Abstract
A method is described whereby biologically active H and L chains, free from each other by immunochemical criteria, can be prepared from guinea-pig γ2-immunoglobulin. The procedure makes use of mild reduction and alkylation in the absence of a denaturing agent, followed by gel filtration in the presence of 4 M guanidine·HCl.
Specific binding of antigen by the method of equilibrium dialysis was found only when specific H chains were mixed with L chains, with more activity resulting when the L chains were also specific. Specific binding by antibody H chains alone was not found.
Full text
PDF


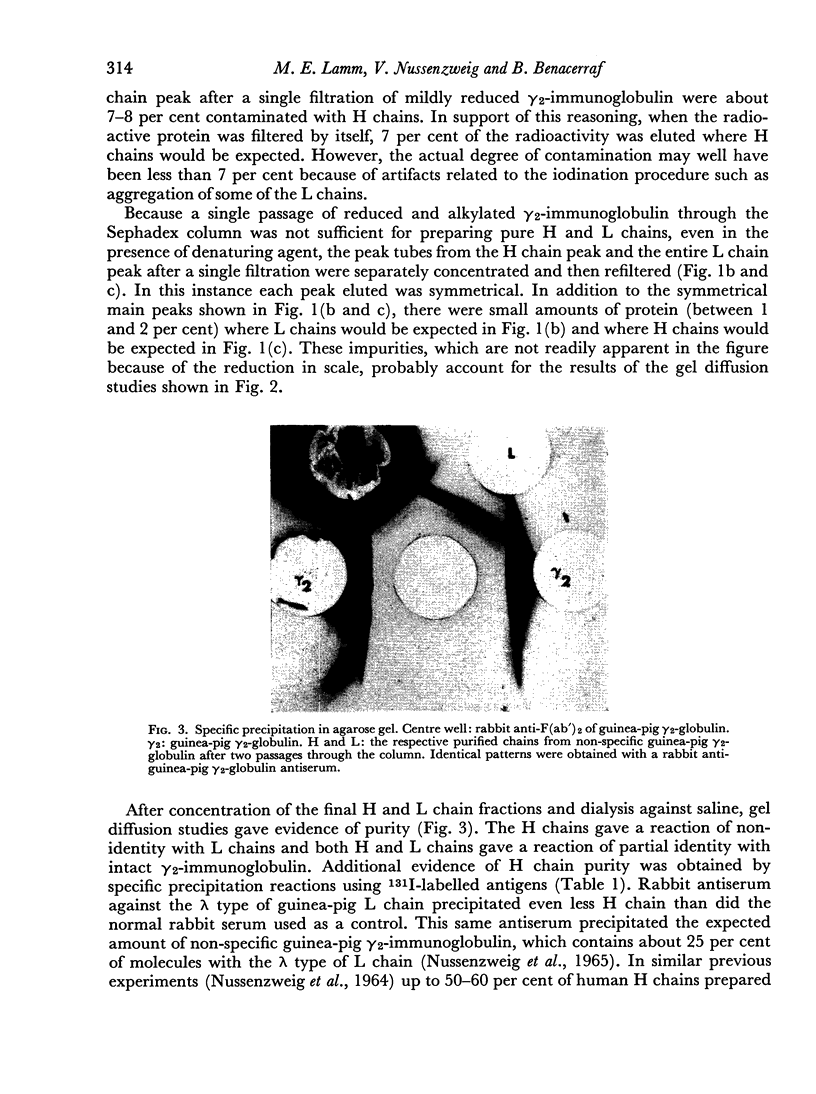
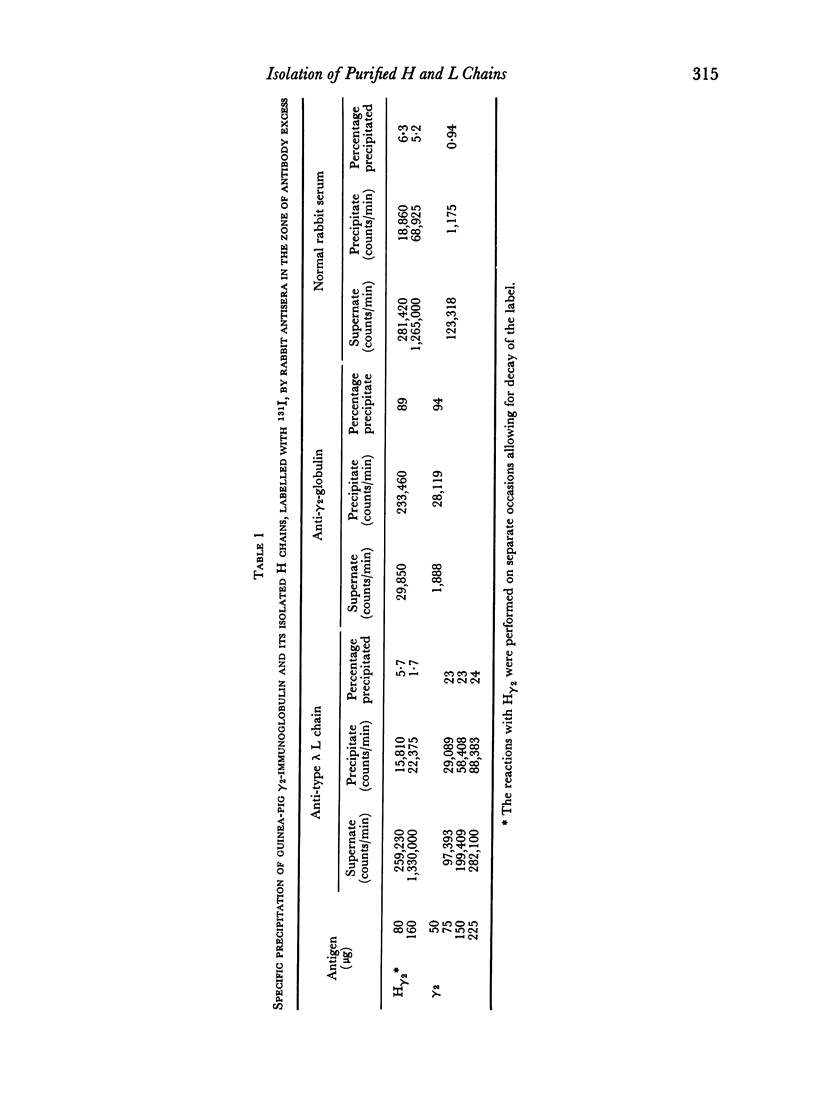

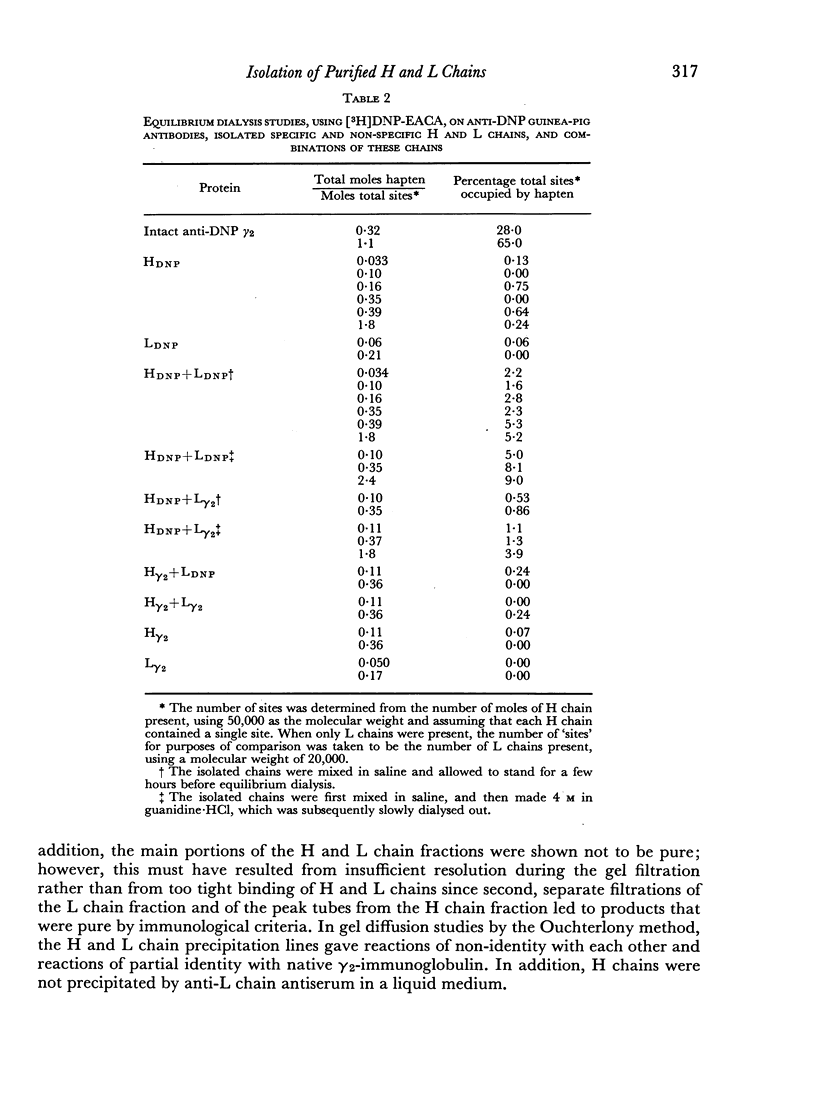


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., OVARY Z., BLOCH K. J., FRANKLIN E. C. Properties of guinea pig 7S antibodies. I. Electrophoretic separation of two types of guinea pig 7S antibodies. J Exp Med. 1963 Jun 1;117:937–949. doi: 10.1084/jem.117.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N. EQUILIBRIUM DIALYSIS FOR MEASUREMENT OF ANTIBODY-HAPTEN AFFINITIES. Methods Med Res. 1964;10:106–114. [PubMed] [Google Scholar]
- FARAH F. S., KERN M., EISEN H. N. The preparation and some properties of purified antibody specific for the 2,4-dinitrophenyl group. J Exp Med. 1960 Dec 1;112:1195–1210. doi: 10.1084/jem.112.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- METZGER H., SINGER S. J. BINDING CAPACITY OF REDUCTIVELY FRAGMENTED ANTIBODIES TO THE 2,4-DINITROPHENYL GROUP. Science. 1963 Nov 8;142(3593):674–675. doi: 10.1126/science.142.3593.674. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- NUSSENZWEIG V., BENACERRAF B. STUDIES ON THE PROPERTIES OF FRAGMENTS OF GUINEA PIG GAMMA-1 AND GAMMA-2 ANTIBODIES OBTAINED BY PAPAIN DIGESTION AND MILD REDUCTION. J Immunol. 1964 Dec;93:1008–1014. [PubMed] [Google Scholar]
- SMALL P. A., Jr, KEHN J. E., LAMM M. E. POLYPEPTIDE CHAINS OF RABBIT GAMMAGLOBULIN. Science. 1963 Oct 18;142(3590):393–394. doi: 10.1126/science.142.3590.393. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., MAURER P. H., BENACERRAF B. The affinity of the reticulo-endothelial system for various modified serum proteins. Br J Exp Pathol. 1960 Apr;41:190–197. [PMC free article] [PubMed] [Google Scholar]
- UTSUMI S., KARUSH F. THE SUBUNITS OF PURIFIED RABBIT ANTIBODY. Biochemistry. 1964 Sep;3:1329–1338. doi: 10.1021/bi00897a024. [DOI] [PubMed] [Google Scholar]