Abstract
In a mouse experimental asthma model, the administration of bacterial lipopolysaccharide (LPS), particularly at low doses, enhances the levels of ovalbumin (OVA)-induced eosinophilic airway inflammation. In an effort to clarify the cellular and molecular basis for the LPS effect, we demonstrate that the OVA-induced eosinophilic inflammation in the lung is dramatically increased by the administration of LPS in wild-type mice, whereas such increase was not observed in mast-cell-deficient mice or Toll-like receptor (TLR)4-deficient mice. Adoptive transfer of bone-marrow-derived mast cells (BMMCs) from wild-type, but not from TLR4-deficient, mice restored the increased eosinophilic inflammation in mast-cell-deficient mice. Wild-type BMMCs pretreated with LPS in vitro also reconstituted the eosinophilic inflammation. Moreover, in vitro analysis revealed that the treatment of BMMCs with LPS resulted in NF-κB activation, sustained up-regulation of GATA1 and -2 expression, and increased the capability to produce IL-5 and -13. Dramatic increases in the expression of IL-5 and -13 and Eotaxin 2 were detected in LPS-treated BMMCs after costimulation with LPS and IgE/Ag. Overexpression of GATA1, but not GATA2, in MC9 mast cells resulted in increased transcriptional activity of IL-4, -5, and -13. Furthermore, the levels of transcription of Th2 cytokines in BMMCs were decreased by the introduction of small interfering RNA for GATA1. Thus, mast cells appear to control allergic airway inflammation after their activation and modulation through TLR4-mediated induction of GATA1 and subsequent increase in Th2 cytokine production.
Keywords: cytokine, GATA1, mast cell, lipopolysaccharide, bone-marrow-derived mast cells
It is well established that mast cells play a central role in anaphylactic reactions. Mast cells are activated by multivalent binding of antigens to receptor-bound IgE and release inflammatory mediators, such as histamine, prostaglandins, and leukotrienes (1–5). It is also known that mast cells regulate the levels of allergic inflammatory responses in the airways by producing cytokines, such as IL-4, -5, -6, -10, and -13 and TNF-α, which are important in the pathogenesis of various allergic reactions (6, 7). In particular, IL-5 production is reported to be critical for the pathogenesis of eosinophilic infiltration in the lung (8, 9).
It is well recognized that respiratory infection modulates allergic airway inflammation (10). However, as for the role for exposure of bacterial components such as lipopolysaccharide (LPS) in allergic inflammation, there is apparent controversy, as evidenced by studies suggesting protective roles for LPS through Th1 cell induction and studies showing the exacerbating effects of LPS on asthma (11–13). LPS, a major component of the outer membrane of Gram-negative bacteria, is ubiquitously distributed in the environment, including household dusts. Recently, Th1/Th2 inflammatory responses were reported to be influenced by the levels of LPS exposure (14). The exposure to high-level LPS with antigens resulted in increased antigen-specific Th1 responses, whereas a low dose of LPS resulted in Th2 sensitization. Collectively, these results suggest a unique biphasic effect for LPS in allergic inflammatory responses.
Recent progress has revealed that innate immune responses are initiated by various pattern-recognition receptors, Toll-like receptors (TLRs) (15). TLRs comprise a family of proteins that enhance certain cytokine gene transcription in response to various pathogenic ligands and control acquired immune responses such as Th1 responses (16, 17). TLR4 was shown to be a receptor for LPS (18, 19). Recent studies on mouse (20–22) and human (23) mast cells suggested that LPS-induced activation was mediated through TLR4 expressed on mast cells. A protective role for mast cells in bacterial infection was first addressed in a bacterial peritonitis animal model, and the infection was suggested to be mediated by the production of TNFα as a consequence of TLR4 activation (21, 24, 25). More recently, LPS-induced production of inflammatory cytokines (IL-1β, TNF-α, IL-6, and IL-13) from mast cells in the peritoneal cavity and the resulting neutrophil recruitment were suggested to be important for protection in septic peritonitis (26). In addition, TNFα produced by mast cells was reported to be involved in hypertrophy of draining lymph nodes during intradermal bacterial infection (27). However, the consequences of LPS-induced mast-cell activation in allergic airway inflammation have not been well elucidated.
In this study, we investigated the role of TLR4 on mast cells in allergic airway inflammation using mast-cell-deficient W/Wv mice and TLR4-deficient [knockout (KO)] mice. The dramatic enhancement of eosinophilic airway inflammation induced by coadministration of a low-dose LPS and ovalbumin (OVA) at the priming phase in wild-type mice was not observed in W/Wv mice. Cell-transfer experiments using TLR4 KO mast cells indicated that the effect of LPS is mediated through TLR4 on mast cells. Furthermore, we demonstrate that bone-marrow-derived mast cells (BMMCs) cultured with LPS for 1 week show an augmented ability to produce Th2 cytokines, which may help explain the enhancement of allergic eosinophilic airway inflammation.
Results
LPS-Mediated Enhancement of Eosinophilic Inflammation Is Not Observed in W/Wv Mice.
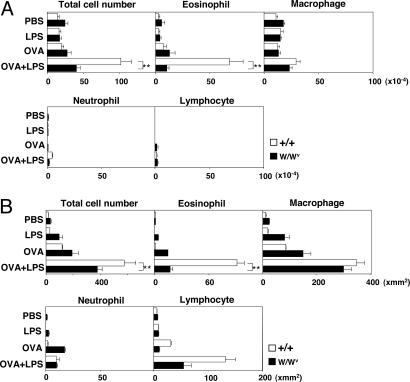
The goal of this study was to clarify molecular targets for LPS in mast-cell activation and allergic eosinophilic airway inflammation. First, we examined the effect of LPS on OVA-induced allergic airway inflammation. Wild-type (+/+) and mast-cell-deficient W/Wv mice were treated intranasally with soluble OVA (10 μg) in conjunction with low-dose LPS (1 μg), and, 2 weeks later, the mice were challenged intranasally with OVA (25 μg). Under these conditions, Th2-skewed eosinophilic inflammation was reproducibly induced in the lung (14). Two days after the final OVA challenge, bronchoalveolar lavage (BAL) fluid was harvested and examined for infiltrating leukocytes. A summary of the types of infiltrating cells is shown in Fig. 1A, and a representative photographic view of May–Giemsa staining in each group can be seen in Fig. 5, which is published as supporting information on the PNAS web site. A modest (≈2-fold) but reproducible increase in the number of eosinophils was detected in mice treated with OVA alone as compared with those in PBS- or LPS-treated mice. The extent of eosinophilic infiltration induced in this protocol was significantly lower than that induced by intraperitoneal immunization of OVA with alum (data not shown) (28, 29). However, the numbers of total cells and eosinophils were significantly increased when wild-type +/+ mice were treated with both OVA and LPS. Intriguingly, when W/Wv mice were treated with OVA plus LPS, the numbers of total infiltrating cells and eosinophils did not increase significantly, as compared with those in +/+ mice. These results point to an important role for mast cells in LPS-mediated enhancement of eosinophilic infiltration in BAL fluid. Furthermore, the expression of Th2 cytokines (IL-4, -5, and -13) and Eotaxin-2 in the BAL fluid of wild-type +/+ and W/Wv mice was examined, and significantly decreased expression was detected in the case of W/Wv mice (see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 1.
LPS-mediated enhancement of eosinophilic inflammation was not observed in W/Wv mice. Wild-type (+/+) and mast-cell-deficient (W/Wv) mice were immunized with OVA (10 μg) in conjunction with 1 μg of LPS intranasally, and, 2 weeks later, OVA (25 μg) was again administrated intranasally. (A) One day after the final OVA administration, BAL fluid was harvested and examined for infiltrating cells. Mean values, with standard deviation, of the numbers of total cells and each cell type are shown. Ten mice were used in each group. ∗∗, <0.01 (Dunnett multiple comparisons test). (B) Histological examination was done 2 days after the final OVA administration. Mean values, with standard deviation, of the numbers of infiltrated leukocytes in 1 mm2 are shown. Ten lung sections per mouse from three mice in each group were examined. ∗∗, <0.01 (Dunnett multiple comparisons test).
Concurrently, changes in lung histology were examined. A summary of the numbers of infiltrating leukocytes calculated by using hematoxylin and eosin (H.E.) staining and Luna staining for eosinophils is shown in Fig. 1B. Among the leukocytes infiltrating, the number of eosinophils was increased dramatically when LPS was coadministered to wild-type mice, and the LPS effect was not observed in W/Wv mice. Sections with H.E. staining can be seen in Fig. 7, which is published as supporting information on the PNAS web site, and Luna staining in Fig. 8, which is published as supporting information on the PNAS web site. Moreover, Masson–trichrome staining revealed substantial levels of subepithelial fibrosis (stained by blue dye) in +/+ mice immunized with OVA plus LPS (see Fig. 9d, which is published as supporting information on the PNAS web site). In W/Wv mice, however, basically no significant fibrosis was detected. Thus, mast cells play a crucial role in low-dose LPS-mediated enhancement of eosinophilic airway inflammation induced by OVA.
Adoptive Transfer of Wild-Type (+/+) BMMCs Reconstitutes LPS-Mediated Enhancement of Eosinophilic Airway Inflammation in W/Wv Mice.
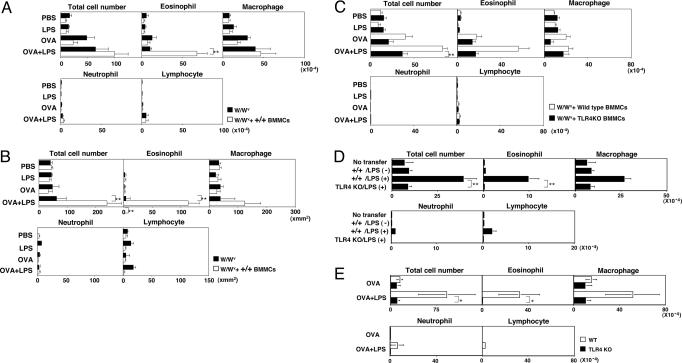
To further investigate the requirement of mast cells in enhanced eosinophilic inflammation induced by OVA plus LPS, wild-type mast cells were adoptively transferred into W/Wv mice. BMMCs were generated by culturing bone marrow cells with IL-3 for 4 weeks. A summary of infiltrating cells in the BAL fluid is presented in Fig. 2A, and representative photographic views of infiltrated cells (May–Giemsa staining) in each group are shown in Fig. 10, which is published as supporting information on the PNAS web site. The administration of +/+ BMMCs resulted in the dramatic eosinophilic infiltration in the BAL fluid of W/Wv mice immunized OVA and LPS when compared with mice not given wild-type (+/+) BMMCs (see Fig. 10h). No apparent effect was observed in the numbers of macrophages, neutrophils, and lymphocytes with the +/+ BMMC transfer.
Fig. 2.
TLR4 on mast cells is required for LPS-induced enhancement of eosinophilic inflammation. (A) BMMCs (1 × 107) were transferred intravenously 2 days before the initial OVA sensitization. The mean values, with standard deviation, of numbers of total cells and each cell type in the BAL fluid of W/Wv mice reconstituted with wild-type (+/+) BMMCs are shown. Ten mice were used in each group. ∗∗, <0.01 (Dunnett multiple comparisons test). (B) Histological analysis of the lung with H.E. and Luna staining. Mean values, with standard deviation, of infiltrated cells in 1 mm2 are shown. Ten lung sections per mouse from three mice in each group were examined. ∗∗, <0.01 (Dunnett multiple comparisons test). (C) Infiltrated cells in BAL fluid of W/Wv mice reconstituted with wild-type (+/+) and TLR4 KO BMMCs are shown. Ten mice were used in each group. ∗∗, <0.01 (Dunnett multiple comparisons test). (D) Infiltrated cells in BAL fluid of W/Wv mice reconstituted with wild-type (+/+) and TLR4 KO BMMCs precultured with or without LPS (10 μg/ml) for 7 days are shown. No immunization was performed. Ten mice were used in each group. ∗∗, P < 0.01 (Student t test). (E) Wild-type and TLR4 KO mice were immunized and challenged, and infiltrated cells in the BAL fluid were assessed as in Fig. 1A. ∗, P < 0.05 (Student t test).
Concurrently, histological analysis showed that the transfer of +/+ BMMCs as described above resulted in the dramatic increase in the levels of eosinophilic infiltration in the airway of W/Wv mice (Fig. 2B). There were also moderate increases in the numbers of total cells. Collectively, these results clearly indicate that mast cells play a critical role in LPS-mediated enhancement of airway eosinophilic inflammation.
TLR4 on Mast Cells Is Required for LPS-Mediated Enhancement of Eosinophilic Inflammation.
The results thus far indicated that mast cells are critical for LPS-mediated enhancement of allergic airway eosinophilic inflammation. Because TLR4 is a known receptor for LPS (30), we next examined the involvement of TLR4 molecules on mast cells in the LPS-mediated enhancement of airway inflammation. BMMCs prepared from TLR4 KO mice showed a normal surface phenotype, e.g., the expression of c-kit and FcεRI (data not shown). Ten million BMMCs prepared from wild-type or TLR4 KO mice were transferred into W/Wv mice. As anticipated, the levels of eosinophilic infiltration were enhanced by the administration of wild-type (+/+), but not in the case of TLR4 KO, BMMCs (Fig. 2C; and see Fig. 11, which is published as supporting information on the PNAS web site). Also, LPS-mediated enhancement in the total numbers of infiltrating leukocytes was marginal in the TLR4 KO BMMC transfer group. It would appear that TLR4 on mast cells is crucial for LPS-mediated enhancement of eosinophilic inflammation.
To further investigate the involvement of TLR4 on mast cells, wild-type and TLR4 KO BMMCs were first treated with LPS (10 μg/ml) for 7 days in vitro and then transferred into W/Wv mice. No immunization was performed. Two days after the challenge with OVA, infiltrated cells in the BAL fluid were assessed. As shown in Fig. 2D, although the levels were modest, a significant increase in the number of eosinophils was detected with the administration of wild-type BMMCs pretreated with LPS. Representative photographic views of infiltrated cells (May–Giemsa staining) are shown in Fig. 12, which is published as supporting information on the PNAS web site. Moreover, TLR4 KO mice were challenged directly with/without LPS and OVA to assess the LPS-mediated enhancement of airway inflammation, and no enhancing effect of LPS on the eosinophilic infiltration was observed in TLR4 KO mice (Fig. 2E; and see Fig. 13, which is published as supporting information on the PNAS web site). The LPS-induced increase in the number of other cell types, including macrophages and neutrophils, was modest, and no increase was seen in TLR4 KO mice.
Treatment with LPS Modulates Cytokine Production Profiles of Mast Cells.
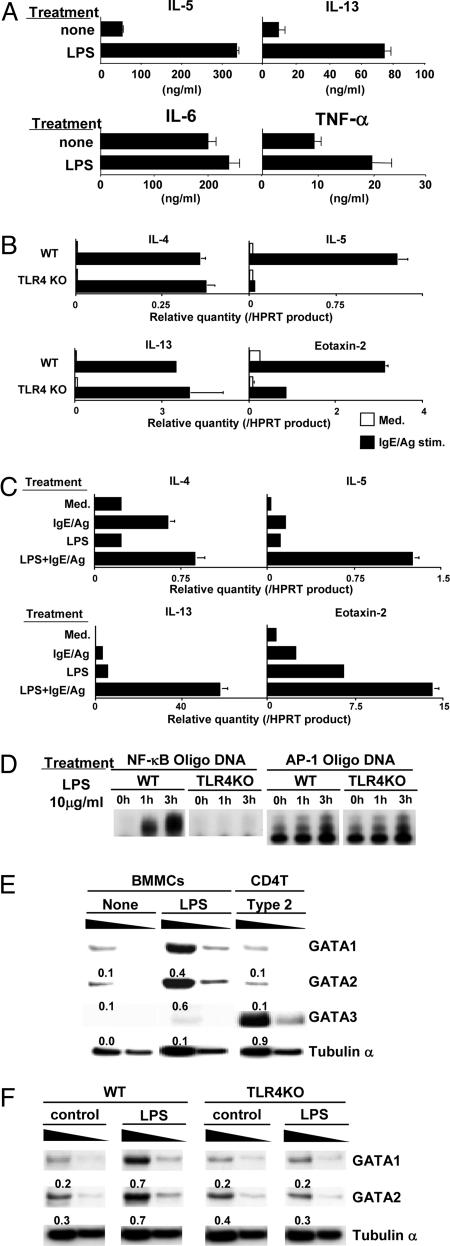
To analyze the molecular changes induced in mast cells after LPS treatment, BMMCs were cultured with or without LPS treatment (10 μg/ml) for 1 week in vitro. The expression levels of FcεRI, c-kit, I-K, CD54, and RIPA/B were similar between wild-type and TLR4 KO BMMCs before and after LPS treatment (data not shown). The LPS-treated BMMCs were then restimulated with phorbol 12-myristate 13-acetate (PMA) plus ionomycin, and the ability to produce various cytokines (IL-5, -13, -6, and -4 and TNF-α) was assessed by ELISA. The levels of IL-5 and -13 were substantially increased after LPS treatment, whereas the production of IL-6 and TNF-α was only slightly increased (Fig. 3A). IL-4 was not detected with or without LPS treatment (data not shown).
Fig. 3.
Cytokine-expression profiles and NF-κB activation in BMMCs treated with LPS. (A) BMMCs were cultured with LPS for 1 week. Then, BMMCs were restimulated with PMA (10 ng/ml) and ionomycin (1 μM) for 24 h to assess the production of IL-5, -13, and -6 and TNF-α by ELISA. Four experiments with individual BMMC preparations were performed, and similar results were obtained. (B) Wild-type (+/+) and TLR4 KO BMMCs were cultured with LPS for 1 week and then stimulated with IgE/Ag. Transcriptional levels of IL-4, -5, and -13 and Eotaxin-2 were determined by real-time RT-PCR analysis. Two independent experiments were performed, and similar results were obtained. (C) Wild-type BMMCs were stimulated with combinations of LPS and IgE/Ag. Transcriptional levels of IL-4, -5, and -13 and Eotaxin-2 were determined by real-time RT-PCR analysis. Two independent experiments were performed, and similar results were obtained. (D) EMSAs for NF-κB and AP-1. Nuclear extracts of wild-type and TLR4 KO BMMCs treated with LPS for 1 and 3 h were subjected to EMSAs with NF-κB and AP-1 probes. Two independent experiments were performed, and similar results were obtained. (E) The levels of protein expression of GATA1 and -2 in LPS-stimulated BMMCs. BMMCs treated with LPS for 3 days and CD4 T cells cultured under Th2-skewed conditions for 3 days were prepared. Nuclear extracts were used for immunoblotting with specific mAbs specific for GATA1, -2, and -3. Arbitrary densitometric units normalized with the band intensity of tubulin α are shown under each band. Three experiments were performed, and similar results were obtained. (F) GATA1 and -2 expression in LPS-treated BMMCs from wild-type and TLR4 KO mice. Two experiments were performed, and similar results were obtained.
Next, we assessed the mRNA expression of IL-4, -5, and -13 and Eotaxin-2 after IgE/Ag stimulation in wild-type and TLR4 KO BMMCs cultured with LPS. The mRNA expression of IL-4, -5, and -13 and Eotaxin-2 was increased dramatically for all of these cytokines in wild-type BMMCs. In the case of TLR4 KO BMMCs, however, the increase was seen in the case of IL-4 and -13 but not in the case of IL-5 and Eotaxin-2 (Fig. 3B). More specifically, the induction in IL-5 was barely detectable, indicating that the Ag/IgE-induced expression of IL-5 was most sensitive to the effect of LPS/TLR4-mediated modification of BMMC function.
To assess more directly whether LPS/TLR4-mediated signaling synergizes with IgE/Ag-dependent responses in BMMCs, BMMCs were stimulated with combinations of LPS and IgE/Ag in vitro. As shown in Fig. 3C, clear synergistic effects were observed in the expression of IL-5 and -13 and some effect on Eotaxin-2 but much less in the case of IL-4.
LPS Treatment Induces NF-κB Activation and Increases Expression of GATA1 and -2 in BMMCs.
Because NF-κB is activated through TLR4 after LPS ligation (18), we wanted to test whether LPS treatment induces NF-κB activation in mast cells. A gel-shift assay for NF-κB was performed with BMMCs treated with LPS. A significant activation of NF-κB was detected in wild-type BMMCs but not in TLR4 KO BMMCs (Fig. 3D Left). In an AP-1 gel-shift assay, a modest increase was detected after LPS treatment, but no difference was observed between wild-type and TLR4 KO groups (Fig. 3D Right).
Although GATA3 is reported to be a downstream target of NF-κB activation (31) and is critical for chromatin remodeling of the Th2 cytokine gene loci (32–34) and transcription of the IL-5 and IL-13 genes in Th2 cells (35–37), GATA3 is not expressed in BMMCs (7). In contrast, GATA1 and -2 play a key role in mast-cell differentiation (38, 39). We examined the levels of protein expression of GATA1 and -2 in BMMCs treated with LPS. As shown in Fig. 3E, the levels of GATA1 and -2, but not -3, were substantially increased in the BMMCs after LPS treatment for 3 days. The LPS-induced increase in the levels of GATA1 and -2 protein was not detected in TLR4 KO BMMCs (Fig. 3F), but increases were detected in STAT6 KO BMMCs (data not shown). The increase in GATA1 and -2 protein was also observed after 7-day cultures (data not shown). Thus, TLR4-mediated signaling is critical for GATA1 and -2 up-regulation in BMMCs upon LPS treatment.
GATA1 Controls the Expression of Th2 Cytokines in Mast Cells.
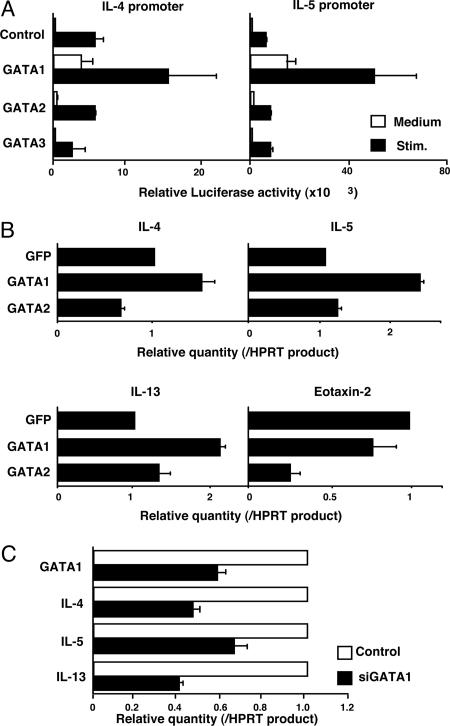
Finally, we studied whether GATA1 and -2 can play a functional role in transcription of IL-5, -13, and -4 in mast cells. We used a MC9 mast-cell line for a reporter gene assay. The introduction of GATA1, but not -2 or -3, into MC9 cells resulted in substantial induction of the reporter activities of IL-4 and -5 promoters (Fig. 4A). When GATA1 was introduced by retrovirus into MC9 cells, the mRNA expression of IL-4, -5, and -13, but not of Eotaxin-2, was increased significantly (Fig. 4B). The increase in Th2 cytokine mRNA was not observed with GATA2 overexpression. Furthermore, we tested whether the inhibition of GATA1 expression in BMMCs would result in decreased expression of Th2 cytokines. BMMCs cultured with LPS were transfected with small interfering (si)RNA specific for GATA1 during the treatment with LPS stimulated with PMA plus ionomycin, and then the transcriptional levels of Th2 cytokines were determined by quantitative PCR. As expected, the mRNA levels of IL-4, -5, and -13 were all decreased significantly (Fig. 4C). These results suggest that GATA1 controls the expression of Th2 cytokines in mast cells, such as MC9 and BMMCs, with LPS stimulation.
Fig. 4.
GATA1 controls the expression of Th2 cytokines in mast cells. (A) A reporter gene assay was done with IL-4 and -5 promoters by using the MC9 mast-cell line. The mean values, with standard deviation, of relative luciferase activity of four different experiments are shown. (B) MC9 cells were infected with retrovirus encoding GATA1 or -2 bicistronically with EGFP. GFP-positive infected cells were enriched by cell-sorting and stimulated with PMA (25 ng/ml) plus ionomycin (500 nM) for 18 h. Quantitative real-time RT-PCR was performed. (C) Inhibition of Th2 cytokine expression by the introduction of siRNA for GATA1. BMMCs were transfected with siRNA specific for GATA1. Two days after transfection, the cells were stimulated with PMA plus ionomycin, and transcriptional levels of GATA1 and Th2 cytokines were determined by quantitative RT-PCR. Two experiments were done with similar results.
Discussion
The results presented here indicate that mast cells and their TLR4 molecules are crucial for LPS-mediated enhancement of allergic airway eosinophilic inflammation. After LPS treatment, BMMCs acquired an increased ability to produce Th2 cytokines, such as IL-5 and -13. Clear synergistic effects in the expression of IL-5 and -13 were detected in LPS-treated BMMCs after costimulation with LPS and IgE/Ag. GATA1 appeared to be important for the transcription of Th2 cytokines in mast cells. These findings suggest that LPS-induced TLR4 signaling modulates mast-cell function and regulates allergic airway inflammation in vivo.
Dendritic cells (DCs) are well recognized to play a central role in inflammatory reactions elicited by LPS (16). When DCs are activated by LPS through TLR4, they become mature and acquire an increased ability to prime T cells (40). In particular, mature DCs produce increased levels of IL-1 and -12 and TNF-α that lead to the promotion of Th1-skewed responses (41). However, it is also well recognized in humans that LPS is a risk factor for asthma (42, 43). In some mouse models, LPS was reported to elicit airway inflammation (14, 22, 44). To investigate the molecular basis underlying the LPS-induced mast-cell activation and regulation of allergic eosinophilic airway inflammation, we used an experimental model with a low-dose LPS administration, in which Th2-dependent eosinophilic airway inflammation is selectively induced (14). As for a mechanism, Eisenbarth et al. (14) suggested the importance of LPS-induced DC activation for the induction of eosinophilic airway inflammation. From the studies presented here, we propose a different mechanism, whereby mast cells, another innate immunity cell type, play a crucial role for regulating eosinophilic airway inflammation. LPS-induced mast-cell activation and modulation with increased production of Th2 cytokines, such as IL-5 and -13, appear to control the severity of eosinophilic airway inflammation.
It is known that IL-5 and -13 play crucial roles in the induction and the severity of eosinophilic airway inflammation in the lung (45). The levels of Th2 cytokines, such as IL-5 and -13, produced by mast cells were increased dramatically after LPS treatment (Fig. 3A). We detected decreased levels of IL-4, -5, and -13 and Eotaxin-2 in BAL fluid cells from mast-cell-deficient W/Wv mice as compared with +/+ mice (Fig. 6). The IgE/Ag-induced IL-5 expression in BMMCs was minimal in TLR4 KO BMMCs (Fig. 3B). Moreover, obvious synergistic effects in the expression of IL-5 and -13 were detected in LPS-treated BMMCs after costimulation with LPS and IgE/Ag (Fig. 3C). Thus, although it is difficult to determine which Th2 cytokines produced by mast cells are most important for LPS-mediated enhancement of allergic eosinophilic inflammation, a direct effect of IL-5 in the activation and migration of eosinophils in the lung appears to be critical. Because BMMCs also produce increased TNF-α (≈2-fold) after LPS treatment (Fig. 3A), it is conceivable that TNF-α produced from activated mast cells may also induce DC maturation and regulate eosinophilic airway inflammation. In addition, Eotaxin-2 expression induced by IgE/Ag stimulation in BMMCs (Fig. 3 B and C) may also contribute to the inflammation by increasing eosinophil migration into the lung.
After in vitro LPS stimulation for a short period (<16 h), Supajatura V. et al. (21) reported that BMMCs produced TNF-α, IL-1β, -6, and -13 but not IL-4 or -5. Similarly Matsuda et al. (22) reported that LPS induced the production of IL-5, -10, and -13, but not of IL-4, in BMMCs. The levels of cytokine production detected in those studies were much lower than those detected in this study (Fig. 3A), due, perhaps, to the different methods of LPS treatment. In our studies, to assess the changes in the ability to produce cytokines in BMMCs, the cells were treated with LPS for 1 week (Fig. 3A). We observed some increase in acetylation of histone H3/K9 at the Th2 cytokine gene loci, suggesting the occurrence of chromatin remodeling (M.Y. and T.N., unpublished observation). Although we do not know whether the LPS treatment induced a true “maturation” of BMMCs, it is clear that BMMCs acquired an ability to produce increased amounts of Th2 cytokines after LPS treatment for 1 week, reminiscent of the maturation process of DCs after LPS stimulation (40).
It has been reported that airway hyperreactivity to methacholine is modulated by the administration of high-dose LPS in rats (44) and mice (46). In the current experimental system, where a mild OVA sensitization protocol was used, we did not observe clear induction of airway hyperreactivity, and treatment with LPS/OVA did not change this situation significantly (M.Y. and T.N., unpublished observation). Further investigation will be needed to clarify the effect of LPS and the contribution of mast cells in the development of airway hyperreactivity.
In summary, in a mouse allergic asthma model, we found that mast cells play a crucial role for LPS-mediated enhancement of eosinophilic airway inflammation. Moreover, TLR4 molecules on mast cells were critical for LPS-induced mast-cell activation and functional modulation. Thus, a search for specific inhibitors acting on the TLR4-mediated signal transduction pathway could lead to an approach for the treatment of inflammation in patients with bronchial asthma, particularly during respiratory infection.
Materials and Methods
Animals.
C57BL/6, WBB6F1 W/Wv mice (47) with (WBxC57BL6)F1 background and WBB6F1 +/+ were purchased from Japan SLC (Shizuoka, Japan). STAT6-deficient and TLR4-deficient (TLR4 KO) mice backcrossed eight times with C57BL/6 mice were kindly provided by Dr. Shizuo Akira (Osaka University) (30, 48). All mice used in this study were maintained under specific-pathogen-free conditions. Animal care was in accordance with the guidelines of Chiba University.
Cell Cultures.
BMMCs were generated as described in ref. 29.
Immunization and Challenge.
Female mice (6–7 weeks old) were anesthetized with ketamine hydrochloride, xylazine, and flunitrazepam i.p. and sensitized intranasally with 10 μg of OVA [Grade V; Sigma Aldrich, LPS contamination <0.5 ng; measured by limulus amebocyte assay (Bio-Whittaker)] and 1 μg of LPS (Escherichia coli O55:B5, List Biological Laboratories, Campbell, CA) in 35 μl of PBS on days 0, 1, and 2. The sensitized mice were challenged on days 14, 15, 18, and 19 intranasally with 25 μg of OVA in 35 μl of PBS under anesthesia as described in ref. 14.
Collection and Analysis of BAL Fluid.
Two days after the last challenge with OVA, BAL was prepared as described in refs. 28 and 29. One-hundred thousand viable BAL cells were cytocentrifuged onto slides by using a Cytospin 3 (Shandon, Pittsburgh) and stained with May–Grunwald–Giemsa solution (Merck) as described in ref. 9. Two hundred leukocytes were counted in each slide. Cell types were identified based on morphological criteria.
Lung Histology.
On day 20, 1 day after the last OVA challenge, mice were killed by CO2 asphyxiation, and the lungs were infused with 10% (vol/vol) formalin in PBS for fixation and then subjected to H.E., Luna, or Masson–trichrome staining as described in refs. 28 and 29.
Treatment of BMMCs with LPS in Vitro.
BMMCs were cultured with or without LPS (10 μg/ml) for 7 days. The LPS-stimulated BMMCs were activated with PMA (10 ng/ml) and ionomycin (1 μM), or with IgE/Ag (anti-DNP IgE and DNP) as described in ref. 49. In the experiments addressing a direct synergistic effect between LPS stimulation and IgE/Ag stimulation, BMMCs were stimulated with a combination of LPS (10 μg/ml) and IgE/Ag in vitro for 24 h. The production of cytokines was assessed by ELISA as described in ref. 50. For transfer experiments, BMMCs (1 × 107) were transferred into W/Wv mice 2 days before the first immunization. In some experiments, BMMCs were pretreated with LPS (10 μg/ml) for 7 days before transfer, and no LPS was used for in vivo priming.
RT-PCR.
Quantitative RT-PCR was performed by using Gene Expression assay (Applied Biosystems) and ABI prism 2000 as described in ref. 34. Hypoxanthine phosphoribosyltransferase was used for a control. The specific primers for detection of cytokines were described in ref. 34. Primer pairs for Eotaxin-2 are tagcctgcgcgtgttgcatcttcc-3′ and 5′-taaacctcggtgctattgccacgg.
Immunoblot Analysis.
Immunoblot analysis for GATA1, -2 and -3 was performed as described in ref. 34. Anti-GATA1 (N1), anti-GATA2 (CG2–96) and anti-GATA3 (HG3–31) (all from Santa Cruz Biotechnology) antibodies were used.
Luciferase Reporter Assay.
Luciferase assay was performed by using Dual Luciferase Reporter instructions as described in ref. 32. A single copy of an IL-4 promoter (−766 bp) and IL-5 promoter (−1,200 bp) in the luciferase reporter plasmid pGL2 Basic (Promega) and MC9 (a mast-cell line expressing some levels of GATA1 and -2 but not GATA3) cells were used.
EMSA
EMSAs were performed by using Gel Shift Assay Systems (Promega) as described in ref. 51.
siRNA.
Introduction of siRNA into BMMCs was performed as described in ref. 52. Predesigned siRNA for GATA1 was purchased from Ambion (#16704). In brief, 2 μl of TransIT-TKO transfection reagent (Mirus, Madison, WI) was diluted in 50 μl of serum-free/antibiotic-free RPMI medium 1640. Ten minutes later, 1 μl of 40 μM siRNA was added to the diluted transfection reagent and incubated for 30 min with gentle agitation. The siRNA solution was added to BMMC cultures containing 5 × 105 cells in 500 μl of medium per well in a 24-well plate. Two days after transfection, expression levels of GATA1 and IL-4, -5, and -13 mRNA were assessed by quantitative RT-PCR.
Supplementary Material
Acknowledgments
We thank Drs. Ralph T. Kubo and Kiyoshi Takeda for helpful comments and constructive criticism in the preparation of the manuscript and Ms. Kaoru Sugaya, Satoko Norikane, and Hikari Asou for excellent technical assistance. This work was supported by Ministry of Education, Culture, Sports, Science and Technology (Japan) Grants 17016010 and 17047007, Grants-in-Aid for Scientific Research in Priority Areas, Scientific Research B 17390139 and Scientific Research C 16616003; Grants-in-Aid for Young Scientists 17790317 and 17790318; and Special Coordination Funds for Promoting Science and Technology, the Ministry of Health, Labor and Welfare (Japan), the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (Japan), the Japan Health Science Foundation, the Kanae Foundation, the Uehara Memorial Foundation, and the Mochida Foundation.
Glossary
Abbreviations:
- BAL
bronchoalveolar lavage
- BMMC
bone-marrow-derived mast cell
- DC
dendritic cell
- H.E.
hematoxylin and eosin
- KO
knockout
- LPS
lipopolysaccharide
- OVA
ovalbumin
- PMA
phorbol 12-myristate 13-acetate
- siRNA
small interfering RNA
- TLR
Toll-like receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Metcalfe D. D., Kaliner M., Donlon M. A. Crit. Rev. Immunol. 1981;3:23–74. [PubMed] [Google Scholar]
- 2.Schwartz L. B., Austen K. F. Prog. Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- 3.Galli S. J. N. Engl. J. Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 4.Boyce J. A. Prostaglandins Leukotrienes Essent. Fatty Acids. 2003;69:195–205. doi: 10.1016/s0952-3278(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 5.Brightling C. E., Bradding P., Pavord I. D., Wardlaw A. J. Clin. Exp. Allergy. 2003;33:550–556. doi: 10.1046/j.1365-2222.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 6.Drazen J. M., Arm J. P., Austen K. F. J. Exp. Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss D. L., Brown M. A. Immunol. Rev. 2001;179:35–47. doi: 10.1034/j.1600-065x.2001.790104.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamelmann E., Gelfand E. W. Immunol. Rev. 2001;179:182–191. doi: 10.1034/j.1600-065x.2001.790118.x. [DOI] [PubMed] [Google Scholar]
- 9.Foster P. S., Hogan S. P., Ramsay A. J., Matthaei K. I., Young I. G. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gern J. E. J. Allergy Clin. Immunol. 2000;105:S497–S502. doi: 10.1016/s0091-6749(00)90050-2. [DOI] [PubMed] [Google Scholar]
- 11.Liu A. H. J. Allergy Clin. Immunol. 2002;109:379–392. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz D. A. Am. J. Respir. Crit. Care Med. 2001;163:305–306. doi: 10.1164/ajrccm.163.2.ed2000a. [DOI] [PubMed] [Google Scholar]
- 13.Braun-Fahrlander C., Riedler J., Herz U., Eder W., Waser M., Grize L., Maisch S., Carr D., Gerlach F., Bufe A., et al. N. Engl. J. Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbarth S. C., Piggott D. A., Huleatt J. W., Visintin I., Herrick C. A., Bottomly K. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeway C. A., Jr., Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 16.Akira S., Takeda K., Kaisho T. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki A., Medzhitov R. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K., Kaisho T., Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 20.McCurdy J. D., Lin T. J., Marshall J. S. J. Leukoc. Biol. 2001;70:977–984. [PubMed] [Google Scholar]
- 21.Supajatura V., Ushio H., Nakao A., Akira S., Okumura K., Ra C., Ogawa H. J. Clin. Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda A., Yoshikai Y., Aiba K., Matsuguchi T. J. Immunol. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 23.Okumura S., Kashiwakura J., Tomita H., Matsumoto K., Nakajima T., Saito H., Okayama Y. Blood. 2003;102:2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 24.Malaviya R., Ikeda T., Ross E., Abraham S. N. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 25.Echtenacher B., Mannel D. N., Hultner L. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 26.Supajatura V., Ushio H., Nakao A., Okumura K., Ra C., Ogawa H. J. Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan J. B., Hart J. P., Pizzo S. V., Shelburne C. P., Staats H. F., Gunn M. D., Abraham S. N. Nat. Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 28.Shibata Y., Kamata T., Kimura M., Yamashita M., Wang C. R., Murata K., Miyazaki M., Taniguchi M., Watanabe N., Nakayama T. J. Immunol. 2002;169:2134–2140. doi: 10.4049/jimmunol.169.4.2134. [DOI] [PubMed] [Google Scholar]
- 29.Kamata T., Yamashita M., Kimura M., Murata K., Inami M., Shimizu C., Sugaya K., Wang C. R., Taniguchi M., Nakayama T. J. Clin. Invest. 2003;111:109–119. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 31.Das J., Chen C. H., Yang L., Cohn L., Ray P., Ray A. Nat. Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita M., Ukai-Tadenuma M., Kimura M., Omori M., Inami M., Taniguchi M., Nakayama T. J. Biol. Chem. 2002;277:42399–42408. doi: 10.1074/jbc.M205876200. [DOI] [PubMed] [Google Scholar]
- 33.Inami M., Yamashita M., Tenda Y., Hasegawa A., Kimura M., Hashimoto K., Seki N., Taniguchi M., Nakayama T. J. Biol. Chem. 2004;279:23123–23133. doi: 10.1074/jbc.M401248200. [DOI] [PubMed] [Google Scholar]
- 34.Omori M., Yamashita M., Inami M., Ukai-Tadenuma M., Kimura M., Nigo Y., Hosokawa H., Hasegawa A., Taniguchi M., Nakayama T. Immunity. 2003;19:281–294. doi: 10.1016/s1074-7613(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee H. J., O’Garra A., Arai K., Arai N. J. Immunol. 1998;160:2343–2352. [PubMed] [Google Scholar]
- 36.Zhang D. H., Yang L., Ray A. J. Immunol. 1998;161:3817–3821. [PubMed] [Google Scholar]
- 37.Schwenger G. T., Fournier R., Kok C. C., Mordvinov V. A., Yeoman D., Sanderson C. J. J. Biol. Chem. 2001;276:48502–48509. doi: 10.1074/jbc.M107836200. [DOI] [PubMed] [Google Scholar]
- 38.Harigae H., Takahashi S., Suwabe N., Ohtsu H., Gu L., Yang Z., Tsai F. Y., Kitamura Y., Engel J. D., Yamamoto M. Genes Cells. 1998;3:39–50. doi: 10.1046/j.1365-2443.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio A. R., Rana R. A., Sanchez M., Lorenzini R., Centurione L., Bianchi L., Vannucchi A. M., Migliaccio G., Orkin S. H. J. Exp. Med. 2003;197:281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banchereau J., Steinman R. M. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 41.Schnare M., Barton G. M., Holt A. C., Takeda K., Akira S., Medzhitov R. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo M. C., Naspitz C. K., Fernandez-Caldas E., Lockey R. F., Mimica I., Sole D. Pediatr. Allergy Immunol. 1997;8:121–126. doi: 10.1111/j.1399-3038.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 43.Michel O., Ginanni R., Duchateau J., Vertongen F., Le Bon B., Sergysels R. Clin. Exp. Allergy. 1991;21:441–448. doi: 10.1111/j.1365-2222.1991.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 44.Tulic M. K., Wale J. L., Holt P. G., Sly P. D. Am. J. Respir. Cell Mol. Biol. 2000;22:604–612. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- 45.Herrick C. A., Bottomly K. Nat. Rev. Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 46.Lefort J., Singer M., Leduc D., Renesto P., Nahori M. A., Huerre M., Creminon C., Chignard M., Vargaftig B. B. J. Immunol. 1998;161:474–480. [PubMed] [Google Scholar]
- 47.Kitamura Y., Go S., Hatanaka K. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 48.Takeda K., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki S., Ishikawa E., Kohno M., Saito T. Blood. 2004;103:3093–3101. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- 50.Kimura M., Koseki Y., Yamashita M., Watanabe N., Shimizu C., Katsumoto T., Kitamura T., Taniguchi M., Koseki H., Nakayama T. Immunity. 2001;15:275–287. doi: 10.1016/s1074-7613(01)00182-0. [DOI] [PubMed] [Google Scholar]
- 51.Kimura M. Y., Hosokawa H., Yamashita M., Hasegawa A., Iwamura C., Watarai H., Taniguchi M., Takagi T., Ishii S., Nakayama T. J. Exp. Med. 2005;201:397–408. doi: 10.1084/jem.20040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita M., Shinnakasu R., Asou H., Kimura M., Hasegawa A., Hashimoto K., Hatano N., Ogata M., Nakayama T. J. Biol. Chem. 2005;280:29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.