptor involved in apoptosis induced in many cell types. Fas have been shown to be expressed by insulin-producing beta cells in mice and humans. However, the importance of Fas in the development of autoimmune diabetes remains controversial. To further evaluate the importance of Fas in pathogenesis of diabetes, we generated NOD mice (nonobese diabetic mice developing spontaneous autoimmune diabetes) with beta cell-specific expression of a dominant-negative point mutation in a death domain of Fas, known as lprcg or Fascg. Spontaneous diabetes was significantly delayed in NOD mice expressing Fascg, and the effect depended on the expression level of the transgene. However, Fascg-bearing mice were still sensitive to diabetes transferred by splenocytes from overtly diabetic NOD mice. At the same time, Fascg expression did neutralize the accelerating effect of transgenic Fas-ligand expressed by the same beta cells. Thus, both Fas-dependent and -independent mechanisms are involved in beta cell destruction, but interference with the Fas pathway early in disease development may retard or prevent diabetes progression.
In autoimmune diabetes, pancreatic beta cells are destroyed by effector lymphocytes, leading to the loss of insulin production and hyperglycemia (1, 2). Both CD8+ and CD4+ T cells contribute to the process (3), and several mechanisms of beta cell death are most likely involved (reviewed in refs. 4 and 5). We and others (6–8) found that triggering of Fas (Apo-1 and CD95) expressed on beta cells leads to their death and, subsequently, to diabetes. In our transgenic model (6), Fas ligand (FasL) was expressed in the beta cells of nonobese diabetic (NOD) mice. Although the original purpose of these experiments was to protect islet cells against autoaggressive T cells, mice that expressed FasL under the control of rat insulin promoter (RIP-FasL) (6) or human insulin promoter (9) demonstrated acceleration of both spontaneous and transferred diabetes. To explain this acceleration, a model has been suggested that predicts that transferred lymphocytes or their products induce Fas expression on beta cells, making them more susceptible to FasL-mediated killing. Normally, FasL involved in beta cell killing is expressed by effector T cells (10, 11). However, in the case of RIP-FasL transgenics, the ectopic expression of FasL accelerates the killing, most likely by a fratricide mechanism.
To further study the involvement of Fas in the destruction of insulin-producing cells, we used mice with a natural Fas mutation −lpr (12). We also produced NOD mice with dominant-negative mutations of Fas-lprcg called Fascg here (13), which expressed the transgene selectively in the islets of Langerhans.
Materials and Methods
Generation of the RIP-Fascg DNA Construct and Evaluation of Its Transgenic Expression.
The plasmid containing the Fas cDNA insert was a generous gift of S. Nagata (Department of Genetics, Osaka University Medical School, Osaka). A single nucleotide change (leading to I246N change in protein sequence) in Fas cDNA coding for the lprcg (Fascg) mutation was introduced by the PCR-directed overlap-extension method, using Fascg 5′-AAATTTGCACGAGAAAATAACAACAAGGAGG-3′ forward and 5′-CCTCCTTGTTGTTATTTTCTCGTGCAAATTT-3′ reverse primers. Simultaneously, 5′-GCATCTCGAGGCCACCATGCTGTGGATCTGGGCT-3′ forward and 5′-CGTACTCGAGTCACTCCAGACATTGTCC-3′ reverse primers were used to introduce flanking XhoI sites for subcloning of the final PCR product into the rat insulin 2 promoter or (RIP)-containing vector (6, 14, 15). Orientation of the insert was verified by restriction analysis and sequencing, and the HinDIII fragment was used for direct injection into NOD oocytes. Several transgene-bearing founders were identified by PCR with the above-listed Fascg forward primer and a reverse 5′-CCACAAACAACCCAAGAGCAC-3′ primer specific for an exon of the Eα gene, which is part of the expression vector.
Transgene expression analysis of RIP-Fascg transgene expression was performed by RT-PCR using RNA isolated from pancreata by the guanidine thiocyanate-cesium chloride centrifugation method (16). cDNA was synthesized using the Superscript First-Strand Synthesis kit (Invitrogen) and amplified using Fascg forward and Eα reverse primers. Amplification with hypoxanthine phosphoribosyltransferase (HPRT)-specific primers (17) was used as a control for cDNA quality.
Mice.
NOD/LtJ (NOD) and NOD.CB17-Prkdcscid/J (NOD-scid) mice were obtained from The Jackson Laboratory. NOD-Tg(Ins2-Tnfrsf6lpr-cg)/Ach (NOD.RIP-Fascg) mice were generated as described above. NOD-Tg(Ins2-Tnfsf6)/24 Ach (NOD.RIP-FasL) mice are described in ref. 6. NOD.MRL-Tnfrsf6lpr mice, abbreviated (NOD-lpr) were a kind gift of L. Matis and Y. Wang (Alexion Pharmaceuticals, New Haven, CT). Mice homozygous for −Prkdcscid and/or −Tnfrsf6lpr mutations on the NOD genetic background and bearing the RIP-FasL transgene, NOD.RIP-FasL-scid, NOD.RIP-FasL-lpr, and NOD.RIP-FasL-scid-lpr, as well as double-transgenic NOD.RIP-FasL RIP-Fascg mice, were bred in The Jackson Laboratory's Research Animal Facility. All animals were housed in a specific pathogen-free research facility.
Diabetes Transfer Experiments.
Diabetes induction was performed using both adoptive transfer of the total splenocyte population from diabetic NOD mice and injection of insulin-specific, Kd-restricted T cells of the TGNFC8 clone (IS-CD8+ cells) (11, 18). Splenocytes (1.5 × 107) were injected i.v. into irradiated (725 rad, 24 h in advance) recipients. Both males and females 6–9 weeks of age were used as recipients. No sex- or age-dependent differences were found in the incidence or time of onset of diabetes, and results were therefore pooled. Transfer into the host animals carrying the scid mutation was performed without irradiation. The recipients of splenocyte transfers were monitored for 50 days for diabetes development. The incidence of diabetes was assessed by monitoring glucose levels in urine by using Diastix reagent strips (Bayer, Elkhart, IN). IS-CD8+ cells were maintained in vitro as described (11, 19) in Click's medium (Irvine Scientific, Santa Ana, CA) supplemented with 5% FCS (Sigma), 2 × 10−5 M 2-mercaptoethanol (Bio-Rad), 100 units/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Rockville, MD), 2 mM l-glutamine (Life Technologies), and 5 units/ml mouse recombinant IL2. Cells were stimulated every 3 weeks by NOD splenocytes loaded with 10 μg/ml of the synthetic insulin B chain peptide (amino acids 15–23, LYLVCGERG) produced by Research Genetics (Huntsville, AL). For diabetes induction, IS-CD8+ cells were washed with PBS, counted, and injected i.v. at 107 cells per animal into irradiated (725 rad, 24 h in advance) recipients. Diabetes was detected by daily monitoring of glucose levels in urine for 21 days.
Immunohistochemical and Histological Studies.
Spleens and pancreata from newly diabetic animals were used. For immunostaining, 7-μm-thick cryostat sections of organs fresh-frozen in OCT compound (Sakura Finetek, Torrance, CA) were fixed in acetone at −20°C for 3 min, air-dried for 2 h, stained for 1 h at room temperature with anti-Gr-1/Ly6G (clone RB6-8C5) and FITC-conjugated anti-CD8 (clone 53.6-72) monoclonal antibodies (both from BD Biosciences), washed, and stained with rhodamine-labeled donkey anti-rat Ig secondary antibodies (Jackson ImmunoResearch). After the final wash, slides were mounted in Slowfade Light mounting medium (Molecular Probes) and examined using fluorescent microscopy. Pancreata for histological studies were fixed overnight in Bouin's fixative (20), paraffin-embedded, sectioned, and stained with hematoxylin/eosin according to standard protocol.
Statistical Analysis.
Analysis of the statistical significance of the observed differences between various groups of mice in the performed experiments was done using superanova software (Microsoft), using Fisher's protected least significant difference (LSD) test at a significance level of 0.05. In addition, statistical analysis of differences in spontaneous diabetes development rates was performed using the Cox proportional hazard model and permutation test (21).
Results and Discussion
Acceleration of Diabetes in NOD.RIP-FasL Mice Is Not Caused by Neutrophil Activation.
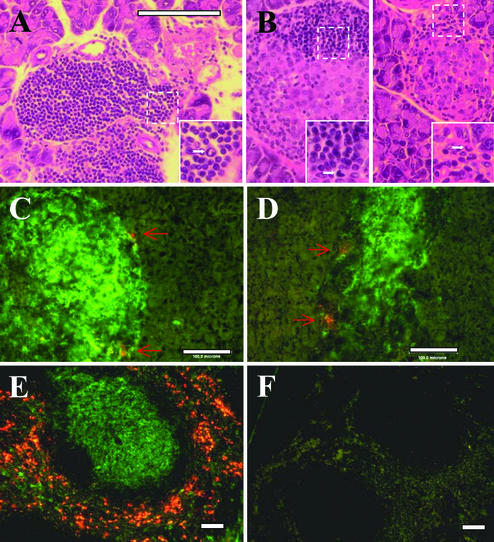
Following our report on RIP-FasL transgenic mice (6), other groups published their results showing that FasL expressed in the islets was causing the death of beta cells and, subsequently, diabetes (9, 22, 23). In some studies, activated neutrophils were found to infiltrate the pancreas (22, 23). It was proposed that FasL activates neutrophils [and it clearly can do so, as shown in other systems (24, 25)], causing infiltration of the islets. These studies differed from ours in the use of different mouse strains as recipients of the FasL transgene, and of an extended promoter region. In one case (22), mice were infected with an adenovirus carrying FasL. All of these factors (the genetic background of transgene carriers, a possible role for additional sequences transferred with a longer promoter region, or the presence of adenoviral sequences) could contribute to neutrophil activation. Clearly, we needed to determine the extent to which neutrophils contribute to the acceleration of diabetes in our RIP-FasL transgenic NOD mice during spontaneous development of disease. Histological examination of spontaneously diabetic NOD.RIP-FasL transgenic mice failed to show any significant neutrophil accumulation compared with that in diabetic nontransgenic NOD animals (Fig. 1), as determined by morphological and immunohistochemical analyses. For the latter, tissues were stained with anti-Gr-1 (Ly6G) monoclonal antibodies, reported to specifically recognize neutrophils (26, 27), and were used for the neutrophil detection in the islets of Langerhans (23). Whereas Gr-1+ cells were evident in the spleen (Fig. 1E), only few GR-1+ cells were found in the infiltrated islets. Overall, only ≈20% of islets in both nontransgenic and RIP-FasL transgenic NOD mice contained detectable neutrophils (1–2 per islet).
Figure 1.
Only a few neutrophils are present in predominantly lymphocytic infiltrates in the islets of both wild-type NOD and NOD.RIP-FasL mice. Paraffin (A and B) or fresh-frozen (C–F) sections of organs from newly diabetic NOD (A and C) and NOD.RIP-FasL (B and D) mice stained with hematoxylin/eosin (A and B) or a combination of anti-Gr-1 (orange)/anti-CD8 (green) antibodies (C and D). (E and F) Splenic sections stained with anti-Gr-1 (orange)/anti-CD8 (green) antibodies (E) or secondary antibodies alone (F). In A and B, areas within dashed-line squares are enlarged (lower right corners). Arrows show cells with typical neutrophil morphology or positive for Gr-1.
Moreover, RIP-FasL transgenic mice homozygous for the scid mutation (NOD.RIP-FasL-scid), which have no lymphocytes but normal neutrophil counts, did not develop spontaneous diabetes (a group of 10 female animals observed for >30 weeks); if neutrophils were responsible, one would anticipate that these mice would develop the disease. It could be argued that prior damage induced by lymphocytes is required for the homing of neutrophils to the islets, but that argument cannot explain why FasL transgenic animals on the B6 background with no prior islet damage experience neutrophil infiltration (22). In summary, we failed to find any prominent role for neutrophils in our model of diabetes accelerated by ectopic expression of FasL in beta cells.
NOD-RIP.FasL Mice Lacking Fas Expression Do Not Show Accelerated Disease in a Transfer Model of Diabetes.
We originally found that NOD mice homozygous for the lpr mutation and thus lacking functional Fas molecules (NOD-lpr mice) were resistant to the development of spontaneous diabetes (6). This was later confirmed by another group (28). We have also found that the transfer of IS-CD8+ cells into NOD-lpr mice does not result in diabetes development (6). Moreover, the NOD.RIP-FasL-lpr mice that we generated were resistant to IS-CD8+ cells; none of five mice became diabetic, whereas all five mice in the control NOD-RIP.FasL group became diabetic within 5 days after transfer. This was an encouraging result, suggesting that FasL expressed in the beta cells requires coexpression of Fas to induce diabetes.
The drawbacks of the lpr system, however, were pointed out in several studies in which mice homozygous for the lpr mutation were shown to express elevated levels of FasL by leukocytes, thus leading to rapid elimination of the transferred T cells (29–31). Although our experiments using the transfer of IS-CD8+ cells demonstrated that these cells were infiltrating the islets of NOD-lpr mice (6), we concurred with the argument and performed a series of experiments using NOD-lpr mice transgenic for FasL, but lacking endogenous lymphocytes expressing FasL. For that, we bred NOD.RIP-FasL-scid mice to NOD-lpr mice and generated NOD.RIP-FasL-scid-lpr mice (together with their FasL− littermates). These groups of mice were injected with 1.5 × 107 splenocytes from diabetic NOD mice. Not all of the mice became diabetic (Table 1), and, most importantly, there was no acceleration of diabetes development in NOD-scid-lpr mice that expressed FasL. All control Fas-sufficient NOD-scid mice became diabetic, and there was an expected acceleration of diabetes in NOD-scid mice expressing FasL in beta cells (Table 1). Three conclusions were drawn from this experiment: first, the delay in diabetes development and reduction of the fraction of NOD-scid-lpr mice that became diabetic is due to the lack of Fas signaling; second, Fas is indeed participating in the killing of beta cells via ectopic RIP-FasL, as predicted by our model (6); and third, mice with Fas deficiency still can become diabetic on adoptive transfer of effector cells (32), indicating that other mechanisms are involved in beta cell damage. These conclusions, however, must be made with caution, because the lpr mutation (insertion of a transposon into an intron of the Fas-encoding gene) does not block Fas expression completely, but instead severely diminishes it.
Table 1.
No acceleration of diabetes development in NOD.RIP-FasL-scid-lpr mice after transfer of diabetogenic splenocytes
| Recipient | Diabetes incidence | Onset, days* |
|---|---|---|
| NOD-scid-lpr | 6/11 | 32.5 ± 2.0 |
| NOD.RIP-FasL-scid-lpr | 10/14 | 39.6 ± 2.6 |
| NOD-scid | 15/15 | 24.1 ± 0.9 |
| NOD.RIP-FasL-scid | 6/6 | 13.4 ± 0.2† |
Mean day of onset ± SE.
Difference versus NOD-scid group is significant (P = 0.0004 by Fisher's LSD test).
Expression of Dominant-Negative Mutant Fascg in Beta Cells Leads to Retardation of Diabetes.
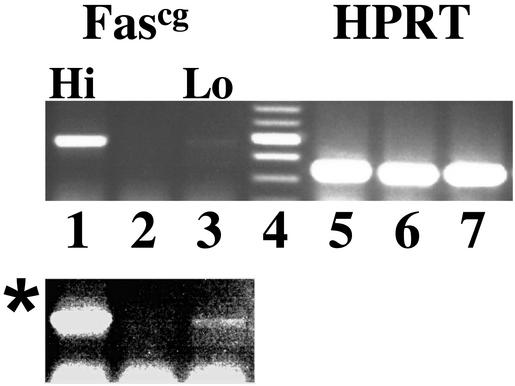
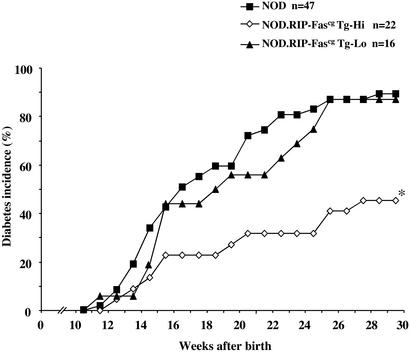
To manipulate Fas signaling in beta cells without systemic disruption of Fas observed in lpr mice, we produced transgenic animals that expressed mutant Fas molecules in beta cells. Fascg is a point mutation in the death domain of Fas that has two important properties: it blocks Fas signaling via interference with Fas oligomerization (33), and does so in a dominant fashion in vitro and in vivo (12, 34, 35), because it was shown to produce an lpr-like phenotype in heterozygotes (36). We introduced this mutation into Fas cDNA by using the PCR overlap-extension method and subcloned this cDNA into a vector containing RIP. We obtained a number of NOD.RIP-Fascg transgenic founder strains that differed in their levels of transgene expression. Transgene expression in two strains is shown in Fig. 2. Subsequent analysis of diabetes frequency in these strains revealed that RIP-Fascg mice showed some level of protection against spontaneous diabetes and that the degree of protection correlated with the level of transgene expression (Fig. 3). This result is exactly what one would anticipate, because Fascg interference with Fas signaling must be dose-dependent, given that the number of wild-type trimers (transducing signal) decreases proportionally with the number of available nontransducing molecules (containing the Fascg monomer).
Figure 2.
Evaluation of pancreatic expression of the RIP-Fascg transgene in mice derived from different transgenic lines. RNA isolated from pancreata of two independent transgenic founders (lanes 1, 3, 5, and 7) and nontransgenic control (lanes 2 and 6) was amplified by RT-PCR using Fascg-specific (lanes 1–3) and hypoxanthine phosphoribosyltransferase (HPRT)-specific (lanes 5–7) primers. Lane 4, molecular weight markers. Transgenic lines with high (lane 1) and low (lane 3) levels of RIP-Fascg expression were identified. *, Enhanced version of lanes 1–3.
Figure 3.
Development of spontaneous diabetes is significantly delayed in NOD.RIP-Fascg transgenic mice with high expression of the transgene. Incidence of spontaneous diabetes was assessed in groups of female RIP-Fascg transgenic mice with high (NOD.RIP-Fascg Tg-Hi) and low (NOD.RIP-Fascg Tg-Lo) levels of expression compared with nontransgenic littermates (NOD). n, number of animals per group. *, The observed delay of diabetes development in NOD.RIP-Fascg Tg-Hi animals was significant compared with that in the NOD.RIP-Fascg Tg-Lo and NOD groups, because P values calculated for variations in the mean time of diabetes onset between indicated groups by protected Fisher's LSD test were <0.005.
Fascg Abolishes the Acceleration of Diabetes Caused by RIP-FasL in a Transfer Model.
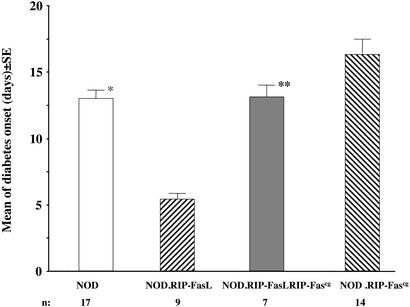
Knowing that the Fascg transgene retards natural diabetes development in NOD mice, we combined this mutation with the RIP-FasL transgene by breeding the two transgenic strains. We reasoned that the resultant double-transgenic NOD.RIP-FasL RIP-Fascg mice would provide additional support for the model of Fas-mediated killing of beta cells. If ectopic FasL is indeed working through Fas expressed by beta cells, then expression of Fascg by the same cells should interfere with Fas signaling and eliminate the acceleration of diabetes observed after adoptive transfer of diabetogenic T cells into NOD.RIP-FasL mice. To test this, we crossed NOD.RIP-FasL and NOD.RIP-Fascg mice, and then injected these two transgenic strains and the generated double transgenics with diabetogenic splenocytes from NOD mice. As expected, diabetes development was twice as rapid in NOD.RIP-FasL mice compared with NOD mice (mean onset of disease 6.3 days and 13 days after transfer, respectively; Fig. 4). This acceleration was abolished by introduction of the dominant-negative Fascg mutation, because double-transgenic NOD.RIP-FasL.RIP-Fascg mice exhibited increased onset times after transfer compared with NOD.RIP-FasL mice (Fig. 4). At the same time, single NOD.RIP-Fascg transgenic mice showed some protection from injected effector cells. Thus, the dominant-negative mutation Fascg neutralized ectopically expressed FasL, which can only be explained by interference with Fas expressed by beta cells. Fascg, however, was not able to completely protect mice in the transfer model of diabetes. This failure most likely results from a combination of factors. First, the level of Fascg expression may not be sufficient to block all Fas-mediated signaling. Second, the effectors from the spleen of diabetic NOD mice are likely to contain cells capable of employing other mechanisms of beta cell destruction. Interestingly, in the natural course of diabetes development, the same mutant Fascg was more successful as a disease protector. Thus, some essential stages of diabetes development may depend more than others on Fas.
Figure 4.
Expression of the dominant-negative Fascg mutation on pancreatic beta cells abolishes the acceleration of diabetes caused by the RIP-FasL transgene on transfer of diabetogenic NOD splenocytes. Data represent the mean time of diabetes onset (days after transfer ± SE). n, number of recipients per group. *, The difference between NOD and NOD.RIP-FasL mice is significant by Fisher's LSD test (P = 0.001); **, the difference between NOD.RIP-FasL RIP-Fascg and NOD.RIP-FasL mice is significant by Fisher's LSD test (P = 0.001).
Taken together, our data indicate a significant contribution of Fas-mediated apoptosis to the destruction of insulin-producing cells and the development of diabetes.
Acknowledgments
We thank Jeffrey Bedrozian for technical assistance, Dr. Susan F. Wong (University of Bristol, Bristol, U.K.) for the gift of IS-CD8+ cells and helpful discussions, Dr. S. Nagata (Osaka University Medical School, Osaka) for the kind gift of Fas cDNA, and Drs. L. Matis and Y. Wang (Alexion Pharmaceuticals, New Haven, CT) for the gift of NOD-lpr mice. This work was supported by National Institutes of Health Grant IDDK53561, Juvenile Diabetes Research Foundation International Grants JDRF-166 and JDRF-546 (to A.V.C.), and a Postdoctoral Fellowship from the Juvenile Diabetes Research Foundation International (to A.Y.S.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- FasL
Fas ligand
- IS-CD8+ cells
insulin-specific CD8+ T cells
- LSD
least significant difference
- NOD
nonobese diabetic
- RIP
rat insulin promoter
References
- 1.Tisch R, McDevitt H O. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 2.Delovitch T L, Singh B. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 3.Wong F S, Janeway C A., Jr J Autoimmun. 1999;13:290–295. doi: 10.1006/jaut.1999.0322. [DOI] [PubMed] [Google Scholar]
- 4.Chervonsky A V. Curr Opin Immunol. 1999;11:684–688. doi: 10.1016/s0952-7915(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 5.Eizirik D L, Mandrup-Poulsen T. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 6.Chervonsky A V, Wang Y, Wong F S, Visintin I, Flavell R A, Janeway C A, Jr, Matis L A. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama M, Nagata M, Yasuda H, Arisawa K, Kotani R, Yamada K, Chowdhury S A, Chakrabarty S, Jin Z Z, Yagita H, et al. Diabetes. 2002;51:1391–1397. doi: 10.2337/diabetes.51.5.1391. [DOI] [PubMed] [Google Scholar]
- 8.Amrani A, Verdaguer J, Thiessen S, Bou S, Santamaria P. J Clin Invest. 2000;105:459–468. doi: 10.1172/JCI8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovsky N, Silva D, Socha L, Slattery R, Charlton B. Ann NY Acad Sci. 2002;958:204–208. doi: 10.1111/j.1749-6632.2002.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 10.Amrani A, Verdaguer J, Anderson B, Utsugi T, Bou S, Santamaria P. J Clin Invest. 1999;103:1201–1209. doi: 10.1172/JCI6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong F S, Visintin I, Wen L, Flavell R A, Janeway C A. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, Katagiri T. J Exp Med. 1990;171:519–531. doi: 10.1084/jem.171.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo D, Burkly L C, Widera G, Cowing C, Flavell R A, Palmiter R D, Brinster R L. Cell. 1988;8:159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- 15.Guerder S, Picarella D E, Linsley P S, Flavell R A. Proc Natl Acad Sci USA. 1994;91:5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 17.Siegling A, Lehmann M, Platzer C, Emmrich F, Volk H D. J Immunol Methods. 1994;177:23–28. doi: 10.1016/0022-1759(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 18.Wong F S, Karttunen J, Dumont C, Wen L, Visintin I, Pilip I M, Shastri N, Pamer E G, Janeway C A. Nat Med. 1999;9:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 19.Savinov A Y, Wong F S, Chervonsky A V. J Immunol. 2001;167:6637–6643. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 20.Yabuki A, Suzuki S, Matsumot M, Kurohmaru M, Hayashi Y, Nishinakagawa H. J Vet Med Sci. 2001;63:1339–1342. doi: 10.1292/jvms.63.1339. [DOI] [PubMed] [Google Scholar]
- 21.Venables W N, Ripley B D. Modern Applied Statistics with S-PLUS. 3rd Ed. New York: Springer; 1999. [Google Scholar]
- 22.Kang S M, Schneider D B, Lin Z, Hanahan D, Dichek D A, Stock P G, Baekkeskov S. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 23.Allison J, Georgiou H M, Strasser A, Vaux D L. Proc Natl Acad Sci USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson D P, Setser E, Hall D G, Schwartz S M, Hewitt T, Klevitsky R, Osinska H, Bellgrau D, Duke R C, Robbins J. J Clin Invest. 2000;105:1199–1208. doi: 10.1172/JCI8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shudo K, Kinoshita K, Imamura R, Fan H, Hasumoto K, Tanaka M, Nagata S, Suda T. Eur J Immunol. 2001;31:2504–2511. doi: 10.1002/1521-4141(200108)31:8<2504::aid-immu2504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Lewinsohn D M, Bargatze R F, Butcher E C. J Immunol. 1987;138:4313–4321. [PubMed] [Google Scholar]
- 27.Lagasse E, Weissman I L. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 28.Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, et al. J Exp Med. 1997;186:613–618. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Kim K A, Hwang D Y, Lee T H, Kayagaki N, Yagita H, Lee M S. J Immunol. 2000;164:2931–2936. doi: 10.4049/jimmunol.164.6.2931. [DOI] [PubMed] [Google Scholar]
- 30.Chu J L, Ramos P, Rosendorff A, Nikolic-Zugic J, Lacy E, Matsuzawa A, Elkon K B. J Exp Med. 1995;181:393–398. doi: 10.1084/jem.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison J, Strasser A. Proc Natl Acad Sci USA. 1998;95:13818–13822. doi: 10.1073/pnas.95.23.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su X, Hu Q, Kristan J M, Costa C, Shen Y, Gero D, Matis L A, Wang Y. J Immunol. 2000;164:2523–2532. doi: 10.4049/jimmunol.164.5.2523. [DOI] [PubMed] [Google Scholar]
- 33.Huang B, Eberstadt M, Olejniczak E T, Meadows R P, Fesik S W. Nature. 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 34.Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 35.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 36.Ogata Y, Kimura M, Shimada K, Wakabayashi T, Onoda H, Katagiri T, Matsuzawa A. Cell Immunol. 1993;148:91–102. doi: 10.1006/cimm.1993.1093. [DOI] [PubMed] [Google Scholar]