Abstract
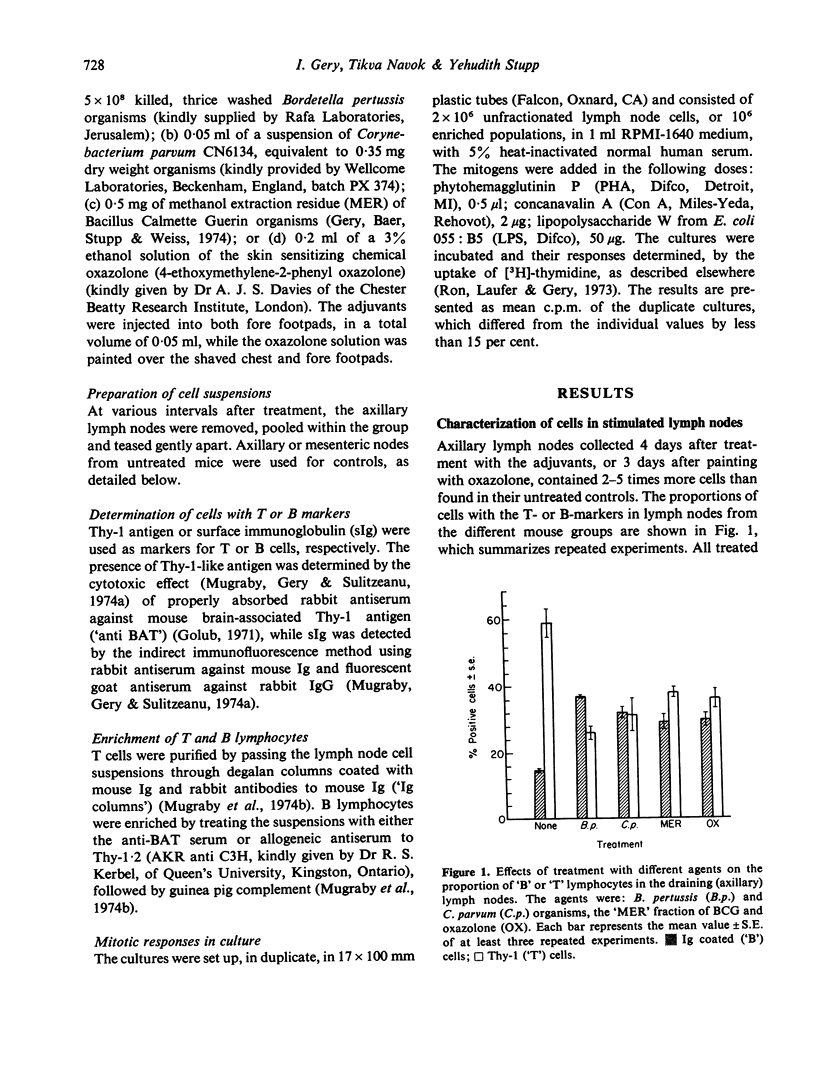
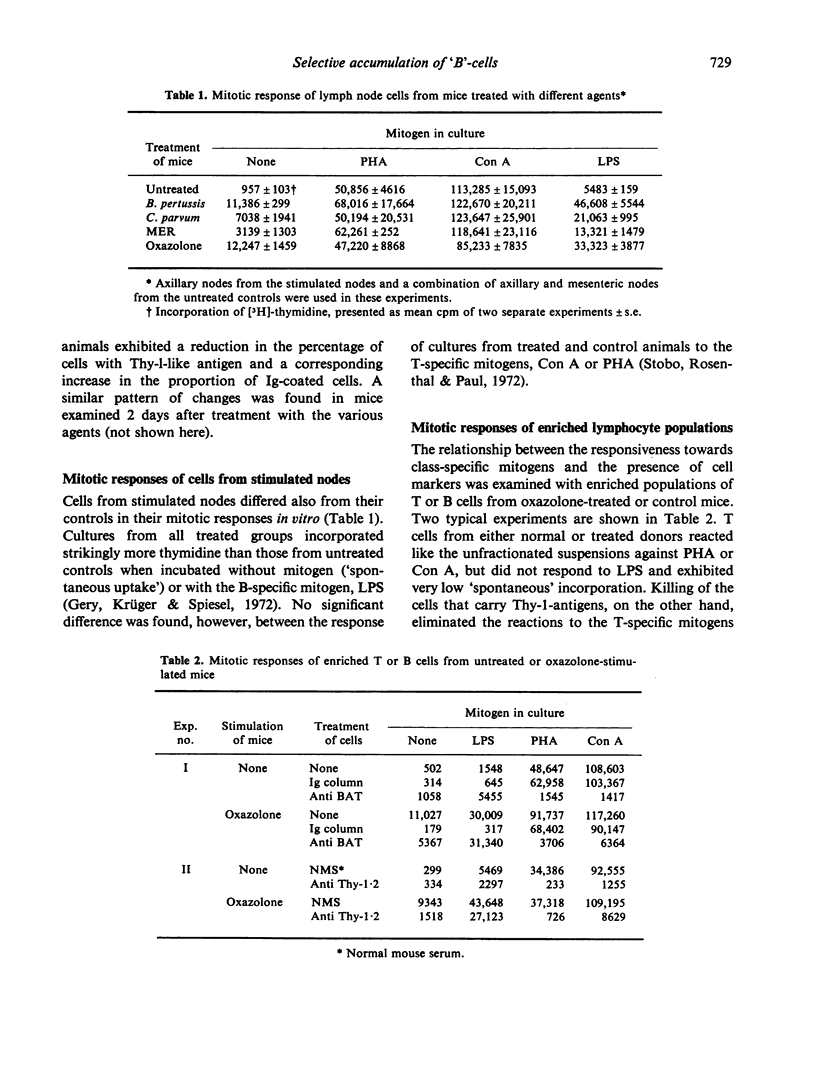
Draining lymph nodes from mice which had been stimulated with bacterial adjuvants or the skin sensitizing agent, oxazolone, showed a marked increase in cell content, presumably due to lymphocyte immigration. A surprisingly large proportion of these cells exhibit properties of B lymphocytes: the presence of surface Ig, lack of Thy-1-like antigen and responsiveness to lopopolysaccharide (LPS). The relationship between the presence of surface markerand responses to class-specific mitogens, of cells from the stimulated nodes, was established by testing fractionated lymphocyte populations. Enriched T cells did not react to LPS, whereas removal of cells with Thy-1 antigen by specific antisera eliminated the reactions to T mitogens but had little or no effect on the LPS response. The data thus suggest that B cells, which make up a small portion of the circulating lymphocyte pool, are selectively accumulated in lymph nodes stimulated by different immunogens, including T-specific stimulants. This interpretation contradicts the generally accepted assumption, that stimulat lymph nodes trap mostly T lymphocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill R. N., Frost H., Trnka Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J Exp Med. 1976 Apr 1;143(4):870–888. doi: 10.1084/jem.143.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V. The morphology of immune reactions in normal, thymectomized and reconstituted mice. II. The response to oxazolone. Immunology. 1969 Jul;17(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Green W. R., Fanger M. W. Complement receptor lymphocytes in the rabbit. II. The effect of immunization on the complement receptor lymphocyte population in the craining lymph node. J Immunol. 1976 Nov;117(5 PT2):1812–1816. [PubMed] [Google Scholar]
- Hedfors E. Activation of peripheral T cells of sarcoidosis patients and healthy controls. Clin Exp Immunol. 1974 Nov;18(3):379–390. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Elliott E. V., Wallis V. J. Assessment of mitotic thymus-derived lymphocytes by their sensitivity to the cytotoxic effects of anti-theta serum. Cell Immunol. 1974 Mar 30;11(1-3):146–161. doi: 10.1016/0008-8749(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Hudson L., Sprent J. Fc-receptors, Ia-antigens, and immunoglobulin on normal and activated mouse T lymphocytes. J Exp Med. 1975 Dec 1;142(6):1403–1415. doi: 10.1084/jem.142.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugraby L., Gery I., Sulitzeanu D. Subpopulations of mouse spleen lymphocytes. I. Dissociation between the reactivities to different "non-specific" mitogens. Immunology. 1974 Apr;26(4):787–795. [PMC free article] [PubMed] [Google Scholar]
- Mugraby L., Gery I., Sulitzeanu D. The participation of thymus-derived and of bone marrow-derived lymphocytes of sensitized mice, in the proliferative response to specific antigen, in vitro. Eur J Immunol. 1974 Jun;4(6):402–405. doi: 10.1002/eji.1830040603. [DOI] [PubMed] [Google Scholar]
- Pernis B., Miller J. F., Forni L., Sprent J. Immunoglobulin on activated T cells detected by indirect immunofluorescence. Cell Immunol. 1974 Mar 15;10(3):476–482. doi: 10.1016/0008-8749(74)90139-7. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Ron N., Laufer A., Gery I. An unusual response of hamster lymphocytes to PHA. Immunology. 1973 Sep;25(3):433–439. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes. I. Detection of a subpopulation of murine T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Sep 1;142(3):611–621. doi: 10.1084/jem.142.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. N., Gershon R. K. The effect of localized injection of adjuvant material on the draining lymph node. 3. Thymus dependence. J Immunol. 1972 Feb;108(2):377–386. [PubMed] [Google Scholar]
- Zatz M. M., Gershon R. K. Thymus dependence of lymphocyte trapping. J Immunol. 1974 Jan;112(1):101–106. [PubMed] [Google Scholar]
- Zatz M. M., Lance E. M. The distribution of 51Cr-labeled lymphocytes into antigen-stimulated mice. Lymphocyte trapping. J Exp Med. 1971 Jul 1;134(1):224–241. doi: 10.1084/jem.134.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


