Abstract
1 The plasma elimination rate of antipyrine, as measured by the salivary concentration decay, and the urinary excretion of antipyrine and its primary metabolites 4-hydroxy-antipyrine, norantipyrine, 3-hydroxymethyl-antipyrine and 3-carboxy-antipyrine was studied in five healthy volunteers, who received 250, 500 and 1000 mg antipyrine orally in a cross-over design.
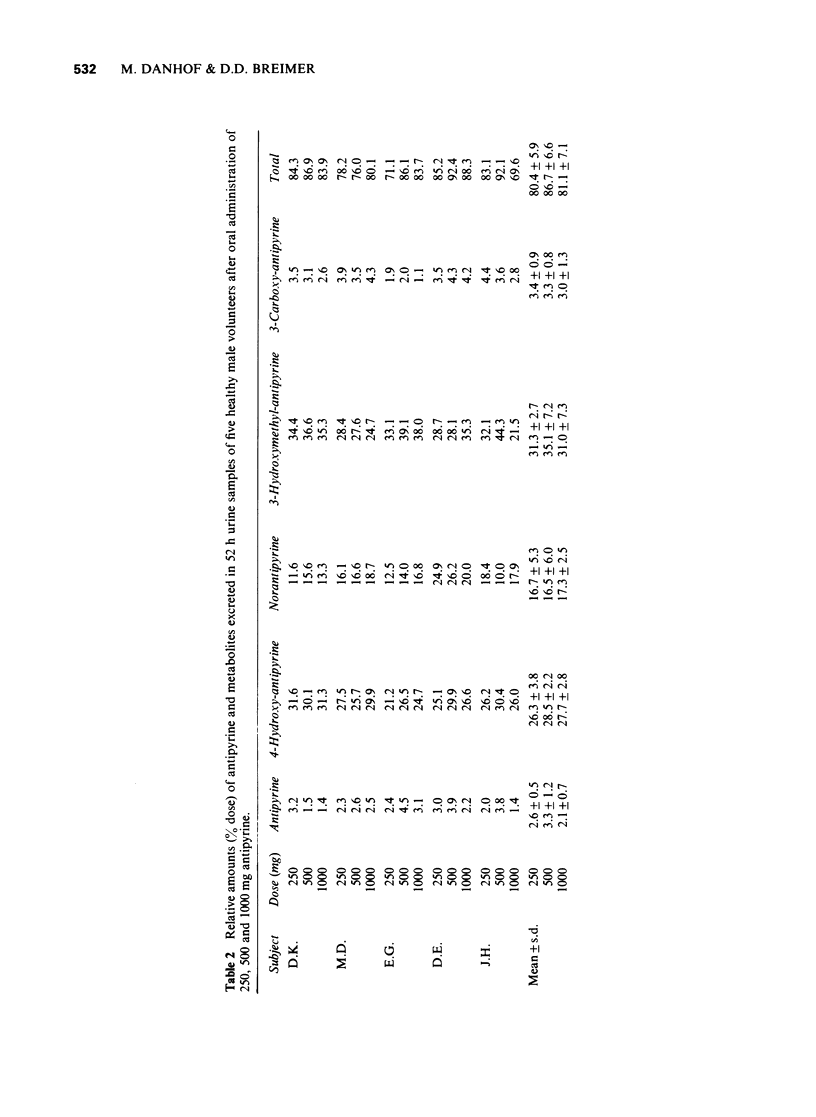
2 The mean antipyrine half-life and metabolic clearance were 11.5 ± 2.5 h (range 10.2-16.9 h) and 3.4 ± 0.9 l/h (range 1.7-4.2 l/h) respectively after 500 mg. These values were not significantly different after 250 or 1000 mg (P > 0.1; paired t-test).
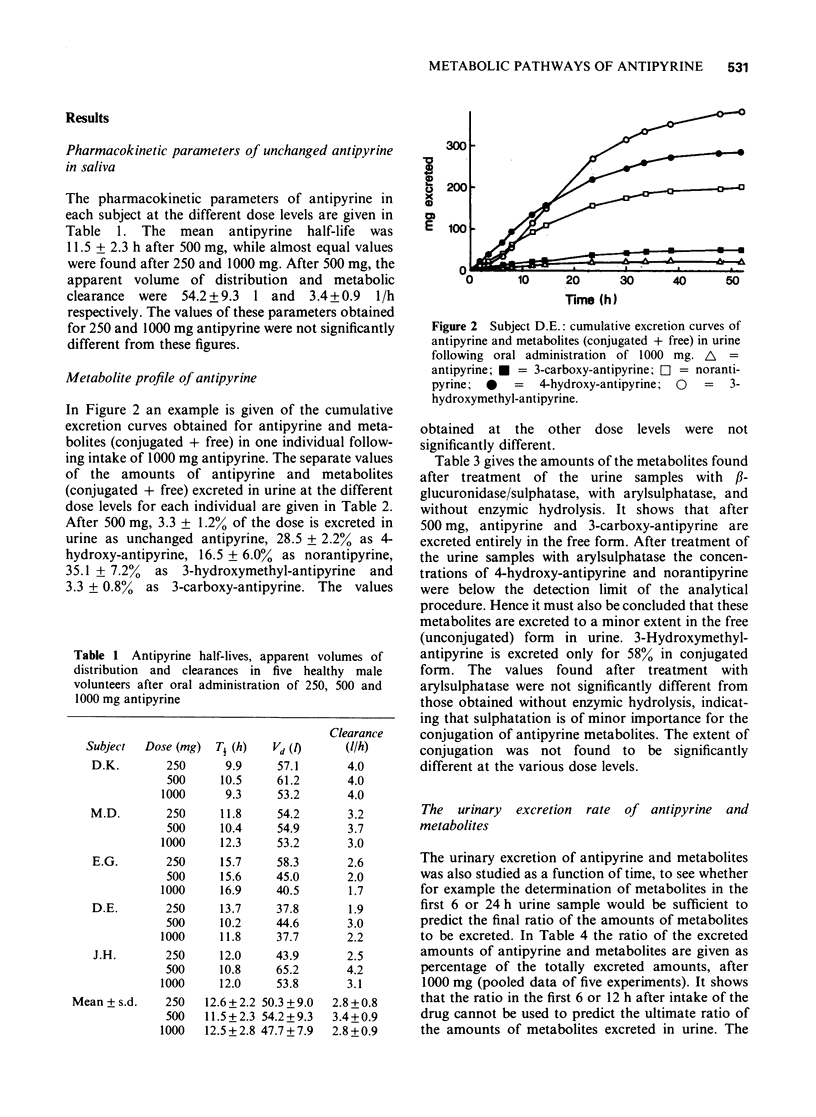
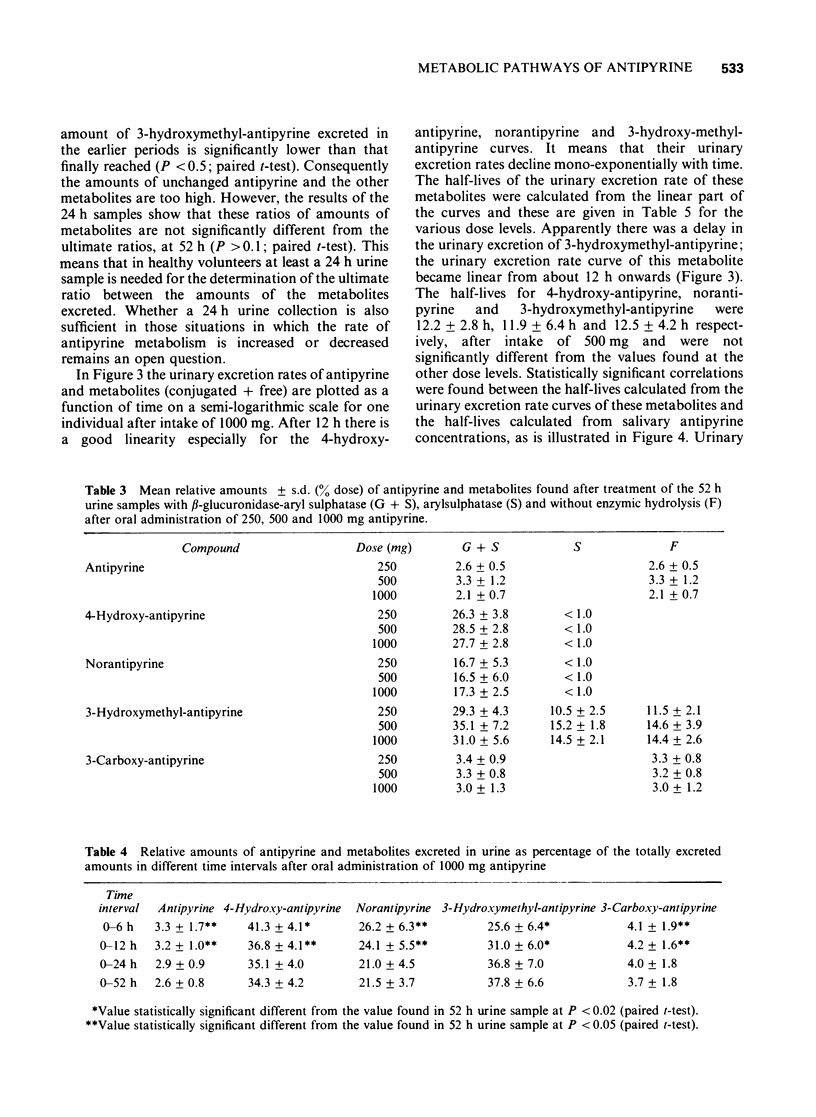
3 In 52 h urine 3.3 ± 1.2% of the dose of 500 mg antipyrine was excreted unchanged as antipyrine, 28.5 ± 2.2% as 4-hydroxy-antipyrine, 16.5 ± 6.0% as norantipyrine, 35.1 ± 7.2% as 3-hydroxymethyl-antipyrine and 3.3 ± 0.8% as 3-carboxy-antipyrine. The values obtained at the other dose levels were not significantly different (P > 0.1; paired t-test).
4 At all dose levels 4-hydroxy-antipyrine and norantipyrine were excreted in urine entirely as glucuronides. After 500 mg antipyrine, 3-hydroxymethyl-antipyrine was excreted as glucuronide to the extent of 58 ± 9% of the total excreted amount. This percentage was not significantly different at the other dose levels. 3-Carboxy-antipyrine was excreted in the free form at all three dose levels.
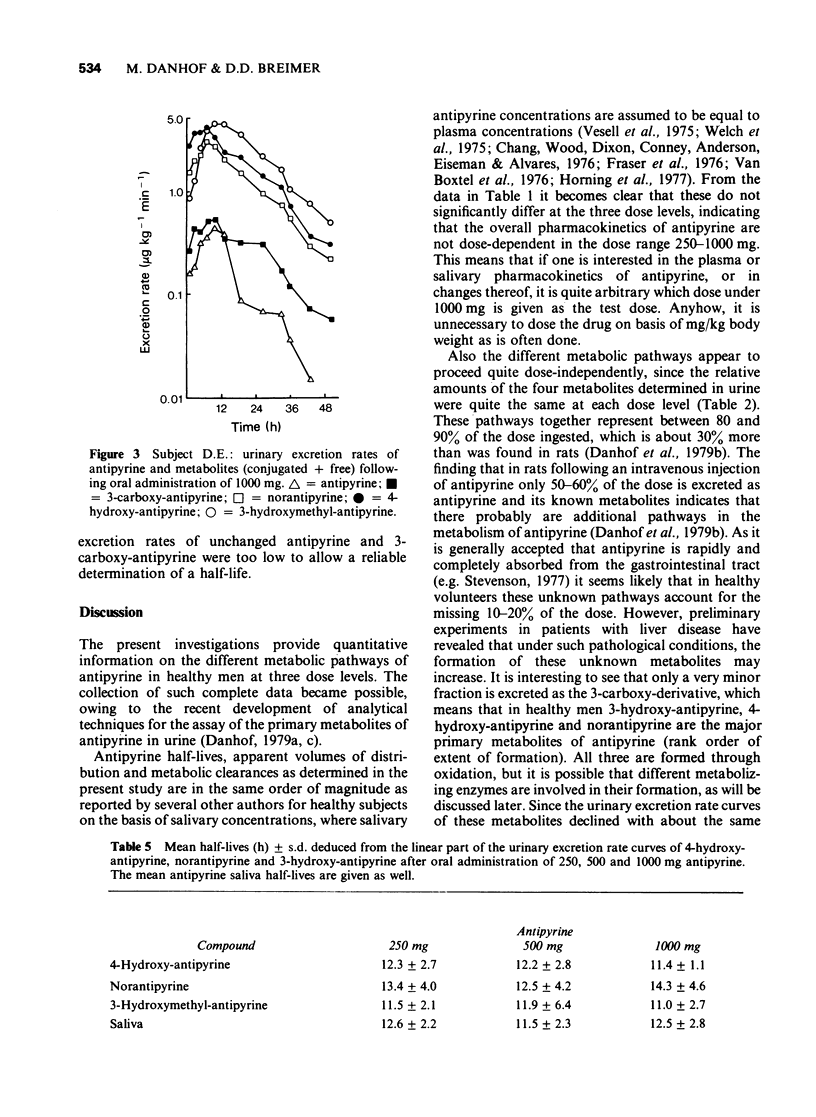
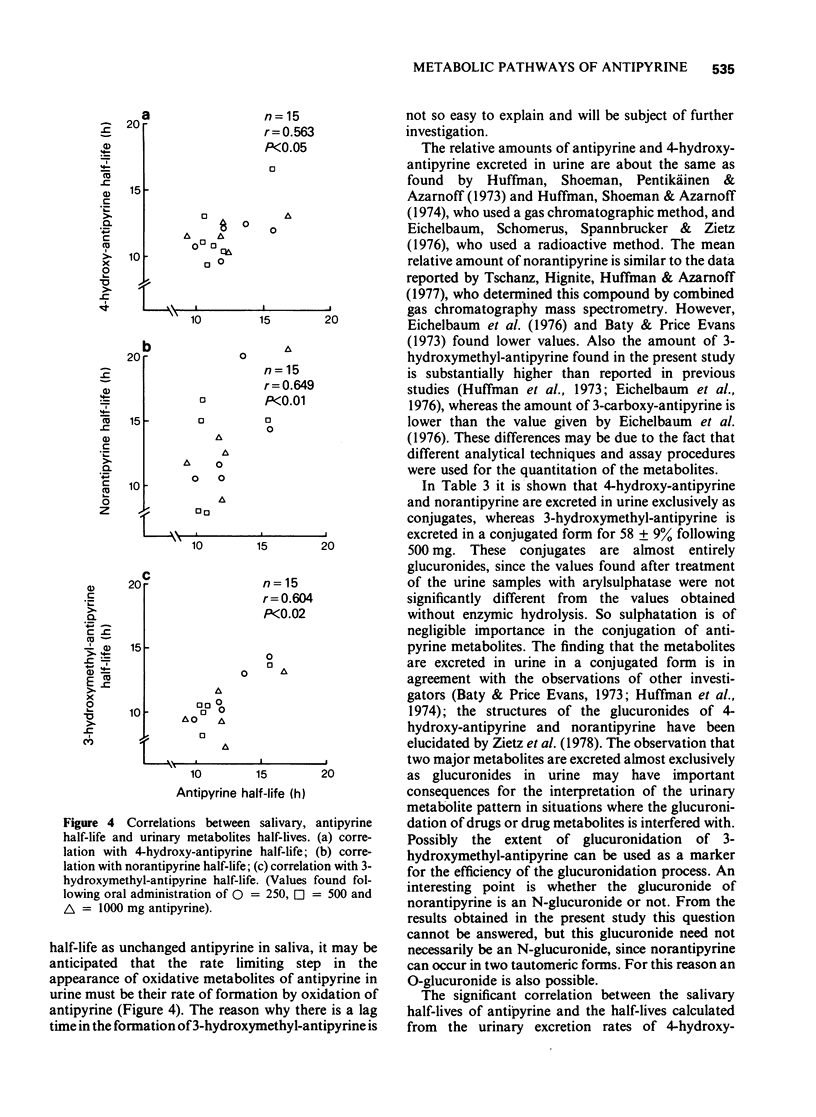
5 From 12 h of drug intake onwards, the urinary excretion rate curves of antipyrine and all its metabolites declined mono-exponentially with about the same half-life as the parent compound in saliva. The half-lives calculated from the excretion rate curves of 4-hydroxy-antipyrine, norantipyrine and 3-hydroxymethyl-antipyrine correlated significantly with the half-life of antipyrine in plasma. At all dose levels a relative delay in urinary excretion of 3-hydroxymethyl-antipyrine was observed compared to the urinary excretion of antipyrine and the other metabolites.
6 The ratios of the cumulative amounts of metabolites excreted in 24 h, were essentially the same as those measured in the 52 h samples.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., AXELROD J. The fate of antipyrine in man. J Pharmacol Exp Ther. 1950 Jan;98(1):97–104. [PubMed] [Google Scholar]
- Baty J. D., Evans D. A. Norphenazone, a new metabolite of phenazone in human urine. J Pharm Pharmacol. 1973 Jan;25(1):83–84. doi: 10.1111/j.2042-7158.1973.tb09124.x. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Wood A. W., Dixon W. R., Conney A. H., Anderson K. E., Eiseman J., Alvares A. P. Antipyrine: radioimmunoassay in plasma and saliva following administration of a high dose and a low dose. Clin Pharmacol Ther. 1976 Aug;20(2):219–226. doi: 10.1002/cpt1976202219. [DOI] [PubMed] [Google Scholar]
- Danhof M., Groot-van der Vis E., Breiner D. D. Assay of antipyrine and its primary metabolites in plasma, saliva and urine by high-performance liquid chromatography and some preliminary results in man. Pharmacology. 1979;18(4):210–223. doi: 10.1159/000137254. [DOI] [PubMed] [Google Scholar]
- Fraser H. S., Mucklow J. C., Murray S., Davies D. S. Assessment of antipyrine kinetics by measurement in saliva. Br J Clin Pharmacol. 1976 Apr;3(2):321–325. doi: 10.1111/j.1365-2125.1976.tb00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning M. G., Brown L., Nowlin J., Lertratanangkoon K., Kellaway P., Zion T. E. Use of saliva in therapeutic drug monitoring. Clin Chem. 1977 Feb;23(2 Pt 1):157–164. [PubMed] [Google Scholar]
- Huffman D. H., Shoeman D. W., Azarnoff D. L. Correlation of the plasma elimination of antipyrine and the appearance of 4-hydroxy antipyrine in the urine of man. Biochem Pharmacol. 1974 Jan 15;23(2):197–201. doi: 10.1016/0006-2952(74)90410-9. [DOI] [PubMed] [Google Scholar]
- Huffman D. H., Shoeman D. W., Pentikäinen P., Azarnoff D. L. The effect of spironolactone on antipyrine metabolism in man. Pharmacology. 1973;10(6):338–344. doi: 10.1159/000136455. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Sjöqvist F., von Bahr C. Interindividual differences in drug oxidation: clinical importance. Drug Metab Dispos. 1973 Jan-Feb;1(1):469–482. [PubMed] [Google Scholar]
- Stevenson I. H. Factors influencing antipyrine elimination. Br J Clin Pharmacol. 1977 Jun;4(3):261–265. doi: 10.1111/j.1365-2125.1977.tb00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschanz C., Hignite C. E., Huffman D. H., Azarnoff D. L. Metabolic disposition of antipyrine in patients with lung cancer. Cancer Res. 1977 Nov;37(11):3881–3886. [PubMed] [Google Scholar]
- Vesell E. S. Genetic and environmental factors affecting drug disposition in man. Clin Pharmacol Ther. 1977 Nov;22(5 Pt 2):659–679. doi: 10.1002/cpt1977225part2659. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. Genetic and environmental factors affecting drug response in man. Fed Proc. 1972 Jul-Aug;31(4):1253–1269. [PubMed] [Google Scholar]
- Vesell E. S., Passananti G. T., Glenwright P. A., Dvorchik B. H. Studies on the disposition of antipyrine, aminopyrine, and phenacetin using plasma, saliva, and urine. Clin Pharmacol Ther. 1975 Sep;18(3):259–272. doi: 10.1002/cpt1975183259. [DOI] [PubMed] [Google Scholar]
- Welch R. M., DeAngelis R. L., Wingfield M., Farmer T. W. Elimination of antipyrine from saliva as a measure of metabolism in man. Clin Pharmacol Ther. 1975 Sep;18(3):249–258. doi: 10.1002/cpt1975183249. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Shimeno H., Tsukamoto H. Metabolism of drugs. LIX. A new metabolite of antipyrine. Biochem Pharmacol. 1968 Aug;17(8):1511–1516. doi: 10.1016/0006-2952(68)90210-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Shimeno H., Tsukamoto H. Metabolism of drugs. LXX. Further study on antipyrine metabolism. Chem Pharm Bull (Tokyo) 1971 Jan;19(1):41–45. doi: 10.1248/cpb.19.41. [DOI] [PubMed] [Google Scholar]
- Zietz E., Eichelbaum M., Dengler H. J., Spiteller G. Zum Metabolismus von Antipyrin (Phenazon) beim Menschen. Arzneimittelforschung. 1978;28(2):315–319. [PubMed] [Google Scholar]
- van Boxtel C. J., Wilson J. T., Lindgren S., Sjöqvist F. Comparison of the half-life of antipyrine in plasma, whole blood and saliva of man. Eur J Clin Pharmacol. 1976 Feb 6;9(4):327–332. doi: 10.1007/BF00561668. [DOI] [PubMed] [Google Scholar]


