Abstract
Cells integrate signals to select the appropriate response from an array of possible outcomes. Signal integration causes the reorganization of signaling pathways by undescribed events. To analyze the molecular changes in signaling pathways that elicit different responses, we focused on the interaction between cyclic AMP (cAMP) and growth factors. We show that the activation of extracellular signal-regulated kinase 5 (ERK5), but not ERK1/2, by growth factors is disrupted by cAMP through cAMP-dependent protein kinase (PKA). Activation of MEKK2, a mitogen-activated protein (MAP) kinase kinase kinase upstream of ERK5 that is required for growth factor activation of ERK5, is also disrupted by PKA. Transcription of c-Jun is induced by ERK5, and like ERK5, c-Jun induction is also blocked by cAMP. Transcription from the serum response element, like activation of ERK1/2, is not blocked by cAMP. Collectively, these results support a model in which cAMP shapes the growth factor-induced cellular response through PKA-dependent uncoupling of selected MAP kinase cascades from activating signals.
Cells are predisposed to respond to stimuli as a consequence of permutations of the signaling network. Alterations in the architecture of signaling pathways can modify how a cell responds to a stimulus (29). During the course of development, cells respond differently to individual stimuli by integrating signals from their environment. For example, the state of integrin ligation determines whether cells proliferate or differentiate in response to growth factor (7). The process of integrating responses to multiple external stimuli is likely to involve reorganizing the connections in the signaling network. To understand how a cell integrates multiple stimuli to achieve a specific response, it is necessary to examine how combinations of stimuli specifically reorganize the signaling network to produce responses distinct from those caused by each individually.
We chose to examine the effect of cyclic AMP (cAMP) on growth factor-induced responses that are coupled to proliferation to develop a paradigm for understanding the molecular mechanisms of signal integration. The addition of cell-permeable, nonhydrolyzable analogs of cAMP reduces serum growth factor-induced cell growth and DNA synthesis in many cell types (1). Similarly, ligands that activate adenylyl cyclase reduce serum-stimulated proliferation of fibroblasts (32). The combination of cAMP and growth factor is integrated to produce a response different from growth factor alone.
Mitogen-activated protein kinase (MAPK) cascades regulate proliferation, migration, nutrient sensing, production of cytokines, and long-term potentiation, for example (18, 25). These cascades consist of a MAP kinase kinase kinase (MAP3K), which phosphorylates a MAP kinase kinase (MAP2K) to then activate a MAPK. There are more than a dozen known MAP3Ks, each of which can regulate one or more MAP2K-MAPK modules (18, 25). The MAPK extracellular signal-regulated kinase 5 (ERK5) is required for growth factor-stimulated proliferation in HeLa cells, focus formation in NIH 3T3 cells, and tumor necrosis factor alpha production in mast cells (6, 9, 14, 24). Overexpression of MEKK2 is sufficient to activate ERK5, and dominant negative MEKK2 blocks activation of ERK5 through the B-cell receptor (6, 36). Mechanisms regulating MEKK2 activity are not known.
ERK5 cooperates with the MAPKs ERK1/2 to regulate cellular responses such as proliferation largely through their abilities to influence transcription (20, 24). We previously identified ERK5 as a target of negative regulation by cAMP in certain cellular contexts (26). In HeLa and NIH 3T3 cells, cAMP regulation of ERK5 appears to be exclusively negative. Although ERK1/2 are often inhibited by cAMP, their activities are unaffected by cAMP in C2C12 myoblasts and stimulated in PC12 and pancreatic beta cells (15, 19, 26, 39). These findings demonstrate that ERK5 and ERK1/2 are differentially sensitive to cyclic nucleotide inhibition. Therefore, we chose to address how signals are integrated in this system. The results led to a model in which cAMP molds the growth factor-induced cellular response by selectively uncoupling MAPK cascades.
MATERIALS AND METHODS
Cell culture and harvest.
NIH 3T3 cells, C2C12 myoblasts, and HEK293 cells were maintained as described previously (26). HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% l-glutamine. Lysates were prepared as described previously (26).
Reagents.
Anti-ERK5, anti-FLAG (M2), and anti-phospho-ERK1/2 antibodies were from Sigma. Anti-MEK5 (C-20), anti-MEKK2 (N-19), anti-Raf-1 (C-12), and anti-c-Jun (H-79) antibodies were from Santa Cruz. The anti-Raf-1 antibody used for immunoprecipitations was from Transduction Labs. Forskolin, isobutyl methyl xanthine (IBMX), and two peptides derived from the protein kinase inhibitor (PKI), PKI 5-24 (#116805) and myristoylated PKI 14-22 amide (myr-PKI; #476485), were from Calbiochem. Epidermal growth factor (EGF) was from Becton Dickinson, and fibroblast growth factor (FGF), prostaglandin E2 (PGE2), and probenecid were from Sigma. The phospho-S153 MEKK2 antibody was obtained from Erik Schaefer (Biosource, Boston, MA).
Plasmids and oligonucleotides.
The plasmids 3X SRE-Luc and PRL-TK were described previously (24); 3X-FLAG-MEKK2 was generated by PCR amplification of mouse MEKK2 from an hemagglutinin epitope-MEKK2 construct (generous gift of G. L. Johnson, University of North Carolina, Chapel Hill) and subsequent subcloning of the MEKK2 PCR product into the 3X-FLAG-CMV-7.1 expression vector (Sigma). The constructs 3X-FLAG-MEKK2 K385M (3X-FLAG-MEKK2KM), 3X-FLAG-MEKK2KM S153A, 3X-FLAG-MEKK2KM S331A, and 3X-FLAG-MEKK2KM S153A, S331A were made by PCR amplification using a Quikchange kit (Stratagene). Small interfering RNA (siRNA) oligonucleotides were generated at the University of Texas Southwestern core facility.
Protein purification.
His6-MEK6KM, glutathione S-transferase (GST)-MEF2C (204-321), His6-MEK1KM, GST-ERK2KR, and GST-PAK (232-544) were expressed and purified by standard methods (26). The catalytic subunit of protein kinase A (PKA) was purified as described previously (30).
Immune complex kinase assays.
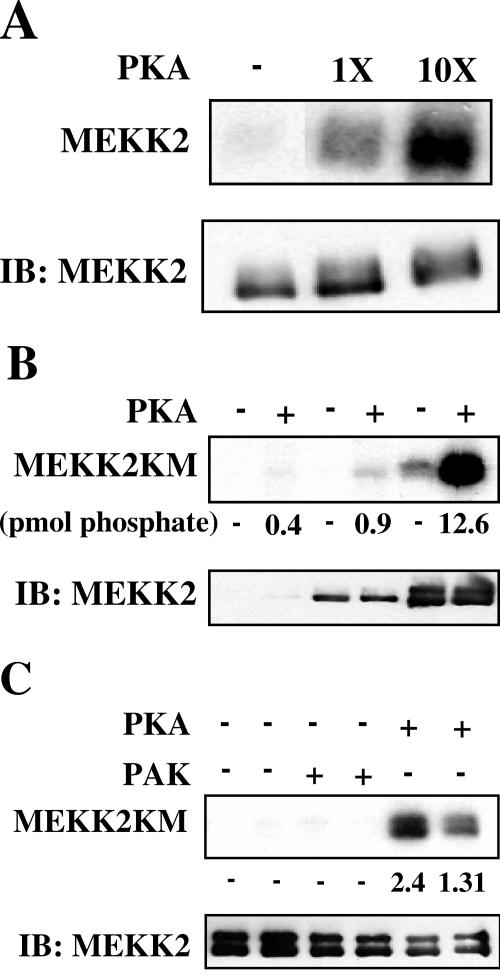
Assays with MEKK2 and Raf-1 were performed using 3 μl of anti-MEKK2 antibody (Santa Cruz) or 5 μl of anti-Raf-1 antibody (Transduction Labs) (23). MEK5 assays were performed with the following modifications: 20 μl of anti-MEK5 antibody (Santa Cruz) was added to precleared lysates at 4°C overnight, after which 40 μl of protein G-Sepharose beads (1:1 slurry) were added for an additional 2 h. Beads were pelleted and washed four times with wash buffer (1 M NaCl, 0.25 M Tris-HCl, pH 7.4, 0.1% Nonidet P-40, 0.1% deoxycholate) and two times with 10 mM HEPES, pH 7.4. Kinase reactions were performed at 30°C in 10 mM MgCl2, 50 μM ATP ([γ-32P]ATP, ∼104 cpm/pmol), and 10 mM HEPES, pH 7.4, with GST-ERK5 (1-451) as the substrate. After 30 min, beads were pelleted, and the supernatant containing phosphorylated GST-ERK5 (1-451) was removed and added to new tubes for a second reaction. A second kinase reaction was performed at 30°C for 30 min in 10 mM MgCl2, 5 μM ATP ([γ-32P]ATP, ∼105 cpm/pmol), and 10 mM HEPES, pH 7.4, with GST-MEF2C (204-321) as the substrate. Reactions were terminated by the addition of 4× sample buffer. MEKK2 kinase assays (see Fig. 8A) were performed with the following modifications: immunoprecipitations were washed as described for MEK5; the first kinase reaction was performed at 30°C in 10 mM MgCl2, 5 μM ATP, 20 mM β-glycerophosphate, 10 mM HEPES, pH 7.4, 0.5 μM pepstatin A, 1 μg/ml leupeptin, 0.1 μM aprotinin, and 0.1 mM dithiothreitol. Either 1 μl of 50% glycerol or 1 μl of PKA was added to the reactions as indicated in the legend to Fig. 8A. After 30 min, beads were washed twice in 0.5 ml of 10 mM HEPES, pH 7.4. After the second wash, the supernatants were removed, and pellets containing MEKK2 were used in a second kinase reaction. The reactions were performed at 30°C for 30 min in 10 mM MgCl2, 5 μM ATP ([γ-32P]ATP, ∼105 cpm/pmol), 1 μM PKI (5-24), 20 mM β-glycerophosphate, 10 mM HEPES, pH 7.4, and protease inhibitors as above with His6-MEK6KM as substrate. Reactions were terminated by the addition of 4× sample buffer. For PKA assays, endogenous MEKK2 or 3X-FLAG-MEKK2 variants were immunoprecipitated using 10 μl anti-MEKK2 (Santa Cruz) antibody or 1 μl anti-FLAG antibody (Sigma). All kinase reactions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized with autoradiography.
FIG. 8.
Phosphorylation of MEKK2 by PKA does not reduce MEKK2 activity. (A) HeLa cells were grown and treated as described for Fig. 4. (Top two panels) MEKK2 was immunoprecipitated from lysates, and two-stage kinase reactions were performed. In the first stage, MEKK2 immunoprecipitates were incubated in kinase reactions containing diluent or PKA. In the second stage, kinase assays were performed using His-MEK6KM as the substrate. (Bottom two panels) MEKK2 immune complex kinase assays using His-MEK6KM as the substrate were performed as described for Fig. 4. (Third from top) Autoradiogram of His-MEK6KM. (Bottom) MEKK2 blot of the immunoprecipitations. (B) Cells were treated with DMSO or 10 μM forskolin and 50 μM IBMX for 15 min prior to stimulation, concurrent with stimulation, 2 min after stimulation, or 5 min after stimulation with 10 ng/ml EGF. ERK5 activity was monitored as described for Fig. 1. Results shown in panels A and B are representative of at least three independent experiments. (C) Cells were treated with the indicated agents using the protocol described for Fig. 1D. (Top panel) Immunoblot of lysates with anti-pERK1/2. (Second panel from top) Immunoblot of lysates with anti-ERK5. (Third panel from top) MEKK2 was immunoprecipitated and immunoblotted with anti-pS153 MEKK2 antibodies. (Bottom panel) The MEKK2 immunoprecipitates were immunoblotted with anti-MEKK2 antibodies. (D) Cells were treated with the indicated agents using the protocol described for Fig. 2B. (Top panel) Immunoblot of MEKK2 immunoprecipitates with anti pS153 MEKK2 antibodies. (Bottom panel) Immunoblot of MEKK2 immunoprecipitates with MEKK2 antibody.
Transfections and other methods.
Transfection of HEK293 cells was performed using calcium phosphate precipitation as described previously (23). Transfection of HeLa cells was performed using FuGENE 6 (Roche) according to the manufacturer's protocol. Transfection of siRNAs was performed using Oligofectamine (Invitrogen) according to the manufacturer's protocol. Luciferase assays were performed as described previously using a Dual-Reporter assay system (Promega) and a Turner Designs TD 20/20 luminometer (23). Western blotting was performed as described previously (26).
RESULTS
Characterization of cyclic nucleotide-dependent inhibition of ERK5.
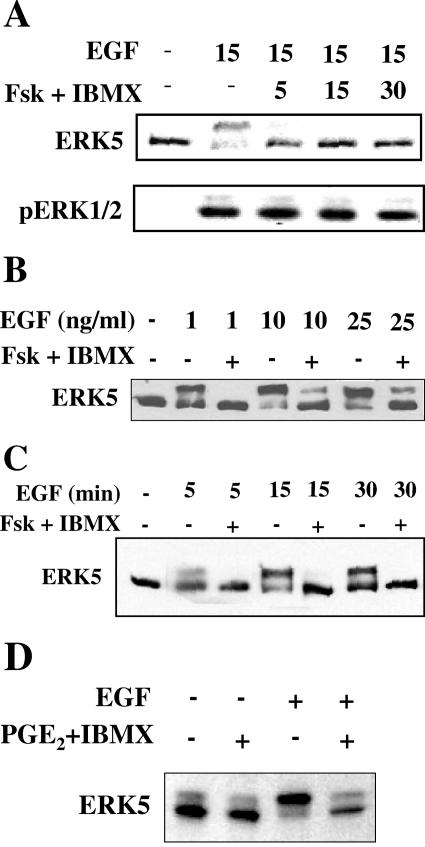
In HeLa, NIH 3T3, and C2C12 cells, cAMP did not activate ERK5. Instead, treatment with forskolin and IBMX prevented activation of ERK5 by EGF in HeLa (Fig. 1A) and C2C12 cells and by FGF in NIH 3T3 cells (not shown). There was no effect of cAMP on ERK1/2 activation. In some cases in which ERK1/2 activation is abolished by cyclic nucleotides, increasing the concentration of growth factor overcomes the inhibition (4). Therefore, we increased the concentration of EGF and found that higher concentrations of EGF did override the inhibitory effect of cAMP on ERK5 activation (Fig. 1B). The result suggests that cAMP raises the threshold for activation of ERK5, so that inhibition may be overcome by increasing the activating signal. An increase in cAMP might also alter the kinetics of ERK1/2 activation (21). In HeLa cells, however, exposure to forskolin and IBMX did not shift ERK5 activation by growth factor to a later time (Fig. 1C). Ligands that stimulate adenylyl cyclase through endogenous receptors also suppressed activation of ERK5 by EGF, as evidenced by the action of prostaglandin E2 (Fig. 1D).
FIG. 1.
Characterization of cyclic nucleotide-dependent inhibition of ERK5. HeLa cells were grown to confluence and cultured in 0.5% FBS prior to stimulation. (A) Cells were treated with dimethyl sulfoxide (DMSO) or 10 μM forskolin and 50 μM IBMX for 5, 15, or 30 min prior to a 15-min stimulation with 10 ng/ml EGF. (Top) ERK5 activation detected by immunoblotting of a slower migrating autophosphorylated ERK5 band in cell lysates. ERK5 autophosphorylation correlates with increased ERK5 activity towards substrate (26). (Bottom) ERK1/2 activity monitored with an antibody which recognizes the dually phosphorylated active form of ERK1/2. (B) Cells were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 15 min with 1, 10, or 25 ng/ml EGF. ERK5 activity was monitored as described for panel A. (C) Cells were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 5, 15, or 30 min with 10 ng/ml EGF. ERK5 activity was monitored as described for panel A. (D) Cells were treated with 15 μM PGE2, 50 μM IBMX, and 1 mM probenecid (to reduce cAMP efflux) for 5 min. ERK5 activity was monitored as described for panel A. Results are representative of at least three independent experiments.
Mechanism of cyclic nucleotide-induced ERK5 inhibition.
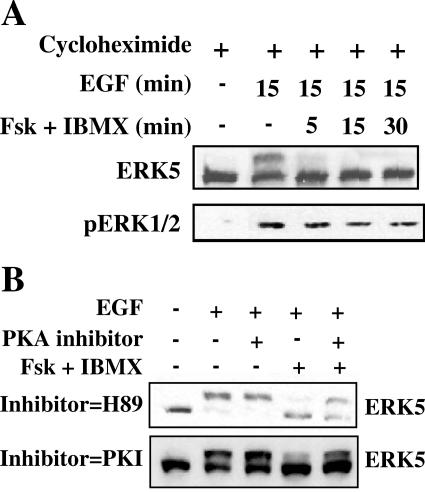
An increase in cAMP concentration might inhibit the activation of upstream regulators of ERK5, increase the direct negative regulation of ERK5, or stimulate a combination of both inhibitory mechanisms. cAMP binds to the regulatory subunits of PKA, promoting their dissociation from the active catalytic subunits (12). Once activated, PKA phosphorylates a range of intracellular targets, including transcription factors, ion channels, adaptor proteins, as well as other kinases (3). To explore the mechanism of cyclic nucleotide-induced ERK5 inhibition, we employed PKA inhibitors and the protein synthesis inhibitor cycloheximide, which should prevent translation of cAMP-induced immediate early genes, such as MKP-1 (35). Treatment of HeLa cells with cycloheximide did not interfere with the inhibitory effect of forskolin and IBMX on growth factor stimulation of ERK5 (Fig. 2A). Incubating cells with H-89 or myr-PKI, a membrane-permeable peptide derived from the PKA kinase inhibitor protein, prior to forskolin and IBMX treatment, however, partially restored activation of ERK5 by EGF (Fig. 2B). This is consistent with a mechanism in which PKA suppresses activation of ERK5.
FIG. 2.
Mechanism of cyclic nucleotide-induced ERK5 inhibition. (A) Cells cultured as described for Fig. 1 were treated with 50 μg/ml of cycloheximide for 30 min, then with DMSO or 10 μM forskolin and 50 μM IBMX for 5, 15, or 30 min, and then for 15 min with 10 ng/ml EGF. ERK5 (top panel) and ERK1/2 (bottom panel) activities were monitored as described for Fig. 1. (B) Cells were first treated with either 5 μM H-89 for 1 h (top panel) or 15 μM myr-PKI peptide (bottom panel) for 4 h or overnight, then with DMSO or 10 μM forskolin and 50 μM IBMX for 15 min, and then for 15 min with 10 ng/ml EGF. Results are representative of at least three independent experiments.
cAMP differentially regulates the MAP2Ks MEK5 and MEK1.
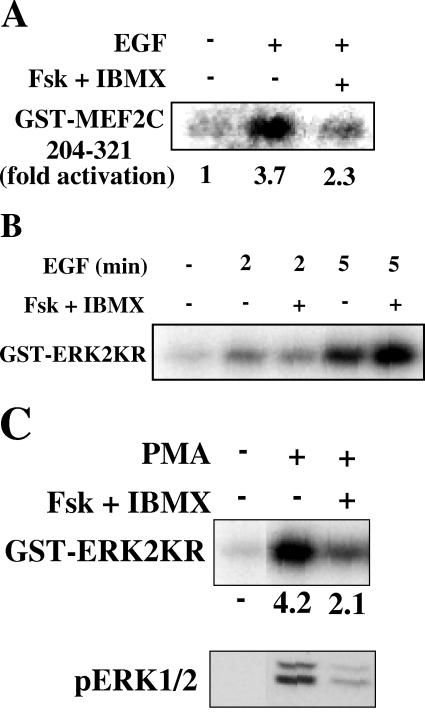
Although PKA can induce MKP-1 expression (5, 8, 34), our results with cycloheximide suggest that PKA does not inhibit ERK5 through increased expression of MKP-1 and subsequent inactivation of ERK5. Instead, we tested the hypothesis that PKA prevents activation of upstream regulators of ERK5. MEK5 immunoprecipitated from cells treated with forskolin and IBMX displayed substantially less activity than MEK5 from cells treated with EGF alone (Fig. 3A).
FIG. 3.
cAMP differentially regulates MEK5 and MEK1. (A) C2C12 myoblasts grown to confluence and cultured in 0.5% FBS for 24 h were treated with DMSO or 10 μM forskolin and 50 μM IBMX for 15 min and then with 1 ng/ml EGF for 5 min. MEK5 was immunoprecipitated from cells, and activity was determined using a coupled in vitro kinase assay with GST-ERK5 (1-451) and GST-MEF2C (204-321). Changes in MEK5 activity are reflected in changes in GST-ERK5 (1-451) activity towards GST-MEF2C (204-321). (B) HeLa cells grown to confluence and cultured in 0.5% FBS for 24 h were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 2 or 5 min with 1 ng/ml EGF. MEK1 activity was determined with immune complex kinase assays using GST-ERK2KR as the substrate. Results are representative of at least two independent experiments. (C) HeLa cells as described for panel B were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 5 min with 100 nM phorbol ester (phorbol myristate acetate). MEK1 activity was determined with immune complex kinase assays using GST-ERK2KR as the substrate. Results are representative of at least two independent experiments.
We found that the pathways involved in growth factor activation of ERK5 and ERK1/2 were differentially sensitive to cAMP concentration (26). EGF stimulation of MEK1 was not altered by an increase in cAMP (Fig. 3B), consistent with the lack of cAMP effect on EGF-stimulated ERK1/2 activity. In contrast, activation of MEK1 and ERK1/2 by phorbol ester was reduced by pretreatment with forskolin and IBMX (Fig. 3C). Thus, mechanisms that activate ERK1/2 are differentially resistant to cAMP inhibition.
cAMP inhibits activation of MEKK2 and Raf-1.
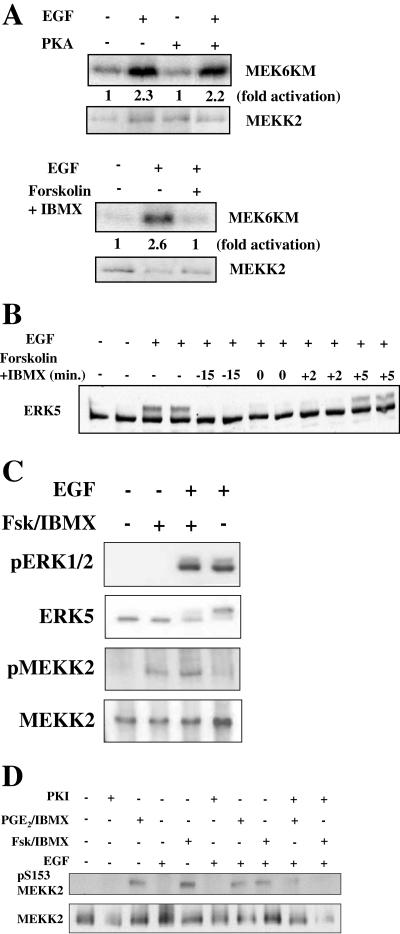
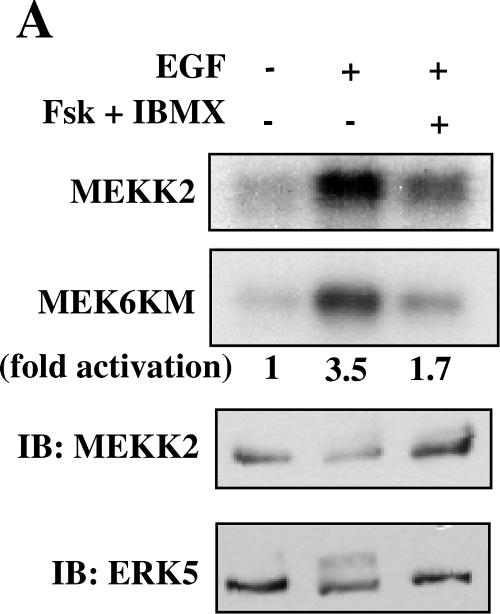
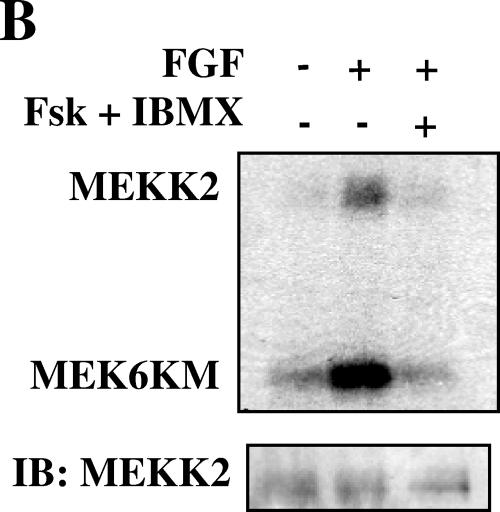
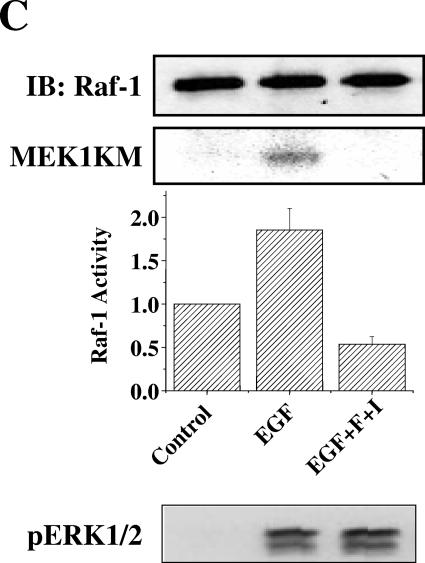
Because MEK5 is inhibited by cAMP, we tested the possibility that cAMP negatively impacts the activity of a MEK5 regulator. MEKK2 has been proposed as an important MAP3K upstream of MEK5 (36). We immunoprecipitated MEKK2 from HeLa and NIH 3T3 cells and found that growth factor-stimulated activity, measured as either MEKK2 autophosphorylation or phosphorylation of substrate, was reduced by cAMP (fourfold activation reduced to twofold by cAMP) (Fig. 4A and B). Interestingly, although MEK1/2 and ERK1/2 activities were the same in cells with or without exposure to cAMP, activation of Raf-1 was inhibited by cAMP in HeLa and NIH 3T3 cells (Fig. 4C and data not shown). Although components of both ERK5 and ERK1/2 signaling pathways are sensitive to cAMP, inhibition of Raf-1 was not accompanied by reduced MEK1/2 and ERK1/2 activity.
FIG.4.
cAMP inhibits activation of MEKK2 and Raf-1. (A) HeLa cells grown to confluence and cultured in 0.5% FBS for 18 h were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 5 min with 100 ng/ml EGF. (Top two panels) MEKK2 immune complex kinase assay using His-MEK6KM as the substrate. MEKK2 autophosphorylation (top) and MEKK2 activity towards MEK6KM (second from top) are shown. (Bottom two panels) MEKK2 immunoblot of MEKK2 immune complex kinase assay (third from top) and ERK5 immunoblot of cell lysates (bottom) are shown. (B) NIH 3T3 cells grown to confluence and cultured in 0.5% calf serum for 24 h were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 5 min with 25 ng/ml FGF. (Top) MEKK2 immune complex kinase assay using His-MEK6KM as the substrate. MEKK2 autophosphorylation and MEKK2 activity towards MEK6KM are shown. (Bottom) MEKK2 immunoblot of MEKK2 immune complex kinase assay. Results shown in panels A and B are representative of at least three independent experiments. (C) HeLa cells grown to confluence and cultured in 0.5% FBS for 18 h were treated for 15 min with DMSO or 10 μM forskolin and 50 μM IBMX and then for 5 min with 100 ng/ml EGF. (Top) Raf-1 immunoblot of Raf-1 immune complex kinase assay. (Second from top) Raf-1 immune complex kinase assay using His-MEK1KM as the substrate. The increase (n-fold) in Raf-1 activity (third panel from top) determined in immune complex kinase assays is the average of results from three independent experiments. Error bars show standard error of the mean. (Bottom) ERK1/2 activity monitored as described for Fig. 1.
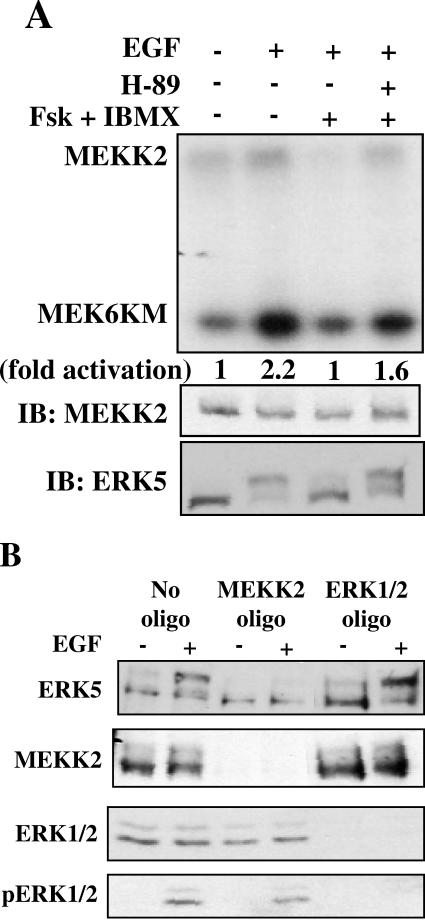
cAMP inhibition of MEKK2 requires PKA.
Our data implicate PKA in the inhibition of ERK5 by cAMP. If cAMP inhibition of ERK5 is the result of impaired MEKK2 activation, then PKA should also be necessary for cAMP inhibition of MEKK2. Changes in ERK5 and MEKK2 activity were directly correlated in HeLa cells that were untreated, stimulated with EGF, treated with forskolin and IBMX prior to EGF stimulation, or treated with the PKA inhibitor H-89 for 1 h and then forskolin and IBMX for 15 min prior to EGF stimulation (Fig. 5A). The same was true in NIH 3T3 cells (data not shown). Under none of these circumstances was there a disconnect between their relative activities, in contrast to what was observed with ERK1/2 and Raf-1. Thus, the effects of cAMP on ERK5 are likely attributable to cAMP-mediated direct or indirect inhibition of MEKK2.
FIG. 5.
cAMP inhibition of MEKK2 requires PKA. (A) HeLa cells grown to confluence and cultured in 0.5% FBS for 18 h were treated with 5 μM H-89 for 1 h, then with DMSO or 10 μM forskolin and 50 μM IBMX for 15 min, and then for 5 min with 100 ng/ml EGF. (Top) MEKK2 immune complex kinase assay using His-MEK6KM as substrate. MEKK2 autophosphorylation and MEKK2 activity towards MEK6KM are seen. (Middle) MEKK2 immunoblot of MEKK2 immune complex kinase assay. (Bottom) ERK5 activity was monitored as described for Fig. 1. Results are representative of at least three independent experiments. (B) HeLa cells were transfected with no siRNA, 10 nM siRNA directed toward MEKK2, or 40 nM siRNA directed toward ERK1/2. After 48 h, cells were transferred to 0.5% FBS for 18 h and then left untreated or stimulated for 15 min with 1 ng/ml EGF. (Top) ERK5 activity monitored as described for Fig. 1. (Middle two panels) MEKK2 (second from top) and ERK1/2 (third from top) immunoblots of cell lysates. (Bottom) ERK1/2 activity monitored as described for Fig. 1. Results shown in panels A and B are representative of at least three independent experiments.
Because inhibition of MEKK2 prevents activation of ERK5 by growth factor, MEKK2 activity appears necessary for the activation of ERK5. To obtain direct evidence that MEKK2 is required for growth factor activation of ERK5 by EGF, we employed siRNA oligonucleotides directed towards MEKK2 to reduce its expression in HeLa cells. We found that MEKK2 expression was necessary for stimulation of ERK5 but not ERK1/2 by EGF (Fig. 5B). Taken together, our findings suggest a model in which cAMP stimulates PKA to inhibit MEKK2, which in turn prevents activation of ERK5 by EGF.
PKA phosphorylates MEKK2 on Ser153.
We explored the possibility that MEKK2 integrates signals from cAMP through its phosphorylation by PKA. Using immunoprecipitated endogenous MEKK2 as a substrate, in vitro kinase reactions were performed with increasing concentrations of purified PKA catalytic subunit (Fig. 6A). Endogenous MEKK2 was an excellent substrate for PKA. The observed phosphorylation was dependent on the amount of MEKK2 present in the immunoprecipitate (Fig. 6B) and was estimated to proceed to at least 1 mol phosphate/mol MEKK2 in vitro. Furthermore, MEKK2 phosphorylation was not catalyzed by another protein kinase with generally similar substrate specificity; constitutively active recombinant PAK, which phosphorylates MEKK1 (10), did not phosphorylate MEKK2 under identical conditions (Fig. 6C).
FIG. 6.
PKA phosphorylates MEKK2 in vitro. (A) HEK293 cells were grown to confluence and cultured in serum-free DMEM for 24 h prior to harvest. Endogenous MEKK2 was immunoprecipitated from cell lysates and used as the substrate in in vitro kinase assays performed with diluent or increasing concentrations of PKA. The autoradiogram (top) and immunoblot of MEKK2 are shown. (B) HEK293 cells were transfected with the following cDNAs: two left lanes, 5 μg of empty vector; two middle lanes, 0.5 μg 3X-FLAG-MEKK2KM; two right lanes, 5 μg 3X-FLAG-MEKK2KM. After 18 h, medium was removed, and cells were cultured for an additional 18 h in serum-free DMEM. 3X-FLAG-MEKK2KM was immunoprecipitated from cell lysates and used as the substrate for in vitro kinase assays performed with PKA. The autoradiogram (top) and immunoblot of 3X-FLAG-MEKK2 are shown. (C) HEK293 cells were transfected with 5 μg cDNA encoding 3X-FLAG-MEKK2KM. After 18 h, cells were transferred to serum-free DMEM for an additional 18 h. 3X-FLAG-MEKK2KM was immunoprecipitated from lysates and used as the substrate for GST-PAK1 (232-454) or PKA. The autoradiogram (top) and immunoblot of 3X-FLAG-MEKK2 (bottom) are shown. Results shown in panels A, B, and C are representative of at least three independent experiments.
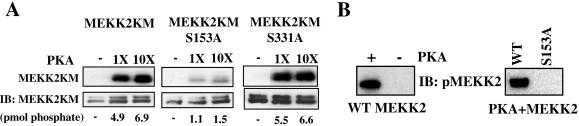
Inspection of the primary sequence of MEKK2 reveals two potential PKA phosphorylation sites, serine 153 and serine 331. We mutated these sites to alanine in a kinase-defective mutant of MEKK2, MEKK2KM. Compared to MEKK2KM, PKA catalyzed a significantly reduced incorporation of phosphate onto MEKK2KM S153A, S331A (not shown). We next determined the relative contribution of these sites to the phosphorylation of MEKK2. Phosphorylation of MEKK2KM S153A was dramatically reduced, while S331A was little changed relative to wild-type MEKK2, suggesting that S153 is the primary phosphorylation site for PKA (Fig. 7A). The capacity of PKA to phosphorylate S153 of MEKK2 with selectivity supports the hypothesis that MEKK2 integrates cAMP signaling through direct phosphorylation by PKA.
FIG. 7.
PKA phosphorylates MEKK2 on serine 153. HEK293 cells were transfected with 5 μg cDNAs encoding the indicated inserts and handled as described for Fig. 6. (A) MEKK2 variants were immunoprecipitated for use in kinase assays with PKA. The autoradiogram (top) and immunoblot of 3X-FLAG-MEKK2 variants (3X-FLAG-MEKK2KM, 3X-FLAG-MEKK2KM S153A, or 3X-FLAG-MEKK2KM S331A) (bottom) are shown. Results are representative of at least three independent experiments. (B) Recombinant GST-MEKK2 N terminus was purified from bacteria and incubated with or without PKA under phosphorylating conditions. The samples were immunoblotted with pS153 MEKK2 (left). 3X-FLAG-MEKK2 wild-type or S153A was phosphorylated in vitro by PKA and immunoblotted with anti-pS153 MEKK2 antibodies (right).
We raised a phospho-specific antibody to MEKK2 S153 to detect phosphorylation of S153 selectively in cells. To characterize the antibody, we expressed an N-terminal fragment of MEKK2 as a GST fusion protein, phosphorylated it with PKA or not, and immunoblotted it with the pS153 MEKK2 antibody (Fig. 7B, left panel). The antibody recognized only the phosphorylated protein. Subsequently, we immunoprecipitated wild-type and S153A MEKK2 overexpressed in cells and phosphorylated the proteins in vitro with PKA. The pS153 antibody recognized wild-type MEKK2 only if it had been phosphorylated by PKA (Fig. 7B, left panel) but did not recognize S153A MEKK2 incubated with PKA (right panel).
Phosphorylation of MEKK2 by PKA does not reduce MEKK2 activity.
To determine if phosphorylation of MEKK2 by PKA could inhibit MEKK2 activity in vitro, MEKK2 was immunoprecipitated from unstimulated or EGF-treated HeLa cells, washed, and incubated with diluent or PKA for 30 min. Under these conditions we estimated at least 1 mol phosphate incorporated per mol MEKK2. The washed immunoprecipitates were assayed for activity. Phosphorylation of MEKK2 from EGF-stimulated cells did not reduce MEKK2 activity (Fig. 8A, top). The activity of MEKK2 from cells treated with forskolin and IBMX prior to EGF stimulation was assayed in parallel to show that MEKK2 from this experiment was inhibited if intracellular cAMP had been raised prior to growth factor stimulation (Fig. 8A, bottom).
These findings suggest that phosphorylation of MEKK2 by PKA must occur prior to stimulation of MEKK2 if MEKK2 activity is to be suppressed. In support of this idea we found that forskolin and IBMX treatment must occur within 2 minutes of stimulation with growth factor to inhibit the activation of ERK5 (Fig. 8B). If cells were treated after a longer period of growth factor treatment had elapsed, ERK5 was refractory to inhibition by cAMP.
To confirm that this site is phosphorylated following an elevation of cAMP in cells, we immunoprecipitated MEKK2 from EGF-treated HeLa cells with or without simultaneous elevation of cAMP. The immunoprecipitated MEKK2 was immunoblotted with the pS153 antibody. We found that MEKK2 from cells with elevated cAMP, whether or not they had been treated with growth factor, migrated slightly differently on gels and was recognized by the pS153 MEKK2 antibody (Fig. 8C). Finally, we inhibited PKA in cells with myr-PKI to determine whether S153 was still phosphorylated (Fig. 8D). In the presence of either forskolin or PGE2 to activate PKA, myr-PKI clearly reduced phosphorylation of MEKK2 on S153.
cAMP inhibits expression of c-Jun but not serum response element activity.
Growth factors stimulate the transcription of a series of genes, including c-fos and c-jun, whose products together contribute to proliferation. ERK5 and ERK1/2 cooperate to regulate cell proliferation (24). ERK1/2 are potent inducers of c-fos through their effects on serum response elements (SREs) (11). ERK5 induces c-jun through its effects on MEF2 transcriptional regulators which bind to the c-jun promoter (13, 28). Thus, one readout of their individual contributions to proliferation can be obtained by measuring activation of the SRE and induction of c-Jun. We have demonstrated that the elevation of intracellular cAMP alters the responsiveness of the MEKK2-MEK5-ERK5 cascade to growth factor activation, while MEK1/2 and ERK1/2 are unaffected.
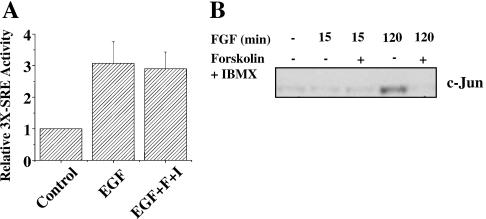
Based on these considerations, we predicted that the SRE would still be activated in growth factor-treated cells in which cAMP is elevated, because ERK1/2 activity is not reduced by cAMP. At the same time, we predicted that c-Jun would not be induced under these conditions, because ERK5 activity is suppressed by cAMP. With respect to the SRE, we found that growth factor activation of a luciferase gene fused to a triple SRE promoter was not reduced by cAMP (Fig. 9A), supporting the hypothesis that outputs downstream of ERK1/2 are not sensitive to cAMP, in spite of the fact that cAMP inhibits growth factor-stimulated proliferation. On the other hand, c-Jun was no longer induced in NIH 3T3 cells (Fig. 9B) or HeLa cells (data not shown) treated with forskolin and IBMX prior to growth factor stimulation, indicating that ERK5-dependent outputs contributing to proliferation are inhibited by cAMP. Changes in degradation or translational efficiency may also contribute to the altered production of c-Jun that we observed. In NIH 3T3 cells, neither p38 nor Jun N-terminal protein kinase family members were detectably activated by growth factor (data not shown). Thus, p38 and Jun N-terminal protein kinase, other enzymes that might otherwise affect the c-jun promoter through MEF2, make little or no contribution to c-Jun expression in these cells. We conclude that cAMP modulates the transcriptional response of the cell to growth factor by selectively restricting activation of growth factor targets.
FIG. 9.
cAMP inhibits expression of c-Jun but not SRE activity. (A) HeLa cells were transfected with cDNA encoding a luciferase gene driven by three tandem copies of the SRE. After 18 h, the culture medium was replaced with 0.5% FBS for an additional 24 h, and then the cells were stimulated with vehicle or 10 ng/ml EGF for 6 h. Relative activity refers to the increase in luciferase activity. Transfection efficiency was monitored with a Renilla firefly luciferase reporter driven by the thymidine kinase promoter (24). The averages of three independent experiments are shown, with error bars representing standard error of the mean. (B) NIH 3T3 cells were grown as described for Fig. 4 and treated with DMSO or 10 μM forskolin and 50 μM IBMX for 15 min and then with 25 ng/ml FGF for 15 min or 120 min. Lysates were immunoblotted with the anti-c-Jun antibody. Results are representative of at least three independent experiments.
DISCUSSION
Growth factors regulate cell proliferation, differentiation, and survival. Cells integrate signals to select the appropriate response to growth factor. Cyclic AMP is one of the physiological switches that determine the cell's choice of response to growth factor. To address how signaling networks can be altered to elicit different responses to a stimulus, we examined how cAMP influences growth factor receptor signaling. The MAPKs ERK5 and ERK1/2 are differentially sensitive to cAMP (26). Thus, we examined the cAMP-induced changes in the signaling network that prevented activation of ERK5 but spared activation of ERK1/2 by growth factor. We found that activation of the components of the pathway regulating ERK5, both MEK5 and MEKK2, was disrupted by cAMP through PKA. Once ERK5 is activated in cells, it is refractory to inhibition by cAMP. Likewise, once activated, MEKK2 is refractory to inhibition by PKA phosphorylation in vitro. Thus, we propose that phosphorylation of MEKK2 by PKA breaks the connection of MEKK2 with its upstream regulatory mechanism, thereby interrupting activation of MEK5 and ERK5. Additional actions of cAMP further upstream may also be involved.
Genes encoding c-Fos and c-Jun are among the most significant of those induced by growth factors to promote cell proliferation (16, 17, 22, 27). Selective suppression of ERK5 by cAMP is correlated with the failure of growth factor to induce c-Jun and the inhibition of proliferation (14). On the other hand, selective retention of the activation of ERK1/2 preserves activation of the downstream protein kinase p90 Rsk. Rsk contributes to induction of c-Fos by phosphorylation of serum response factor (31). In a number of systems, activation of Rsk also correlates with a growth factor-dependent survival response through interference with the proapoptotic protein Bad (2, 33). It seems likely then that cAMP switches the output produced by growth factor from proliferation to cell survival. Preliminary data examining confluent cells treated with growth factor with or without forskolin and IBMX suggest that fewer of the growth factor-treated cells with increased cAMP underwent apoptosis than cells exposed to growth factor alone (G. Pearson, unpublished data), consistent with this hypothesis. We conclude that changes in cAMP shape the response of a cell to growth factor by selectively blocking MAPK pathways. When cAMP concentration is low, growth factors activate ERK1/2 and ERK5, inducing both c-Fos and c-Jun to lead to cell proliferation. When the concentration of cAMP is high, growth factors lose the ability to stimulate ERK5 and c-Jun production but maintain the ability to activate ERK1/2, Rsk, and SREs, leading to a redirection of the growth factor signal. The response is presumably more appropriate for cells that are contact inhibited.
Finally, an implication of our findings is that the robustness of activation of a MAPK is dependent on the number of MAP3Ks that can activate a MAP2K-MAPK combination in response to a given stimulus. If multiple MAP3Ks control a MAPK cascade, the cascade will be relatively insensitive to events that impact only one of the MAP3Ks in that cascade. This would explain the apparent paradox in our findings that activation of Raf-1, like MEKK2, is sensitive to inhibition by cAMP, even though ERK1/2 is not. MEK1 can be activated not only by Raf-1 but also by A-Raf and B-Raf in response to EGF in HeLa cells (38), and both A-Raf and B-Raf are reportedly resistant to cAMP inhibition under conditions that inhibit Raf-1 (19, 37). The observation that stimulation of ERK1/2 by phorbol ester is inhibited by cAMP suggests that phorbol ester works only through Raf-1, not the other Raf isoforms. Thus, each stimulus has a different ability to access the MAP3Ks that have the potential to regulate a pathway. If a single MAP3K controls a MAPK cascade, any event that modulates the activity of the MAP3K will have an impact on the MAPK in the cascade. This is the case not only for activation of ERK1/2 by phorbol ester but also for activation of ERK5 by growth factors. One might also arrive at this idea from studies in which MAPK signaling networks have been manipulated at the level of protein expression. In whole animals and in culture, elimination of Raf-1 does not prevent activation of MEK1/2-ERK1/2 under proliferative conditions. On the other hand, we show here that abolishing expression of MEKK2 alone is sufficient to block activation of ERK5.
Acknowledgments
We thank Mike White (University of Texas Southwestern, Department of Cell Biology), Malavika Raman, Angelique Whitehurst, Bing-e Xu, and other current and former members of the Cobb laboratory for comments about the manuscript, Kathleen McGlynn for technical assistance in the early stage of this work, and Dionne Ware for administrative assistance.
This work was supported by grants from the National Institutes of Health (DK34128) and the Welch Foundation (I1243). Gray Pearson was supported by an NIGMS Predoctoral Training Grant in the pharmacological sciences.
REFERENCES
- 1.Abell, C. W., and T. M. Monahan. 1973. The role of adenosine 3′,5′-cyclic monophosphate in the regulation of mammalian cell division. J. Cell Biol. 59:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballif, B. A., and J. Blenis. 2001. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 12:397-408. [PubMed] [Google Scholar]
- 3.Brandon, E. P., R. L. Idzerda, and G. S. McKnight. 1997. PKA isoforms, neural pathways, and behaviour: making the connection. Curr. Opin. Neurobiol. 7:397-403. [DOI] [PubMed] [Google Scholar]
- 4.Burgering, B. M., G. J. Pronk, P. C. van Weeren, P. Chardin, and J. L. Bos. 1993. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 12:4211-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgun, C., L. Esteve, N. Humblot, D. Aunis, and J. Zwiller. 2000. Cyclic AMP-elevating agents induce the expression of MAP kinase phosphatase-1 in PC12 cells. FEBS Lett. 484:189-193. [DOI] [PubMed] [Google Scholar]
- 6.Chayama, K., P. J. Papst, T. P. Garrington, J. C. Pratt, T. Ishizuka, S. Webb, S. Ganiatsas, L. I. Zon, W. Sun, G. L. Johnson, and E. W. Gelfand. 2001. Role of MEKK2-MEK5 in the regulation of TNF-alpha gene expression and MEKK2-MKK7 in the activation of c-Jun N-terminal kinase in mast cells. Proc. Natl. Acad. Sci. USA 98:4599-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colognato, H., W. Baron, V. Avellana-Adalid, J. B. Relvas, A. Baron-Van Evercooren, E. Georges-Labouesse, and C. French-Constant. 2002. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4:833-841. [DOI] [PubMed] [Google Scholar]
- 8.De Cesare, D., G. M. Fimia, and P. Sassone-Corsi. 1999. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem. Sci. 24:281-285. [DOI] [PubMed] [Google Scholar]
- 9.Dong, F., J. S. Gutkind, and A. C. Larner. 2001. Granulocyte colony-stimulating factor induces ERK5 activation, which is differentially regulated by protein-tyrosine kinases and protein kinase C. Regulation of cell proliferation and survival. J. Biol. Chem. 276:10811-10816. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher, E. D., S. Xu, C. Moomaw, C. A. Slaughter, and M. H. Cobb. 2002. Binding of JNK/SAPK to MEKK1 is regulated by phosphorylation. J. Biol. Chem. 277:45785-45792. [DOI] [PubMed] [Google Scholar]
- 11.Gille, H., A. D. Sharrocks, and P. E. Shaw. 1992. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358:414-416. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, D. A., P. Akamine, E. Radzio-Andzelm, M. Madhusudan, and S. S. Taylor. 2001. Dynamics of cAMP-dependent protein kinase. Chem. Rev. 101:2243-2270. [DOI] [PubMed] [Google Scholar]
- 13.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J. D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16:7054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato, Y., R. I. Tapping, S. Huang, M. H. Watson, R. J. Ulevitch, and J. D. Lee. 1998. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395:713-716. [DOI] [PubMed] [Google Scholar]
- 15.Khoo, S., and M. H. Cobb. 1997. Activation of MAP kinase by glucose is not required for insulin secretion. Proc. Natl. Acad. Sci. USA 94:5599-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortenjann, M., O. Thomae, and P. E. Shaw. 1994. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol. Cell. Biol. 14:4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Cellular oncogenes and multistep carcinogenesis. Science 222:771-778. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 19.MacNicol, M. C., and A. M. MacNicol. 1999. Nerve growth factor-stimulated B-Raf catalytic activity is refractory to inhibition by cAMP-dependent protein kinase. J. Biol. Chem. 274:13193-13197. [DOI] [PubMed] [Google Scholar]
- 20.Marinissen, M. J., M. Chiariello, M. Pallante, and J. S. Gutkind. 1999. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol. Cell. Biol. 19:4289-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mckenzie, F. R., and J. Pouyssegur. 1996. cAMP-mediated growth inhibition in fibroblasts is not mediated via mitogen-activated protein (MAP) kinase (ERK) inhibition. cAMP-dependent protein kinase induces a temporal shift in growth factor-stimulated MAP kinases. J. Biol. Chem. 271:13476-13483. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 23.Pearson, G., R. Bumeister, D. O. Henry, M. H. Cobb, and M. A. White. 2000. Uncoupling Raf1 from MEK1 impairs only a subset of cellular responses to Raf activation. J. Biol. Chem. 275:37303-37306. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, G., J. M. English, M. A. White, and M. H. Cobb. 2001. ERK5 and ERK2 cooperate to regulate NF-kappaB and cell transformation. J. Biol. Chem. 276:7927-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, G., F. Robinson, G. T. Beers, B. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 26.Pearson, G. W., and M. H. Cobb. 2002. Cell condition-dependent regulation of ERK5 by cAMP. J. Biol. Chem. 277:48094-48098. [DOI] [PubMed] [Google Scholar]
- 27.Perucho, M., M. Goldfarb, K. Shimizu, C. Lama, J. Fogh, and M. Wigler. 1981. Human-tumor-derived cell lines contain common and different transforming genes. Cell 27:467-476. [DOI] [PubMed] [Google Scholar]
- 28.Price, M. A., F. H. Cruzalegui, and R. Treisman. 1996. The p38 and ERK MAP kinase pathways cooperate to activate Ternary Complex Factors and c-fos transcription in response to UV light. EMBO J. 15:6552-6563. [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos, J. W., P. E. Hughes, M. W. Renshaw, M. A. Schwartz, E. Formstecher, H. Chneiweiss, and M. H. Ginsberg. 2000. Death effector domain protein PEA-15 potentiates Ras activation of extracellular signal receptor-activated kinase by an adhesion-independent mechanism. Mol. Biol. Cell 11:2863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimann, E. M., and R. A. Beham. 1983. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 99:51-55. [DOI] [PubMed] [Google Scholar]
- 31.Rivera, V. M., C. K. Miranti, R. P. Misra, D. D. Ginty, R. H. Chen, J. Blenis, and M. E. Greenberg. 1993. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol. Cell. Biol. 13:6260-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheppard, J. R., and D. M. Prescott. 1972. Cyclic AMP levels in synchronized mammalian cells. Exp. Cell Res. 75:293-296. [DOI] [PubMed] [Google Scholar]
- 33.Shimamura, A., B. A. Ballif, S. A. Richards, and J. Blenis. 2000. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr. Biol. 10:127-135. [DOI] [PubMed] [Google Scholar]
- 34.Sommer, A., H. Burkhardt, S. M. Keyse, and B. Luscher. 2000. Synergistic activation of the mkp-1 gene by protein kinase A signaling and USF, but not c-Myc. FEBS Lett. 474:146-150. [DOI] [PubMed] [Google Scholar]
- 35.Sun, H., C. H. Charles, L. F. Lau, and N. K. Tonks. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487-493. [DOI] [PubMed] [Google Scholar]
- 36.Sun, W., K. Kesavan, B. C. Schaefer, T. P. Garrington, M. Ware, N. L. Johnson, E. W. Gelfand, and G. L. Johnson. 2001. MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway. J. Biol. Chem. 276:5093-5100. [DOI] [PubMed] [Google Scholar]
- 37.Sutor, S. L., B. T. Vroman, E. A. Armstrong, R. T. Abraham, and L. M. Karnitz. 1999. A phosphatidylinositol 3-kinase-dependent pathway that differentially regulates c-Raf and A-Raf. J. Biol. Chem. 274:7002-7010. [DOI] [PubMed] [Google Scholar]
- 38.Wu, X., S. J. Noh, G. Zhou, G. Dixon, J. E. Dixon, and K. Guan. 1996. Selective activation of MEK1 but not MEK2 by A-Raf from epidermal growth factor-stimulated HeLa cells. J. Biol. Chem. 271:3265-3271. [DOI] [PubMed] [Google Scholar]
- 39.Young, S. W., M. Dickens, and J. M. Tavaré. 1994. Differentiation of PC12 cells in response to a cAMP analogue is accompanied by sustained activation of mitogen-activated protein kinase. Comparison with the effects of insulin, growth factors and phorbol esters. FEBS Lett. 338:212-216. [DOI] [PubMed] [Google Scholar]