Abstract
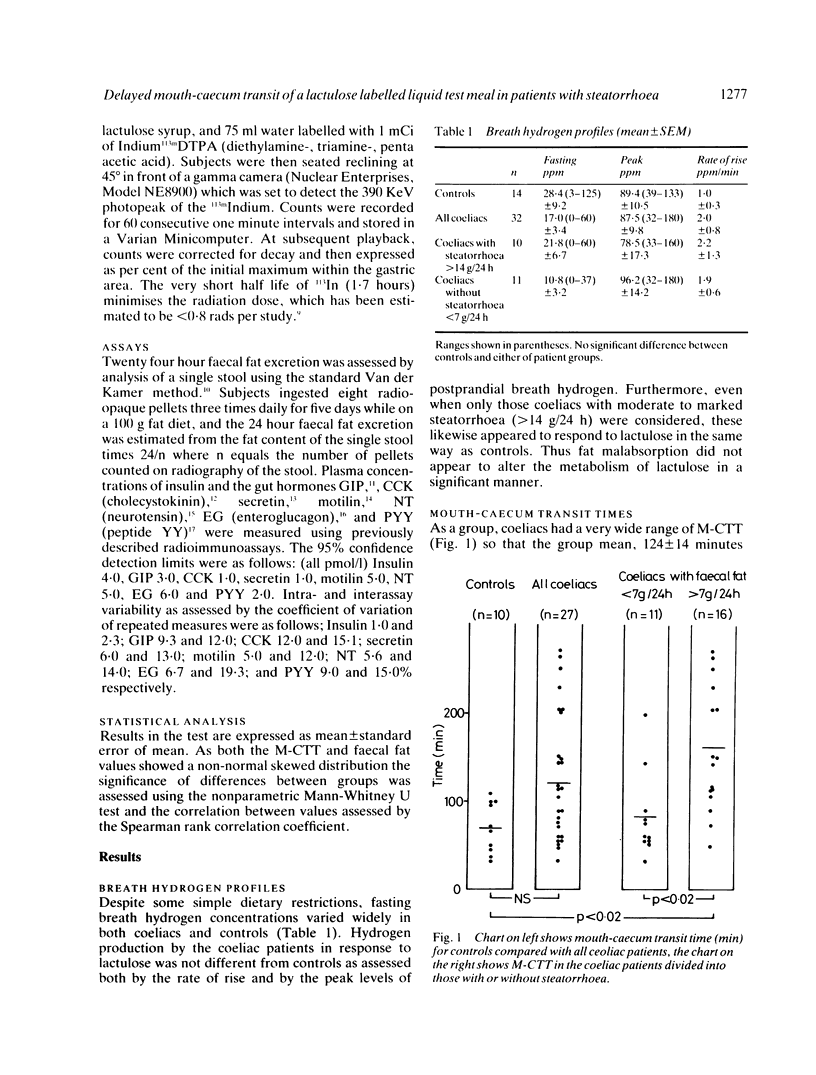
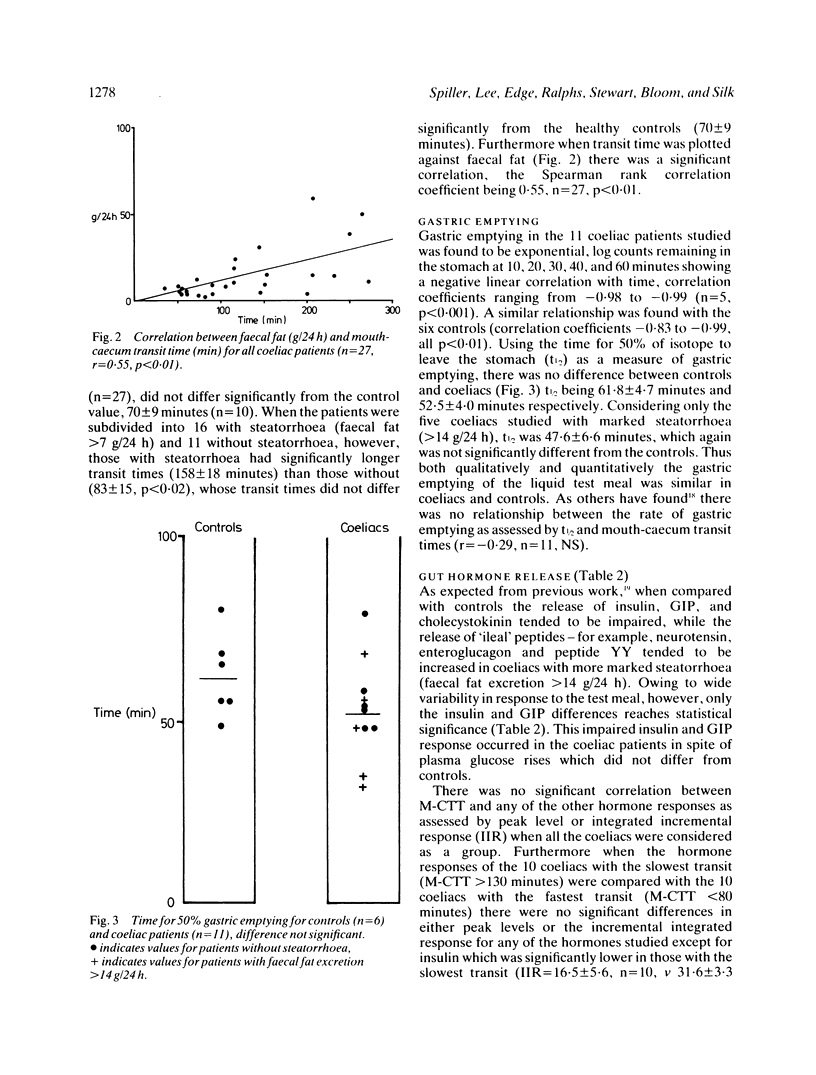
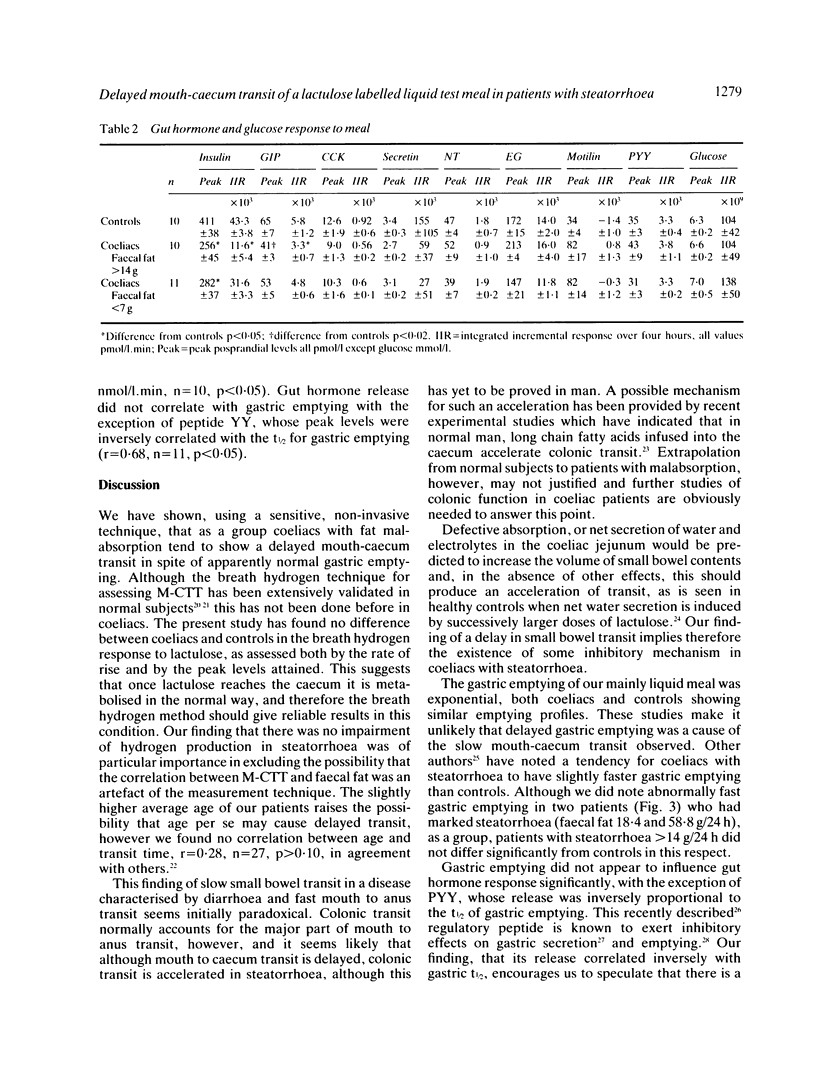
Mouth-caecum transit time (M-CTT) of a lactulose labelled liquid test meal has been measured in 27 coeliac patients and 10 healthy controls using the breath hydrogen technique. Although all patients were urged to maintain a gluten free diet, not all did, and there was, therefore, a wide range in the severity of fat malabsorption within the patient group. Gastric emptying of a 113Indium DTPA-labelled liquid test meal was also assessed in separate studies on six healthy controls and 11 of the coeliac patients. Fasting breath hydrogen concentrations and the response to lactulose, as assessed both by the rate of rise, and the peak breath hydrogen concentration reached, showed no difference between coeliacs and controls, regardless of the presence or absence of steatorrhoea. Mouth-caecum transit time in the 16 coeliac patients with steatorrhea (faecal fat greater than 7 g/24 h) was, however, significantly prolonged being 158 +/- 18 minutes (mean +/- SEM), compared with 70 +/- 9 minutes for the controls (p less than 0.02), and 83 +/- 15 minutes for the 11 coeliacs without steatorrhoea (p less than 0.002). Mouth-caecum transit time in the coeliac patients was linearly related to the 24 hour faecal fat excretion, r = 0.55, n = 27, p less than 0.01. Slow mouth-caecum transit in the coeliacs with steatorrhoea was not caused by delayed gastric emptying as the t1/2 for coeliacs with steatorrhoea was within the normal range. Coeliacs with delayed mouth-caecum transit had impaired insulin release but the postprandial profiles of the other peptides measured (cholecystokinin, GIP, secretin, motilin, neurotensin, enteroglucagon, and peptide YY) were all within the normal range in this group of partially treated coeliac patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman H. S., Bloom S. R., Sarson D. L., Blackburn A. M., Johnston D. I., Patel H. R., Stewart J. S., Modigliani R., Guerin S., Mallinson C. N. Gut-hormone profile in coeliac disease. Lancet. 1978 Apr 15;1(8068):785–788. doi: 10.1016/s0140-6736(78)92994-x. [DOI] [PubMed] [Google Scholar]
- Blackburn A. M., Bloom S. R. A radioimmunoassay for neurotensin in human plasma. J Endocrinol. 1979 Nov;83(2):175–181. doi: 10.1677/joe.0.0830175. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Mitznegg P., Bryant M. G. Measurement of human plasma motilin. Scand J Gastroenterol Suppl. 1976;39:47–52. [PubMed] [Google Scholar]
- Bond J. H., Jr, Levitt M. D., Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975 Apr;85(4):546–555. [PubMed] [Google Scholar]
- Caride V. J., Prokop E. K., Troncale F. J., Buddoura W., Winchenbach K., McCallum R. W. Scintigraphic determination of small intestinal transit time: comparison with the hydrogen breath technique. Gastroenterology. 1984 Apr;86(4):714–720. [PubMed] [Google Scholar]
- Feibusch J. M., Holt P. R. Impaired absorptive capacity for carbohydrate in the aging human. Dig Dis Sci. 1982 Dec;27(12):1095–1100. doi: 10.1007/BF01391447. [DOI] [PubMed] [Google Scholar]
- Ghatei M. A., Jung R. T., Stevenson J. C., Hillyard C. J., Adrian T. E., Lee Y. C., Christofides N. D., Sarson D. L., Mashiter K., MacIntyre I. Bombesin: action on gut hormones and calcium in man. J Clin Endocrinol Metab. 1982 May;54(5):980–985. doi: 10.1210/jcem-54-5-980. [DOI] [PubMed] [Google Scholar]
- Ghatei M. A., Uttenthal L. O., Christofides N. D., Bryant M. G., Bloom S. R. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab. 1983 Sep;57(3):488–495. doi: 10.1210/jcem-57-3-488. [DOI] [PubMed] [Google Scholar]
- Heading R. C., Tothill P., Laidlaw A. J., Shearman D. J. An evaluation of indium DTPA chelate in the measurement of gastric emptying by scintiscanning. Gut. 1971 Aug;12(8):611–615. doi: 10.1136/gut.12.8.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate A. M., Read N. W. Relationship between small bowel transit time and absorption of a solid meal. Influence of metoclopramide, magnesium sulfate, and lactulose. Dig Dis Sci. 1983 Sep;28(9):812–819. doi: 10.1007/BF01296904. [DOI] [PubMed] [Google Scholar]
- Ingelfinger F. J., Moss R. E. THE MOTILITY OF THE SMALL INTESTINE IN SPRUE. J Clin Invest. 1943 May;22(3):345–352. doi: 10.1172/JCI101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz G., Gassull M. A., Leeds A. R., Blendis L. M., Jenkins D. J. A simple method of measuring breath hydrogen in carbohydrate malabsorption by end-expiratory sampling. Clin Sci Mol Med. 1976 Mar;50(3):237–240. doi: 10.1042/cs0500237. [DOI] [PubMed] [Google Scholar]
- Moberg S., Carlberger G. Gastric emptying in healthy subjects and in patients with various malabsorptive states. Scand J Gastroenterol. 1974;9(1):17–21. [PubMed] [Google Scholar]
- PERMAN G., MATTSSON O. The small intestine transit time in steatorrhoea. Acta Med Scand. 1962 Mar;171:273–281. doi: 10.1111/j.0954-6820.1962.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Peña A. S., Truelove S. C., Whitehead R. Disaccharidase activity and jejunal morphology in coeliac disease. Q J Med. 1972 Oct;41(164):457–476. [PubMed] [Google Scholar]
- Pirk F. Changes in the motility of the small intestine in digestive disorders. Gut. 1967 Oct;8(5):486–490. doi: 10.1136/gut.8.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Cammack J., Edwards C., Holgate A. M., Cann P. A., Brown C. Is the transit time of a meal through the small intestine related to the rate at which it leaves the stomach? Gut. 1982 Oct;23(10):824–828. doi: 10.1136/gut.23.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Miles C. A., Fisher D., Holgate A. M., Kime N. D., Mitchell M. A., Reeve A. M., Roche T. B., Walker M. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980 Dec;79(6):1276–1282. [PubMed] [Google Scholar]
- Ritchie J. A., Salem S. N. Upper intestinal motility in ulcerative colitis, idiopathic steatorrhoea, and the irritable colon syndrome. Gut. 1965 Aug;6(4):325–337. doi: 10.1136/gut.6.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarson D. L., Bryant M. G., Bloom S. R. A radioimmunoassay of gastric inhibitory polypeptide in human plasma. J Endocrinol. 1980 Jun;85(3):487–496. doi: 10.1677/joe.0.0850487. [DOI] [PubMed] [Google Scholar]
- Spiller R. C., Brown M. L., Phillips S. F. Decreased fluid tolerance, accelerated transit, and abnormal motility of the human colon induced by oleic acid. Gastroenterology. 1986 Jul;91(1):100–107. doi: 10.1016/0016-5085(86)90445-2. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980 Jun 5;285(5764):417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]


