Abstract
Phylogenetic inferences drawn from comparative data on mammalian β-globin gene clusters indicate that the ancestral primate cluster contained a locus control region (LCR) and five paralogously related β-type globin loci (5′-LCR-ε-γ-ψη-δ-β-3′), with ε and γ expressed solely during embryonic life. A γ locus tandem duplication (5′-γ1-γ2-3′) triggered γ’s evolution toward fetal expression but by a different trajectory in platyrrhines (New World monkeys) than in catarrhines (Old World monkeys and apes, including humans). In platyrrhine (e.g., Cebus) fetuses, γ1 at the ancestral distance from ε is down-regulated, whereas γ2 at increased distance is up-regulated. Catarrhine γ1 and γ2 acquired longer distances from ε (14 and 19 kb, respectively), and both are up-regulated throughout fetal life with γ1’s expression predominating over γ2’s. On enlarging the platyrrhine expression data, we find Aotus γ is embryonic, Alouatta γ is inactive at term, and in Callithrix, γ1 is down-regulated fetally, whereas γ2 is up-regulated. Of eight mammalian taxa now represented per taxon by embryonic, fetal, and postnatal β-type globin gene expression data, four taxa are primates, and data for three of these primates are from this laboratory. Our results support a model in which a short distance (<10 kb) between ε and the adjacent γ is a plesiomorphic character that allows the LCR to drive embryonic expression of both genes, whereas a longer distance (>10 kb) impedes embryonic activation of the downstream gene.
Keywords: embryonic activation, gene duplication, gene expression, hemoglobin
There are considerable comparative genomic and functional data on the gene loci within mammalian β-globin gene clusters and on the locus control region (LCR) at the 5′ end of the clusters (1–7). Phylogenetic reconstructions carried out on these data depict the following scenario. All genes found within the clusters trace back to a single gene that tandemly duplicated in the early stem-mammalian lineage (8, 9). In the subsequent evolution of the resultant two-gene cluster, the 5′ gene (proto ε) became expressed solely in the embryonic stage of life, whereas the 3′ gene (proto β) became expressed primarily in postembryonic stages. In placental mammalian evolution, further tandem duplications on the cluster’s 5′ side gave rise to three genes (5′-LCR-ε-γ-η-) that trace back to proto ε, whereas on the cluster’s 3′ side, a tandem duplication gave rise to two genes (-δ-β-3′) that trace back to proto β. Not long after these duplications, the early primate η gene became a pseudogene. The linkage order of the LCR and β-type globin genes in the ancestral primate cluster (5′-LCR-ε-γ-ψη-δ-β-3′) still exists in the strepsirrhine primate galago (Fig. 1; refs. 10 and 11). It is likely that in the early primates as in galago (Otolemur), up-regulation of both ε and γ occurred during embryonic life, followed by down-regulation at the start of fetal life and, concomitantly, up-regulation of δ and β (7, 14).
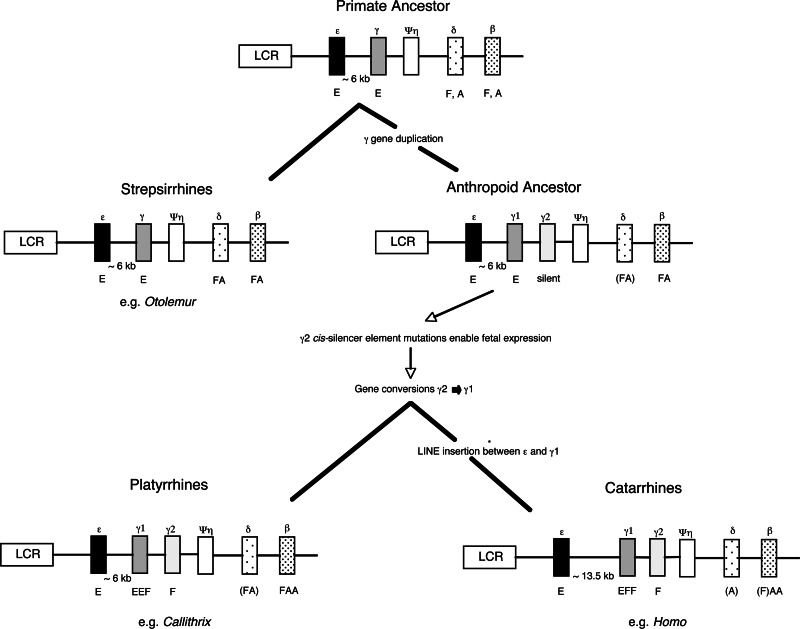
Fig. 1.
Major events in the proposed evolution of β-globin cluster gene expression in primates. The linkage order shown in the primate ancestor is retained in strepsirrhines, represented by the bush baby (Otolemur sp.), along with predominantly ε and γ expression during embryonic life and δ and β during fetal and adult developmental stages. Based on the existence of only one γ gene in tarsiers (12), this pattern likely persisted until the duplication of the γ gene in stem anthropoids. In the anthropoid ancestor, γ2 was relatively silent because of its separation from the LCR by two intervening embryonically expressed genes and to the retention of postembryonic cis-acting silencer elements, such as those found in the bush baby promoter (13). In its silent condition, γ2 was free to acquire the base changes that inactivated those cis elements that bound fetal repressors. Gene conversions from γ2 to γ1 then conferred the capacity for fetal expression on γ1. In platyrrhines, the retained proximity of γ1 to ε (and, by extension, to the LCR) promotes predominantly embryonic expression of γ1; in this clade, represented by the marmoset genus Callithrix, γ2 is the primary fetally expressed gene. In contrast, in stem catarrhines, LINE element insertions between ε and γ1 weakened the LCR’s ability to promote embryonic expression of γ1; in the catarrhine clade, represented here by humans (Homo sapiens), γ1 is the primary fetally expressed gene. E, F, and A indicate embryonic, fetal, and adult stage gene expression, respectively. Where a gene is expressed in more than one stage, the stage of predominant expression, if any, is indicated by double letters. Stages shown in parentheses indicate trace levels of expression.
A γ-globin locus tandem duplication that produced the gene pair 5′-γ1-γ2-3′ occurred in the stem-anthropoid lineage ancestral to both platyrrhines (New World monkeys) and catarrhines (Old World monkeys and apes including humans) (15). A proposed model had the increased distance of the γ2 gene from the LCR as a precondition for the acquisition of fetal expression (14, 16). According to this model, when the γ gene duplication occurred, both ε and close by γ1 remained subject to embryonic activation by the LCR, but γ2 at decreased proximity to the LCR and at increased distance from ε was less open to embryonic activation. The two anthropoid γ genes like the ancestral primate γ had cis-silencer elements that could combine with postembryonic repressors. Thus, initially, the nascent γ2 gene was poorly expressed or silent throughout all developmental stages. The LCR could fetally activate the γ2 gene once mutations in the gene’s cis-silencer elements destroyed the ability to combine with fetal repressors.
The original model had the γ1 locus acquire via gene conversions the cis mutations at the γ2 locus that favored fetal expression, i.e., both γ1 and γ2 acquired a predominant fetal expression pattern in the stem-anthropoid lineage (14). That platyrrhines could have retained an embryonically expressed γ1 gene, however, was suggested (16, 17). Here we present further evidence that agrees with this suggestion and allows us to modify the model by having platyrrhines retain, as a plesiomorphic character, embryonic expression of the γ gene adjacent to ε. We support the modified model both with previously undescribed results about platyrrhine genera Alouatta, Aotus, and Callithrix and with accumulated results on the developmental expression patterns of the globin genes of different primates and other mammals. We also summarize the data on genomic distances within mammalian β-globin gene clusters and observe that a short downstream distance separating a γ gene from ε (<10 kb) is consistent with the LCR embryonically activating this γ gene along with ε, whereas a much longer distance (>20 kb) is consistent with inhibition of embryonic activation of this downstream gene.
Results
Globin Expression During Platyrrhine Development.
Real-time PCR was used to determine the levels of mRNA expression for ε-, γ-, and β-globin genes in the three tissue samples (Table 1). Product was not a mixture of globin gene products. All amplified fragments were found to be homogenous, with the exception of the Callithrix γ PCR products, where each amplicon contained a mixture of γ1 and γ2.
Table 1.
Globin gene expression in platyrrhine embryonic, fetal, and placental tissues
| Species | Tissue | Gene | Percent of total β-type globin message |
|---|---|---|---|
| Aotus | Embryo | ε | 61.6, 67.1 |
| γ | 35.4, 30.2 | ||
| β | 2.9, 2.7 | ||
| Callithrix | Fetus | ε | 0.3, 0.6 |
| γ1 | 6.7*, 12.6* | ||
| γ2 | 91.3*, 84.4* | ||
| β | 1.7, 2.4 | ||
| Alouatta | Term placenta | ε | 0, 0 |
| γ | 0, 0 | ||
| β | 100, 100 |
Two values are shown for two real-time PCR assays.
*Callithrix PCR products were mixtures of both γ genes.
Restriction Digests of γ Amplicons.
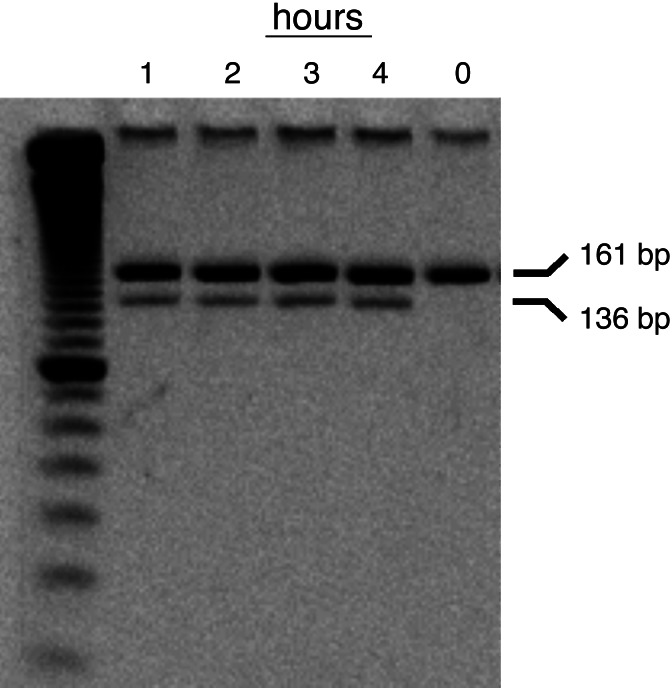
We used restriction digestion coupled with densitometry to define the ratio of γ1 and γ2 mRNA expression in the Callithrix fetal tissue more precisely. A primer set was designed that would hybridize to identical sequences on Callithrix γ1 and γ2 that flank a base difference that creates a restriction site for EcoICRI in the γ1 sequence (Data Set 1, which is published as supporting information on the PNAS web site). After digestion with EcoICRI, the γ2 amplicon remains intact, whereas the γ1 amplicon yields fragments of 136 bp and 24 bp. The digests were separated on a polyacrylamide gel and quantitated by SYBR staining (Fig. 2). No further cleavage occurred after an hour. The average γ2/γ1 ratio in three runs was 6.6 ± 0.7, indicating that in the Callithrix fetus, the γ2 gene is expressed at a much higher level than γ1.
Fig. 2.
Representative time course of digestion of amplicons from Callithrix γ globin genes. PCR of RNA from a C. penicillata fetus was performed by using a primer set that complements sequences that are identical in C. jacchus γ1 and γ2, but which enclose a region with a base pair difference between γ1 and γ2 that produces an EcoICRI cleavage site in γ1. The mixture of amplified fragments from fetal mRNA was digested with EcoICRI, yielding two fragments of 136 and 24 bp from the γ1 amplicon. To ensure that digestion was complete, a time course across four time points (1 h, 2 h, 3 h, and 4 h) was run. A 10-bp ladder is in lane 1. The mean γ2/γ1 ratio for three digestions was 6.6 ± 0.7.
γ Expression in an Aotus Embryo.
Evidence for embryonic expression of a platyrrhine γ gene was obtained in Aotus embryonic tissue. Aotus has only a single fused γ gene, 5′-γ1/γ2-3′ (18), and, notably, the promoter of the fused gene is that of γ1. In the Aotus infulatus embryo that we assayed (Table 1), we found a significant level of γ expression (ε, γ, and β expression levels of 62%, 36%, and 3%, respectively). Combining these data with earlier findings (Table 2) clearly shows the progression of Aotus γ expression from embryonic to fetal and its early, prenatal, down-regulation. In this progression, the fused γ gene with its γ1 promoter is more strongly expressed in the embryo than in the fetus. It should be noted that the Aotus fetal and postnatal samples that were examined in ref. 19 were from A. azarae, a close relative of A. infulatus.
Table 2.
Globin gene expression during platyrrhine development
| Species | Tissue | ε | γ | δ | β |
|---|---|---|---|---|---|
| Aotus | Embryo* | 65 | 32 | nd | 3 |
| Fetus† | 0 | 24 | 1 | 75 | |
| Newborn† | 0 | 0 | 1 | 99 | |
| Adult† | 0 | 0 | 1 | 99 | |
| Callithrix | Embryo‡ | high | high/0 | ? | ? |
| Fetus* | 0 | 13/85 | 0 | 2 | |
| Adult† | 0 | 0/0 | 4 | 96 | |
| Cebus | Fetus†,‡ | 0 | 0/46 | trace | 54 |
| Newborn† | 0 | 0/1 | 1 | 98 | |
| Adult† | 0 | 0/1 | 1 | 98 | |
| Alouatta | Term placenta* | 0 | 0 | 0 | 100 |
The values are the percent of the total β-type globin expression. γ values reflect fused γ1/γ2 in Aotus, γ1 and γ2 in Callithrix and Cebus, and γ in Alouatta. nd, no data.
*This work. Additional Callithrix fetuses were examined earlier by using HPLC (19). The progression from γ to β expression was further advanced in these samples, but it was not possible to determine which γ gene was being expressed.
†Data from ref. 19.
‡Data from ref. 16.
Early Down-Regulation of γ Expression in Alouatta.
We added to the evidence for a prenatal switch from γ to β expression in platyrrhines by observing that all of the β-cluster globin message was β in Alouatta term placental tissue (Table 1). The platyrrhines so far studied have a hemomonochorial placenta with a trabecular maternofetal interdigitation and a multivillous fetomaternal blood flow interrelationship (20). Although maternal capillaries empty into the intervillous/intertrabecular space (21, 22), the term placenta of Ateles (spider monkey), a close relative of Alouatta, has little maternal blood in the intervillous space (K. Benirschke, http://medicine.ucsd.edu/cpa/mon.html). Because there is no indication that the howler monkey deviates from the placentas of other platyrrhines, it is likely that nearly all of the β-globin cluster message in the Alouatta term placenta is of fetal origin. These data provide additional support for our earlier conclusion that the γ to β switch is complete before birth in platyrrhines.
Discussion
The expression of each functional β-type globin gene is shaped by the close-range effects of proximal cis-acting regulatory elements, the trans-acting environment, and, crucially, the long-range effects of the LCR. In erythroid cells, the LCR activates high levels of β-type globin gene expression. The mechanism for activation by the LCR is not known, however, although various models, including tracking and looping, have been proposed (1, 4, 23–25). In tracking models, transcription spreads from DNase1 hypersensitive sites within the LCR, first, to encompass the β-type globin genes closest to the LCR, and then, as development proceeds, the complete β-globin gene cluster. In looping models, the LCR interacts physically with each expressed β-type globin gene. By either tracking or looping, the LCR preferentially activates in the embryo the cluster’s most 5′ genes, and when these genes are deactivated by postembryonic repressors, the LCR activates the more distal 3′ genes. Transgenic mice experiments have established for large cosmid or YAC transgenes spanning the entire β-globin gene cluster that the gene closest to the LCR is always expressed in embryonic erythroid cells (26–28), even when the gene order is reversed, making β, rather than ε, closest to the LCR (29). Similarly, when an extra adult β-globin gene is placed just 5′ of the ε-globin gene, this extra β is expressed embryonically (30).
Comparative data on the LCR and genes in mammalian β-globin gene clusters reveal that in both marsupials and placental mammals, the ε-globin gene is closest to the LCR and is indeed solely an embryonic gene (Table 3). The distance of ε from the LCR, as measured from the highly conserved hypersensitive 2 site, ranges from 6 kb to 11 kb, with the distance to ε being smallest in murids (6–7 kb) and largest in anthropoids (10–11 kb). Marsupial β, which is >15 kb downstream from marsupial ε, is nonexpressed in the embryo but is fully expressed in the fetus and in all subsequent developmental stages. This developmentally and genomically differentiated two-gene cluster probably already existed in the last common ancestor of marsupials and placental mammals, with the high expression level of each β-type globin gene dependent on long-range effects of the LCR. Of course, differences in expression between embryonic proto ε and postembryonic proto β could not have been solely due to the greater distance of proto β from the LCR but would also have depended on cis-regulatory evolution and corresponding trans-factor binding differences between the two loci. The ε promoter has an assortment of cis-acting elements (44) that bind postembryonic repressors (13). Clearly, by either tracking or looping mechanisms, differences in the trans-acting environment between embryonic and postembryonic erythroid cells are likely to be involved in the longer reach of the LCR in postembryonic erythroid cells.
Table 3.
Intergenic distances and expression patterns of the major β-type globin genes expressed during embryonic (e), fetal (f), and postnatal (pn) developmental stages
| Taxon | HS2-ε | ε expression |
ε-γ, ε-γ1, or ε-η | γ, γ1, or η expression |
ε-γ2 | γ2 expression |
ε-β | β expression |
Expression references | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e | f | pn | e | f | pn | e | f | pn | e | f | pn | ||||||
| Homo sapiens* | 10,446 | ✓ | x | x | 13,569 | ✓ | ✓✓ | x | 18,493 | x | ✓ | x | 41,279 | x | x | ✓ | 1, 2 |
| Macaca mulatta | 10,504 | nd | x | x | 13,642 | nd | ✓ | x | 18,566 | nd | ✓ | x | 41,299 | nd | ✓ | ✓✓ | 31 |
| Papio anubis | 11,049 | nd | nd | nd | 14,456 | nd | ✓ | x | 19,347 | nd | ✓ | x | 42,051 | nd | ✓ | ✓✓ | 32‡, 33‡ |
| Cebus albifrons | nd | nd | nd | nd | 5,331 | nd | x | x | 10,240 | nd | ✓ | x | nd | nd | ✓ | ✓✓ | 16, 19 |
| Callithrix jacchus* | 10,651 | ✓ | x | x | 7,152 | ✓✓ | ✓ | x | 12,183 | x | ✓ | x | 33,940 | nd | ✓ | ✓✓ | 16, 19, this work |
| Aotus trivirgatus*† | nd | ✓ | x | x | 7,000 | ✓✓ | ✓ | x | — | — | — | — | 28,600 | x | ✓ | ✓✓ | 19, this work |
| Otolemur crassicaudatus* | 9,809 | ✓ | x | x | 6,182 | ✓ | x | x | — | — | — | — | 29,193 | — | ✓ | ✓ | 7 |
| Mus musculus* | 7,690 | ✓✓ | ✓ | x | 8,597 | ✓✓ | ✓ | x | — | — | — | — | 43,404 | x | ✓ | ✓✓ | 34, 35 |
| Rattus norvegicus | 6,038 | nd | ✓ | x | 9,685 | nd | ✓ | x | — | — | — | — | 31,299 | nd | ✓ | ✓✓ | 36 |
| Oryctolagus cuniculus* | 10,265 | ✓✓ | ✓ | x | 7,978 | ✓ | x | x | — | — | — | — | 23,427 | x | ✓ | ✓✓ | 37 |
| Bos taurus | 9,947 | nd | nd | nd | 7,524 | nd | nd | nd | — | — | — | — | 23,456 | nd | nd | nd | — |
| Capra hircus*† | 8,560 | ✓ | x | x | 7,220 | ✓ | x | x | — | — | — | — | 21,100 | x | x | ✓ | 38–41 |
| Sminthopsis macroura* | 7,420 | ✓✓ | ✓ | x | — | — | — | — | — | — | — | — | 15,916 | x | ✓ | ✓✓ | 42, 43 |
GenBank IDs of sequences used to determine intergenic distances are provided as Table 4, which is published as supporting information on the PNAS web site. Where species-specific data were not available, distances were obtained by bioinformatically probing one taxon with sequences from another closely related taxon; results from this approach are underlined. Underlined data on expression patterns are always derived from species within the same genus. ✓ indicates a gene is expressed at more than trace levels during a particular developmental stage. If expressed in more than one stage, the relative amounts are denoted by the number of ✓s indicated. x, nd, and — indicate no expression, no data, and not applicable, respectively.
*Indicates taxa for which there are complete β-globin cluster expression data available, i.e. from embryonic, fetal and postnatal stages.
†Indicates taxa for which intergenic distances are based on physical measurements from published data.
‡Reported only total γ chain synthesis levels, with no distinction between γ1 and γ2.
More complex β-globin gene clusters with complex gene expression patterns emerged in the placental mammals where, because of tandem duplications on the cluster’s 5′ side, ε, γ, and η trace back to proto ε and, on the cluster’s 3′ side, δ and β trace back to proto β. Thus γ and η as well as ε probably retained cis-acting elements associated with embryonic expression, whereas δ and β retained the elements associated with postembryonic expression. Whether either γ or η is embryonic would then have depended on that gene’s closeness to ε, i.e., its proximity to and distance from the LCR. The data in Table 3 document for placental mammals that when a downstream β-type globin gene is separated from ε by only a short distance, <10 kb, this downstream gene and ε is mainly expressed during embryonic life. Murids, rabbits, and noncatarrhine primates have γ-globin genes that are within 10 kb of the ε-globin gene and are embryonic in their expression patterns. Goats and cows lack the γ locus but have a functional η-globin gene that is close to ε (<10 kb apart) and has the properties of an embryonic gene. The data in Table 3 further show that when a downstream functional β-type globin gene is separated from ε by >10 kb, postembryonic expression of this downstream gene predominates over embryonic expression, and when the distance from ε is >20 kb, the β-type globin gene at the increased distance is inactive during embryonic life.
At least several independent increases in downstream distance from the LCR appear to be associated with embryonic down-regulation. As pointed out above, the last common ancestor of marsupials and placental mammals probably had a two-gene cluster in which the embryonically expressed gene (ε) was closest to the LCR, whereas the postembryonically expressed gene (β) at >15kb downstream from ε was furthest from the LCR. During primate evolution, to elaborate on the scenario sketched out in the Introduction, in the stem anthropoid lineage, a 5.5-kb DNA fragment containing the γ-globin gene locus tandemly duplicated, which left γ1 at the ancestral distance, ≈6 kb, from ε but placed γ2 at ≈12 kb from ε. This more-downstream locus (γ2) then acquired the predominating fetal expression patterns that have been retained in platyrrhines and catarrhines. In the stem-catarrhine primate lineage, LINE insertions between ε and γ1 increased the intergenic distance between the two loci to 14 kb (45) and the γ1 gene in catarrhines then became the primary fetally expressed gene (refs. 1, 2, 31, and 45; see also Table 4, which is published as supporting information on the PNAS web site). During bovid evolution, block duplications that did not include the LCR gave the goat β-globin gene cluster three sets of cluster genes (46). As a result, the goat has three ε orthologs and three η orthologs but only ε and η in the most 5′ block, thus most proximal to the LCR, are expressed and both are exclusively expressed in the embryonic yolk sac (38–41). None of the goat’s three β orthologs are expressed in the embryo (39, 40). Cis-regulatory mutations resulted in the 5′ block having βc expressed in the juvenile goat, the middle block having β expressed postnatally (i.e., in the adult), and the 3′ block having βF expressed during fetal life (46).
We suggest that during evolution increases in the downstream distance from the LCR and from ε to another β-type globin gene greatly weakened the LCR’s ability to promote embryonic expression of this downstream gene (Table 3). In turn, the developmental stage-specific expression of the downstream gene largely depended on nearby cis-regulatory elements and the trans-factors that bind to these elements. This scenario could explain the goat results in which βF is at the longest downstream distance from the LCR and is expressed at an earlier ontogenetic stage (fetal) than is the adult β (postnatal). Results obtained on platyrrhine γ1 genes suggest that gene-proximal cis-regulatory elements may also shape the postembryonic expression patterns of embryonically expressed genes. The Callithrix γ1 promoter provides a possible example. In addition to base changes in known phylogenetic footprints, we find that a truncated LINE insertion occurred just upstream of the Callithrix γ1 core promoter at −576 (Fig. 3C, which is published as supporting information on the PNAS web site). LINE elements can inhibit transcription by acting as a start site for repressive transcripts (47). This −576 LINE insertion lacks a transcriptional start site but could introduce cis-binding sites or inhibit transcription by altering the relation between the promoter and enhancer elements. In the deletional form of hereditary persistence of fetal hemoglobin, deletions within the γ promoter lead to overexpression of fetal hemoglobin. It has been suggested that these deletions bring downstream enhancer elements closer to the core promoter, augmenting their ability to drive transcription (48, 49). We suggest that the LINE insertion here may have the complementary effect, moving LCR enhancers away from the core promoter to down-regulate fetal transcription. This truncated LINE insertion lies upstream of −169, the 5′ limit of the Callithrix γ conversion (17) and, therefore, has escaped any corrective gene conversion from γ2.
The results obtained in mice emphasize that the trans-acting environment also plays a major role in determining whether a β-type globin gene is expressed embryonically. Yeast artificial chromosome transgenes that contain the entire human β-globin gene cluster plus extensive 5′ and 3′ flanking sequences express the human γ1 gene to a greater extent in the mouse embryo than in the mouse fetus (27, 50), a pattern that simulates the embryonic expression levels of the mouse’s endogenous γ and ε genes. Yet, in humans, the γ1 gene is expressed to a much greater extent in the fetus than in the embryo. This difference in expression patterns probably results from the mouse’s trans-acting environment being different from the human’s. For example, GATA1, an erythroid transcription factor that interacts with the LCR, resides at a locus with marked differences in chromatin structure in mice and humans (51). Moreover, cis-elements in the GATA1 locus show greater binding affinity for trans-factors in mouse than in human erythroid cells (51). If this pattern holds for embryonic erythroid cells as well, then the different trans-acting transcriptional environments that apparently exist in human and mouse may account for human γ1 being a major embryonic gene in transgenic mice but only a minor embryonic gene in humans, where it is the major β-type globin fetal gene.
Our updated model for the regulatory evolution of primate γ-globin genes predicts that the short distance between ε and the adjacent γ gene in platyrrhines allows the LCR to activate both genes in embryonic erythroid cells of the yolk sac. Accordingly, the platyrrhine γ1 genes never were primarily fetally, but rather have remained embryonically, expressed despite gene conversions from the fetal γ2 gene. This conclusion can account for earlier observations that were difficult to fit into a coherent model. Several platyrrhine clades have what appear to be harmful mutations in the proximal CCAAT box of the γ1 promoter (18), as judged by findings in which the wild-type CCAAT motif is a promoter element needed for expression of β-globin gene (52). Yet, these platyrrhine γ1 genes have none of the characteristics of pseudogenes. However, if the genes are primarily embryonic, the CCAAT mutations would not be inactivating, because the proximal CCAAT box is not required for embryonic expression (52, 53) but may be required for fetal expression. Fetal inactivation might also be caused, as pointed out above, by the truncated LINE sequence found in the Callithrix γ1 promoter.
An additional feature of platyrrhine γ expression is that the switch from γ to β expression occurs during midfetal life, whereas in catarrhines, the full switch to β does not occur until months after birth (31, 54). The results reported here for Alouatta reconfirm this conclusion. It may be noted that among the platyrrhines examined during midportions of fetal life, the switch from γ to β expression was most pronounced in Aotus (Table 2), and the distance of β from ε is shortest in Aotus (Table 3). Catarrhines show the most delayed switch from γ to β expression and the longest distance between ε and β. These results indicate that in primates, the greater the distance of a downstream β-type globin gene from ε, and from the LCR, the greater the delay in ontogeny before up-regulation of the downstream gene. Selection of the appropriate gene-proximal cis-regulatory elements would still be required. However, it would fit either a tracking or looping mechanism for LCR activation if the β-type globin gene chosen to be fetally expressed (γ2 in the stem-anthropoid lineage) was genomically the next in line on moving downstream from the embryonically expressed γ1 gene.
Materials and Methods
The tissue samples used in the analysis were obtained from the Centro Nacional de Primatas, Ananindeua, Parà, Brazil, and were as follows: whole embryonic tissue from the owl monkey Aotus infulatus, whole fetal tissue from the marmoset Callithrix penicillata, and term placental tissue from a hybrid offspring of the howler monkeys Alouatta belzebul and Alouatta caraya. The samples were received in 1998 and have been stored in liquid nitrogen.
PCR.
Total RNA was isolated from each tissue sample by using the RNAqueous-4PCR isolation kit (Ambion, Austin, TX) and treated with RNase-free DNase. The quantity and purity of RNA in each sample was determined by absorbance at 260 and 280 nm. Primers (sequences are given in Data Set 1; see also Data Sets 2 and 3, which are published as supporting information on the PNAS web site) were designed to amplify fragments of <200 bp to maximize efficiency. In most cases, interference by any remaining genomic DNA was avoided by using primer pairs that amplify genomic DNA segments across an exon-intron boundary. To test the primers, reverse transcription reactions were performed by using the SuperScript One Step RT-PCR with Platinum Taq (Invitrogen Life Technologies). All reactions contained ≈1 μg of total RNA, 200 units of enzyme, and 5 pM of each primer in a total volume of 50 μl. The RT-PCR sequence was 1 cycle of reverse transcription at 45°C for 40 min, followed by PCR cycles consisting of an initial denaturation of 94°C for 5 min for 32 cycles of touchdown PCR amplification (4 cycles for each °C) 94°C for 45s; 52°C-45°C for 45 s; 68°C for 2 min, and a final extension of 68°C for 10 min. The resulting fragments were sequenced to determine that the correct globin mRNA was amplified.
With tested primers, quantitative real-time RT-PCR assay of transcripts was carried out in a Light Cycler (Roche) with SYBR green fluorescence (55) by using the Quantitect SYBR Green RT-PCR kit (Qiagen, Valencia, CA) as described by the manufacturer. To determine the relation between relative concentration and Ct, dilutions of the RNA preparation were amplified with a β-actin primer set that had been designed in this laboratory by using publicly available primate sequences. It was found that a one-log increase in RNA concentration lowered Ct by 3.27 cycles. Replicate PCR using primer sets for the globin genes for each species was performed on the same dilutions, and the relative amounts of each globin message were calculated.
Restriction Digest and Electrophoresis of Callithrix Amplicons.
We designed a primer set (GgloU35869 in exon 3 and GgloL36029 in the 3′ UTR) to complement sequences, which are identical in γ1 and γ2 but flank a region with five base pair differences. PCR produced a mixture of two amplified fragments, one from the γ1 mRNA and one from γ2 mRNA. Because the fragments are nearly the same size (160 and 161 bp) and are obtained by using the same primer pair, it is reasonable to conclude that they are amplified with equal efficiency. To determine the amount of each fragment, we took advantage of the fact that the γ1 sequence contains a restriction site for EcoICRI, which is not found in γ2. Thus, digestion with EcoICRI yields two fragments from the γ1 amplicon (136 and 24 bp), whereas the γ2 amplicon remains intact. For the restriction digest, 1 μg of DNA (obtained from amplified RT-PCR product) was digested with EcoICRI (5 units) in a final volume of 20 μl. Each time point sample was electrophoresed on a 12% polyacrylamide gel, with five aliquots of the undigested cDNA as standards. The bands were detected by ChemiImager (Alpha Innotech, San Leandro, CA) after staining with SYBR green and quantified by using a densitometer (Molecular Dynamics).
DNA Sequencing.
DNA sequencing reactions were done by using the Big-Dye primer cycle sequencing kit (Applied Biosystems) according to the manufacturer’s specifications. Sequencing was performed on the ABI 3700 (Applied Biosystems).
Callithrix Promoter Analysis.
Phylogenetic footprints are sequences of six or more bases that are evolutionarily highly conserved and have been shown to bind nuclear factors or to have a regulatory function in transgenic mice (7, 12, 13, 44, 56–59). Using clustalx (60), the 5′-flanking regions of Callithrix γ1 (AH010019) and γ2 (AF016991) were aligned from the cap site to position −1,200 to identify potential sequence differences that may have led to a relative down-regulation of γ1 (Fig. 3). We focused on those base changes within 12 regulatory motifs, including nine in the +1 to −235 region (61) that have been characterized by molecular biological techniques, and an additional three in the −235 to −1,200 region identified by phylogenetic footprinting (12, 44, 56, 62).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 DK56927 and National Science Foundation Grant BCS-0318375.
Abbreviation
- LCR
locus control region.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bunn H. F., Forget B. G. Hemoglobin: Molecular, Genetic, and Clinical Aspects. Philadelphia: Saunders; 1986. [Google Scholar]
- 2.Collins F. S., Weissman S. M. Prog. Nucleic Acid Res. Mol. Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- 3.Fraser P., Pruzina S., Antoniou M., Grosveld F. Genes Dev. 1993;7:106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
- 4.Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 5.Hardison R., Slightom J. L., Gumucio D. L., Goodman M., Stojanovic N., Miller W. Gene. 1997;205:73–94. doi: 10.1016/s0378-1119(97)00474-5. [DOI] [PubMed] [Google Scholar]
- 6.Li Q., Peterson K. R., Fang X., Stamatoyannopoulos G. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagle D. A., Koop B. F., Goodman M., Slightom J. L., Hess D. L., Jones R. T. J. Mol. Biol. 1988;203:439–455. doi: 10.1016/0022-2836(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 8.Goodman M., Koop B., Czelusniak J., Weiss M. J. Mol. Biol. 1984;180:802–823. doi: 10.1016/0022-2836(84)90258-4. [DOI] [PubMed] [Google Scholar]
- 9.Koop B. F., Goodman M. Proc. Natl. Acad. Sci. USA. 1988;85:3893–3897. doi: 10.1073/pnas.85.11.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagle D. A., Stanhope M. J., Siemieniak D. R., Benson P., Goodman M., Slightom J. L. Genomics. 1992;13:741–760. doi: 10.1016/0888-7543(92)90150-q. [DOI] [PubMed] [Google Scholar]
- 11.Slightom J. L., Bock J. H., Tagle D. A., Gumucio D. L., Goodman M., Stojanovic N., Jackson J., Miller W., Hardison R. Genomics. 1997;39:90–94. doi: 10.1006/geno.1996.4458. [DOI] [PubMed] [Google Scholar]
- 12.Hayasaka K., Skinner C. G., Goodman M., Slightom J. L. Genomics. 1993;18:20–28. doi: 10.1006/geno.1993.1422. [DOI] [PubMed] [Google Scholar]
- 13.Gumucio D. L., Shelton D. A., Blanchard-McQuate K., Gray T., Tarle S., Heilstedt-Williamson H., Slightom J. L., Collins F., Goodman M. J. Biol. Chem. 1994;269:15371–15380. [PubMed] [Google Scholar]
- 14.Goodman M., Slightom J. L., Gumucio D. L. In: Gene Families: Structure, Function, Genetics, and Evolution. Holmes R. S., Lim H. A., editors. Singapore: World Scientific; 1996. pp. 43–52. [Google Scholar]
- 15.Fitch D. H., Bailey W. J., Tagle D. A., Goodman M., Sieu L., Slightom J. L. Proc. Natl. Acad. Sci. USA. 1991;88:7396–7400. doi: 10.1073/pnas.88.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu C.-H., Gregoire L., Gumucio D. L., Muniz J., Lancester W., Goodman M. J. Exp. Zool. 1999;285:27–40. [PubMed] [Google Scholar]
- 17.Chiu C. H., Schneider H., Slightom J. L., Gumucio D. L., Goodman M. Gene. 1997;205:47–57. doi: 10.1016/s0378-1119(97)00476-9. [DOI] [PubMed] [Google Scholar]
- 18.Chiu C.-h., Schneider H., Schneider M. P. C., Sampaio I., Meireles C., Slightom J. L., Gumucio D. L., Goodman M. Proc. Natl. Acad. Sci. USA. 1996;93:6510–6515. doi: 10.1073/pnas.93.13.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson R. M., Buck S., Chiu C., Schneider H., Sampaio I., Gage D. A., Shen T. L., Schneider M., Muniz J. A., Gumucio D. L., Goodman M. J. Biol. Chem. 1996;271:14684–14691. doi: 10.1074/jbc.271.25.14684. [DOI] [PubMed] [Google Scholar]
- 20.Benirschke K., Kauffman P. Pathology of the Human Placenta. New York: Springer; 2000. [Google Scholar]
- 21.Hill J. P. Philos. Trans. Roy. Soc. London B. 1932;221:45–178. [Google Scholar]
- 22.Luckett W. In: Reproductive Biology of the Primates. Luckett W., editor. Vol. 3. Basel: Karger; 1974. pp. 142–234. [Google Scholar]
- 23.Routledge S. J., Proudfoot N. J. J. Mol. Biol. 2002;323:601–611. doi: 10.1016/s0022-2836(02)01011-2. [DOI] [PubMed] [Google Scholar]
- 24.Tolhuis B., Palstra R., Splinter E., Grosveld F., de Laat W. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 25.Tuan D., Kong S., Hu K. Proc. Natl. Acad. Sci. USA. 1992;89:11219–11223. doi: 10.1073/pnas.89.23.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillon N., Trimborn T., Strouboulis J., Fraser P., Grosveld F. Mol. Cell. 1997;1:131–139. doi: 10.1016/s1097-2765(00)80014-3. [DOI] [PubMed] [Google Scholar]
- 27.Gaensler K. M. L., Kitamura M., Kan Y. W. Proc. Natl. Acad. Sci. USA. 1993;90:11381–11385. doi: 10.1073/pnas.90.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson K. R., Stamatoyannopoulos G. Mol. Cell. Biol. 1993;13:4836–4843. doi: 10.1128/mcb.13.8.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimoto K., Liu Q., Bungert J., Engel J. Nature. 1999;398:344–348. doi: 10.1038/18698. [DOI] [PubMed] [Google Scholar]
- 30.Harju S., Navas P. A., Stamatoyannopoulos G., Peterson K. R. Mol. Cell. Biol. 2005;25:8765–8778. doi: 10.1128/MCB.25.20.8765-8778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R. M., Buck S., Chiu C. H., Gage D. A., Shen T. L., Hendrickx A. G., Gumucio D. L., Goodman M. J. Exp. Zool. 2000;288:318–326. doi: 10.1002/1097-010X(20001215)288:4<318::AID-JEZ4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.DeSimone J., Mueller A. Blood. 1978;53:19–27. [Google Scholar]
- 33.DeSimone J., Mueller A. L. J. Lab. Clin. Med. 1978;91:862–871. [PubMed] [Google Scholar]
- 34.Bender M. A., Bulger M., Close J., Groudine M. Mol. Cell. 2000;5:387–393. doi: 10.1016/s1097-2765(00)80433-5. [DOI] [PubMed] [Google Scholar]
- 35.Hu X., Bulger M., Roach J. N., Eszterhas S. K., Olivier E., Bouhassira E. E., Groudine M. T., Fiering S. Proc. Natl. Acad. Sci. USA. 2003;100:1111–1115. doi: 10.1073/pnas.0337404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwahara S. I., Abe Y., Okazaki T. J. Biochem. (Tokyo) 1996;119:360–366. doi: 10.1093/oxfordjournals.jbchem.a021248. [DOI] [PubMed] [Google Scholar]
- 37.Rohrbaugh M. L., Hardison R. C. J. Mol. Biol. 1983;164:395–417. doi: 10.1016/0022-2836(83)90058-x. [DOI] [PubMed] [Google Scholar]
- 38.Kitchen H., Brett I. Ann. N.Y. Acad. Sci. 1974;241:653–671. doi: 10.1111/j.1749-6632.1974.tb21921.x. [DOI] [PubMed] [Google Scholar]
- 39.Schon E. A., Cleary M. L., Haynes J. R., Lingrel J. B. Cell. 1981;27:359–369. doi: 10.1016/0092-8674(81)90419-0. [DOI] [PubMed] [Google Scholar]
- 40.Lingrel J. B., Townes T. M., Shapiro S. G., Spence S. E., Liberator P. A., Wernke S. M. Prog. Clin. Biol. Res. 1983;134:131–139. [PubMed] [Google Scholar]
- 41.Menon A. G., Lingrel J. B. Gene. 1986;42:141–150. doi: 10.1016/0378-1119(86)90290-8. [DOI] [PubMed] [Google Scholar]
- 42.Cooper S., Murphy R., Dolman G., Hussey D., Hope R. Mol. Biol. Evol. 1996;13:1012–1022. doi: 10.1093/oxfordjournals.molbev.a025651. [DOI] [PubMed] [Google Scholar]
- 43.Cooper S. J. B., Hope R. M. Proc. Natl. Acad. Sci. USA. 1993;90:11777–11781. doi: 10.1073/pnas.90.24.11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gumucio D. L., Shelton D. A., Bailey W. J., Slightom J. L., Goodman M. Proc. Natl. Acad. Sci. USA. 1993;90:6018–6022. doi: 10.1073/pnas.90.13.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogan P. K., Pan J., Weissman S. M. Mol. Biol. Evol. 1987;4:327–342. doi: 10.1093/oxfordjournals.molbev.a040448. [DOI] [PubMed] [Google Scholar]
- 46.Townes T., Shapiro S., Wernke S., Lingrel J. J. Biol. Chem. 1984;259:1896–1900. [PubMed] [Google Scholar]
- 47.Han J., Szak S., Boeke J. Nature. 2004;429:268–274. doi: 10.1038/nature02536. [DOI] [PubMed] [Google Scholar]
- 48.Feingold E. A., Forget B. G. Blood. 1989;74:2178–2186. [PubMed] [Google Scholar]
- 49.Forget B. G. Ann. N.Y. Acad. Sci. 1998;850:38–44. doi: 10.1111/j.1749-6632.1998.tb10460.x. [DOI] [PubMed] [Google Scholar]
- 50.Peterson K. R., Clegg C. H., Huxley C., Josephson B. M., Haugen H. S., Furukawa T., Stamatoyannopoulos G. Proc. Natl. Acad. Sci. USA. 1993;90:7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valverde-Garduno V., Guyot B., Anguita E., Hamlett I., Porcher C., Vyas P. Blood. 2004;104:3106–3116. doi: 10.1182/blood-2004-04-1333. [DOI] [PubMed] [Google Scholar]
- 52.Fang X., Han H., Stamatoyannopoulos G., Li Q. J. Biol. Chem. 2004;279:5444–5449. doi: 10.1074/jbc.M306241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronchi A., Berry M., Raguz S., Imam A., Yannoutsos N., Ottolenghi S., Grosveld F., Dillon N. EMBO J. 1996;15:143–149. [PMC free article] [PubMed] [Google Scholar]
- 54.Alter B. Blood. 1979;54:1158–1163. [PubMed] [Google Scholar]
- 55.Higuchi R., Dollinger G., Walsh P. S., Griffith R. Biotechnology. 1992;10:413–417. doi: 10.1038/nbt0492-413. [DOI] [PubMed] [Google Scholar]
- 56.Gumucio D. L., Heilstedt-Williamson H., Gray T. A., Tarle S. A., Shelton D. A., Tagle D. A., Slightom J. L., Goodman M., Collins F. S. Mol. Cell. Biol. 1992;12:4919–4929. doi: 10.1128/mcb.12.11.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayasaka K., Fitch D. H., Slightom J. L., Goodman M. J. Mol. Biol. 1992;224:875–881. doi: 10.1016/0022-2836(92)90568-5. [DOI] [PubMed] [Google Scholar]
- 58.TomHon C., Zhu W., Millinoff D., Hayasaka K., Slightom J. L., Goodman M., Gumucio D. L. J. Biol. Chem. 1997;272:14062–14066. doi: 10.1074/jbc.272.22.14062. [DOI] [PubMed] [Google Scholar]
- 59.Zhu W., TomHon C., Mason M., Campbell T., Shelden E., Richards N., Goodman M., Gumucio D. L. Blood. 1999;93:3540–3549. [PubMed] [Google Scholar]
- 60.Jeanmougin F., Thompson J. D., Gouy M., Higgins D. G., Gibson T. J. Trends Biochem. Sci. 1998;10:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 61.Stamatoyannopoulos G., Grosveld F. In: The Molecular Basis of Blood Diseases. 3rd Ed. Stamatoyannopoulos G., Majerus P., Perlmutter R., Varmus H., editors. New York: Saunders; 2001. pp. 135–182. [Google Scholar]
- 62.Gumucio D. L., Blanchard-McQuate K., Heilstedt-Williamson H., Tagle D. A., Gray T. A., Tarlé S. A., Gragowski L., Goodman M., Slightom J. L., Collins F. S. In: Stamatoyannopoulos G., Nienhuis A., editors. The Regulation of Hemoglobin Switching; Proceedings of the Seventh Conference on Hemoglobin Switching, Airlie, Virginia, 1990; Baltimore: Johns Hopkins Univ. Press; 1991. pp. 277–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.