Abstract
Primary infection by herpes simplex virus type 1 (HSV-1) can cause clinical symptoms in the peripheral and central nervous system, upper respiratory tract, and gastrointestinal tract. Recurrent ocular shedding leads to corneal scarring that can progress to vision loss. Consequently, HSV-1 is the leading cause of corneal blindness due to an infectious agent. Bovine herpesvirus 1 (BHV-1) has similar biological properties to HSV-1 and is a significant health concern to the cattle industry. Latency of BHV-1 and HSV-1 is established in sensory neurons of trigeminal ganglia, but latency can be interrupted periodically, leading to reactivation from latency and spread of infectious virus. The ability of HSV-1 and BHV-1 to reactivate from latency leads to virus transmission and can lead to recurrent disease in individuals latently infected with HSV-1. During latency, the only abundant HSV-1 RNA expressed is the latency-associated transcript (LAT). In latently infected cattle, the latency-related (LR) RNA is the only abundant transcript that is expressed. LAT and LR RNA are antisense to ICP0 or bICP0, viral genes that are crucial for productive infection, suggesting that LAT and LR RNA interfere with productive infection by inhibiting ICP0 or bICP0 expression. Numerous studies have concluded that LAT expression is important for the latency-reactivation cycle in animal models. The LR gene has recently been demonstrated to be required for the latency-reactivation cycle in cattle. Several recent studies have demonstrated that LAT and the LR gene inhibit apoptosis (programmed cell death) in trigeminal ganglia of infected animals and transiently transfected cells. The antiapoptotic properties of LAT map to the same sequences that are necessary for promoting reactivation from latency. This review summarizes our current knowledge of factors regulating the latency-reactivation cycle of HSV-1 and BHV-1.
HSV-1 AND BHV-1 PATHOGENESIS
A high percentage of the world’s population are infected with herpes simplex virus type 1 (HSV-1), and infection can cause a variety of disorders (35, 187). Recurrent ocular HSV-1 is the leading cause of infectious corneal blindness in industrialized nations (190). In a murine model, ocular infection induces autoimmune disorders, leading to corneal antigen destruction and stromal keratitis (275). HSV-1 infections also cause gastrointestinal disorders, esophageal disorders, and approximately 25% of the genital herpes infections (67, 158).
HSV-1 infections can cause sporadic encephalitis, but this is relatively rare compared to other diseases resulting from infection. Further evidence for its involvement in central nervous disorders comes from epidemiological studies that suggest a link between Alzheimer's disease and HSV-1 infection (108, 151). The apolipoprotein E type 4 allele is hypothesized to be a cofactor because it makes an individual susceptible to HSV-1 spread in the brain. The same regions of the brain affected by acute HSV-1 encephalitis are those most severely affected in Alzheimer's disorder. Finally, infection of neonate mice with an attenuated virus strain leads to hyperactivity and learning deficits, suggesting that this could be a concern when infants become infected (34). In summary, HSV-1 continues to be a significant public health problem.
Bovine herpesvirus 1 (BHV-1) also belongs to the Alphaherpesvirinae subfamily and shares a number of biological properties with HSV-1 and HSV-2. BHV-1 infection can cause conjunctivitis, pneumonia, genital disorders, abortions, and an upper respiratory infection referred to as shipping fever (258). BHV-1 is not the sole infectious agent associated with shipping fever, but it initiates the disorder by immunosuppressing infected cattle. BHV-1-induced immunosuppression frequently leads to secondary bacterial infections (with Pasteurella haemolytica, Pasteurella multocida, and Haemophilus somnus for example) that can cause pneumonia. Increased susceptibility to secondary infection correlates with depressed cell-mediated immunity after BHV-1 infection (23, 74-76). CD8+ T-cell recognition of infected cells is impaired by repressing the expression of major histocompatibility complex class I and the transporter associated with antigen presentation (89, 99, 189). CD4+ T-cell function is impaired during acute infection of calves because BHV-1 infects CD4+ T cells and induces apoptosis (267). BHV-1 infection costs the cattle industry at least $500 million per year in the United States (18). Although vaccines are available, they can cause disease in young calves and abortions in cows.
OVERVIEW OF THE STEPS OF THE LATENCY-REACTIVATION CYCLE
Despite a vigorous immune response during acute infection, HSV-1 establishes latency in ganglionic sensory neurons, typically trigeminal ganglia (TG) or sacral dorsal root ganglia (116, 263). Although TG are primary sites of latency following ocular, oral, or intranasal infection (7, 8), latent HSV-1 can also be detected in human adult nodose ganglia and the vagus nerve (67, 158). As many as 40 to 60% of sensory neurons can be latently infected (166, 172, 208, 209, 223). HSV-1 genomic DNA has also been detected in the central nervous system of a significant percentage of humans (6, 108, 145).
The steps of the latency-reactivation cycle have been operationally divided into three major steps: establishment, maintenance, and reactivation (Table 1). Establishment of latency includes entry of the viral genome into a sensory neuron and acute infection. Viral gene expression is then extinguished, with the exception of the latency-associated transcript (LAT). For further details regarding viral gene expression during acute infection and establishment of latency, see the following section.
TABLE 1.
Major steps during the latency-reactivation cycle
| Establishment of latency |
| Entry of viral genome into ganglionic neurons |
| Abundant viral gene expression and DNA replication (acute infection) |
| Extinction of viral gene expression |
| Abundant LAT expression |
| Maintenance of latency |
| Expression of LAT |
| No detection of abundant lytic viral gene expression |
| No detection of abundant viral DNA replication |
| Reactivation from latency |
| External stimulus (stress and immunosuppression for example) |
| Productive infection (extensive viral gene expression, DNA replication, and infectious virus) |
| Survival of latently infected cell? |
| LAT expression |
Maintenance of latency is a phase that lasts for the life of the host and can be operationally defined as a period when infectious virus is not detected by standard virus isolation procedures. In general, abundant expression of viral genes that are required for productive infection does not occur. LAT is the only known viral transcript that is abundantly expressed during this stage of latency.
Reactivation from latency is initiated by external stimuli (stress and immunosuppression, for example) that stimulate viral gene expression. Abundant viral gene expression is detected in sensory neurons, and infectious virus can be isolated from TG, eye swabs, and/or nasal swabs. It is not clear whether the neuron that undergoes reactivation survives and resumes latency or is killed by the virus as a result of productive infection. For further discussions of factors that regulate reactivation from latency, see below. The ability of HSV-1 to reactivate from latency results in recurrent disease and virus transmission.
VIRAL GENE EXPRESSION DURING PRODUCTIVE INFECTION AND LATENCY
Expression during Productive Infection
Binding and entry of HSV-1 to cells are mediated by viral glycoproteins and cellular factors (247). A cellular mediator of viral entry (HveA or HVEM) is expressed primarily in activated T cells and belongs to the tumor necrosis factor (TNF) receptor family (180). Entry of HSV-1 into epithelial and other nonlymphoid cells is mediated by an unrelated membrane glycoprotein that resembles the poliovirus receptor (HveB and HveC) (66). HveC is active as an entry mediator for all herpesviruses tested so far (HSV- 1, BHV-1, and pseudorabies virus, PRV). HveC is abundantly expressed in neurons and can block viral entry in several neuronlike cell lines (66). After uncoating, the viral genome is present in the nucleus and viral gene expression ensues. HSV-1 gene expression is temporally regulated in three distinct phases: immediate-early (IE), early (E), and late (L) (101). IE RNA expression does not require protein synthesis and is stimulated by the tegument protein VP16 (193) and by active cyclin-dependent kinases (227, 228). E RNA expression is dependent on at least one IE protein, and generally E genes encode nonstructural proteins that play a role in viral DNA synthesis. L RNA expression is maximal after viral DNA replication and requires IE protein expression, and most L proteins are structural proteins that comprise the virion particle.
Five IE genes encode ICP0, ICP4, ICP22, ICP27, or ICP47. ICP4 (22, 39, 40, 49) and ICP27 (168, 171, 218) are required for virus growth in tissue culture. In general, ICP4 represses IE gene expression (40, 78, 79, 175, 194, 212) and activates E or L RNA expression by interacting with RNA polymerase II transcription factors (78, 245). ICP22 is important for viral growth in some cultured cells (232) and modifies RNA polymerase II (211). ICP27 redistributes small nuclear ribonucleoprotein complexes, interferes with splicing of IE transcripts, and promotes E and L poly(A) site selection (87, 88, 221, 222). Thus, ICP27 is required for transition from IE gene expression to E and L RNA expression. ICP47 prevents transport of antigenic peptides into the endoplasmic reticulum (97) and is crucial for neurovirulence because it inhibits CD8+ T-cell responses (72).
The amino terminus of ICP0 is required for IE promoter activation, but a separate domain activates E or L promoters (155, 156). Thus, ICP0 can activate the expression of all classes of viral genes, in large part because it increases steady-state levels of mRNA (117). ICP0 also binds several cellular proteins: (i) elongation factor 1α (119), (ii) cyclin D3 (120), and (iii) ubiquitin-specific protease (173, 174). These activities promote virus replication in differentiated cells (20).
Infection of permissive cells (46) or calves (267) with BHV-1 leads to rapid cell death, in part due to apoptosis. Viral gene expression is temporally regulated in three distinct phases: IE, E, and L. IE gene expression is stimulated by a virion component, bTIF. bTIF interacts with a cellular transcription factor (Oct-1) and transactivates IE gene expression (49). Two IE transcription units exist: IE transcription unit 1 (IEtu1) and IEtu2. IEtu1 encodes functional homologues of two HSV-1 IE proteins, ICP0 and ICP4. IEtu2 encodes a protein that is similar to an essential HSV IE protein, ICP22 (69). IE proteins activate E gene expression, and viral DNA replication ensues. L gene expression is also activated by bICP0, culminating in virion assembly and release. Thus, IE genes are essential for virus growth because they regulate viral gene expression. In particular, bICP0 is very important for productive infection because it activates all classes of viral promoters and is expressed at high levels throughout infection (62, 270, 272). Although there are differences in the organization of IE gene expression during HSV-1 and BHV-1 productive infection, the same general cascade of viral gene expression occurs.
Repression of Viral Gene Expression after Infection of Sensory Neurons
Following infection of rodents, rabbits, or humans with HSV-1, productive infection is initiated in the mucosal epithelium. Virus particles or subparticles then enter sensory neurons and are transported intra-axonally to the sensory ganglia. Since HSV-1 infection typically occurs via the oral, ocular, or nasal route, the trigeminal ganglia (TG) are a primary site for latency (7). In general, viral transcription from the HSV-1 genome is silent in sensory neurons following acute infection (establishment of latency). During latency, two changes occur in the organization of the viral genome that may influence viral gene expression. First, the viral genome is present as a circular episome (215, 216). Second, the viral genome is associated with cellular histones and thus exists as chromatin in latently infected neurons (42).
Extensive viral gene expression and replication occur within TG for approximately 1 week following infection of animal models that support HSV infection (124, 125). Productive viral gene expression that occurs in TG appears to be different from what is seen in cultured cells (248). Infectious virus can readily be detected in homogenates prepared from TG during acute infection. However, it is difficult to conclude whether this infectious virus is the result of productive infection in sensory neurons or the result of transport from peripheral sites of infection. Events during this time are likely to play a critical role in establishment of latency. Replication is not required for establishment of latency, because mutants that cannot replicate will establish latency but at a reduced level (32, 54, 118, 138, 167, 234, 249, 262).
Regulation of IE Promoters in Sensory Neurons versus Nonneuronal Cell Types
Several studies using transgenic mice that contain IE promoters linked to a reporter gene have concluded that IE promoters are differentially regulated by neuron-specific factors. For example, the HSV-1 ICP4 promoter is active in Schwann cells but not sensory neurons in TG (251). As expected, the ICP4 promoter in transgenic mice is activated in TG neurons following infection with HSV-1. In contrast to the ICP4 promoter, transgenic mice containing the ICP0 or ICP27 promoters are active in certain neurons within the brain and TG (159). The ICP0 promoter is also differentially regulated in TG neurons depending on the age of the mouse. The ICP0 promoter contains a cis-acting element that can bind a neuron-specific transcription factor, Olf-1, which is differentially and developmentally expressed in specific subsets of sensory neurons (47), suggesting that the Olf-1 site plays a role in activating ICP0 promoter activity in certain neurons.
All IE promoters contain a common cis-acting sequence (TAATGARAT) that is required for VP16-mediated trans activation (193). VP16 must interact with two cellular proteins, Oct-1 and HCF, to efficiently induce IE promoter activity. A cellular transcription factor, Zhangfei, binds to HCF and prevents activation of the ICP0 promoter (163). Another cellular transcription factor, Luman, also binds to HCF and sequesters HCF in the cytoplasm of sensory neurons, suggesting that Luman plays a role in latency (162). Zhangfei and Luman have basic domain-leucine zippers (bZIP) regions, acidic activation domains, and consensus HCF-binding motifs, yet have little amino acid similarity. In nonneuronal cells, HCF has a nuclear localization (129), but in sensory neurons it appears to be localized predominantly to the cytoplasm (131). If the relative levels of Luman and Zhangfei are high, the availability of “free” HCF that could interact with VP16 would be reduced and consequently IE gene expression would be repressed. It has also been hypothesized that VP16 is not present in sufficient quantities in the nuclei of infected sensory neurons to stimulate efficient productive infection (130). However, inducible expression of VP16 in the context of the viral genome or in transgenic mice does not lead to enhanced viral replication (233). In summary, several factors exist that may inhibit the formation of a functional VP16 transcriptional complex in sensory neurons.
TAATGARAT elements from HSV-1 IE genes have also been reported to be directly repressed by specific isoforms of Oct-2 (41, 58, 121, 137, 147-150). At least five Oct-2 isoforms are generated by differential splicing of a single transcript (146). The proteins translated from these transcripts all bind to a consensus Oct site but can either repress or activate simple promoters containing Oct binding sites or multimers of the TAATGARAT motif. Oct-2.1 and Oct-2.5 are not able to repress the transcriptional activity of an intact IE promoter in transient-transfection assays (81). Two studies have concluded that Oct-2 isoforms are not expressed in sensory neurons (82, 260), suggesting that Oct-2 isoforms do not ordinarily regulate IE gene expression in the peripheral nervous system.
Sensory neurons express other proteins, such as Brn-3.0 (261) and N-Oct3, that have the potential to regulate IE gene expression (82). Brn-3.0 binds to noncoding sequences in the HSV-1 genome (261), but the binding sites for Brn-3.0 are not identical to those for Oct-1 or other related transcription factors that also include Brn-3.1 and Brn-3.2 (77). Brn-3.0 is important in the peripheral nervous system of mice because null mutations in the brn-3.0 locus result in neonatal death with defects in sensory ganglia and specific central nervous system nuclei (169, 231), brn-3.2 is required for differentiation of certain retinal ganglion cells (57). One study has concluded that Brn-3.1 and Brn-3.2 have opposite effects on a target promoter (181). Considering that the Brn-3 family of transcription factors is expressed in the peripheral nervous system, these proteins may regulate HSV gene expression during the latency-reactivation cycle.
Following infection of primary neurons, ICP0 does not accumulate in the nuclei of infected cells (25). An independent study also concluded that the function of ICP0 is impaired in human neuron-like cells because a nuclear structure (ND10) that ICP0 interacts with is different from that in nonneuronal cells (103). The same neuron-like cells do not support efficient viral replication, in part because ICP0-expressing plasmids do not activate viral transcription efficiently. These studies argue that ICP0 does not function efficiently in neuronal cells and thus productive infection is inhibited. In summary, several key steps in the productive infection cycle are impaired in sensory neurons, thus promoting the establishment of latency.
The Promoter That Regulates LAT Expression Is a Neuron-Specific Transcriptional Element
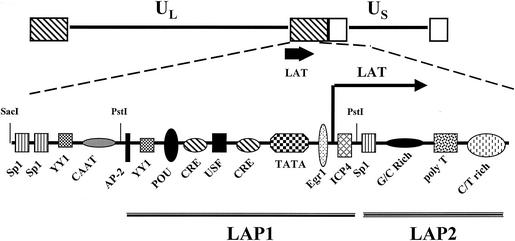
In sharp contrast to other HSV-1 promoters, the promoter that directs the expression of LAT is activated in sensory neurons (Fig. 1 gives a schematic of the HSV-1 LAT promoter). Two separate promoter fragments that are upstream of the start site of LAT, latency-associated promoters 1 and 2 (LAP1 and LAP2), can cis activate a reporter gene in transiently transfected cells (26, 70). Several studies have demonstrated that sequences spanning the TATA box, LAP1, are critical for directing LAT expression in sensory neurons (26, 43, 50, 51, 179). LAP2 promotes the expression of the stable 2-kb LAT during productive infection of cultured cells (26, 192). Although the LAT promoter elements have neuronal specificity in transient-transfection assays, they can also direct the expression of a reporter gene in nonneural cells (9-11, 276, 277). This may reflect the abundance of cellular transcription factor-binding sites within the LAT promoter (Fig. 1). Many of these transcription factors are present in nonneural cells and thus have the potential to activate expression in transiently transfected cells. For example, the two cyclic AMP (cAMP)-responsive element (CRE)-binding sites in the LAT promoter are functional because cAMP activates the promoter (122, 141). The CRE motif that is proximal to the TATA box is important for expression in neurons, and its presence has a positive effect on reactivation from latency (15, 141, 207). Furthermore, Sp1, YY1, USF, and CAAT are frequently found in RNA polymerase II promoters that are not neuron specific. Neuron-specific factors have been identified that bind to the LAT promoter (9-11, 276, 277). The finding that the IE protein ICP4 binds to DNA sequences downstream of the TATA box and represses the LAT promoter is one important reason why LAT is not an abundant transcript during productive infection (11).
FIG. 1.
Schematic of the HSV-1 LAT promoter. The LAT promoter contains numerous cis-acting sites that can be bound by cellular transcription factors. Binding of ICP4 to the ICP4-binding site in the LAT promoter inhibits promoter activity (11). In transient-transfection assays, the LAT promoter can be divided into a strong promoter (LAP1) and a weaker promoter (LAP2) (26, 70). For details of transcripts encoded by LAT, see Fig. 2.
Long-term expression of LAT has also been examined in the context of the viral genome (12, 160, 161). These studies have demonstrated that LAP2 sequences function as a long-term enhancer (Fig. 1) in latently infected mice. LAP2 is also required for maintaining LAP1 promoter activity. Although DNA sequences within the LAT promoter activate RNA expression in sensory neurons, it is clear that neuronal specificity is not located in a single cis-acting motif.
ANALYSIS OF THE GENE ENCODING LAT
LAT Is Abundantly Expressed in Sensory Neurons during Latency
In situ hybridization has revealed that LAT is abundantly transcribed in latently infected neurons (35, 37, 38, 127, 178, 217, 250, 264, 265) (Fig 2). Mice, rabbits, and humans latently infected with HSV-1 express LAT, and LAT is detected predominantly in the nucleus. Sensitive reverse transcription-PCR (RT-PCR) assays have also detected thymidine kinase and ICP4 transcripts, in addition to LAT, in TG of mice latently infected with HSV-1 (126). These transcripts may result from low levels of spontaneous reactivation or unsuccessful reactivation events (73, 236). Viral genome-positive neurons that are LAT RNA negative have been detected in latently infected mice (209). Since in situ PCR was used to detect the viral genome but in situ hybridization was used for LAT RNA detection, neurons expressing low levels of LAT may have been missed. Thus, it is likely that all viral genome-positive neurons express LAT during latency. LAT is complementary to ICP0 and overlaps the ICP0 transcript, suggesting that LAT inhibits ICP0 expression by an antisense mechanism. Although the ability of LAT to repress ICP0 expression is probably important, LAT sequences that promote spontaneous reactivation in a rabbit ocular model do not overlap ICP0 (201). The simplest interpretations of these data are that LAT has more than one function or the ability of LAT to repress ICP0 expression is not that important in the small-animal models used to study latency.
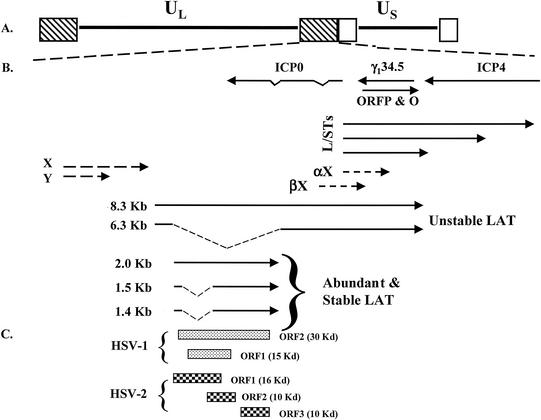
FIG. 2.
Location of genes within the HSV-1 repeats. (A) UL and US denote the unique sequences of the long (L) and short (S) components of the genome. The boxes depict repeat sequences. (B) Transcription map of the repeat region. The location and orientation of LAT (217, 250), ICP0, γ134.5 (29, 30), ORFP (136), and L/STs (274) are indicated by solid lines. Partially mapped transcripts αX and βX are denoted by dashed arrows (16, 17). (C) Positions of potential ORFs within the abundant and stable LAT of HSV-1 strain 17 syn+, and HSV-2 strain 333 (128). The approximate size of the respective ORF is given in parentheses.
In productively infected cells or latently infected rabbits, an 8.5-kb transcript is expressed that has the same sense as LAT (38, 217, 277) (Fig. 2 gives a schematic of the LAT region). The majority of LAT is not capped, lacks a consensus poly(A) addition site, and appears to be circular. The most abundant LAT is a 2-kb intron, which is a spliced product of the 8.5-kb LAT (59, 133). The 2-kb LAT has a half-life of approximately 24 h in human neuroblastoma or monkey kidney cells (253). Mutatgenesis of a potential stem-loop structure adjacent to the nonconsensus intron branch is crucial for 2-kb LAT stability. The 2-kb LAT can also be spliced in neurons to yield 1.4- and 1.5-kb transcripts (165). Mutation of the splice acceptor site or the splice donor site that generates the 2-kb LAT drastically reduces expression of the 2-kb LAT during productive infection but has little effect on expression during latency (3). The relationship of LAT to the other transcripts in the inverted repeats that have the same polarity as LAT (L/STs, αX, or βX transcripts [Fig. 2]) is not clear. In summary, it is thought that splicing of the unstable 8.5-kb transcript yields the abundant stable 2-kb LAT that can be further processed by splicing.
Although LAT has a nuclear localization, it can also be detected in the cytoplasm and other compartments (2, 192, 253). LAT is associated with polyribosomes, suggesting that it can be translated or can regulate translation (2, 71). The association between LAT and polyribosomes is more like rRNA than mRNA, supporting the concept that LAT can regulate protein expression. LAT is also associated with splicing factors (2), suggesting that it can regulate the splicing of certain transcripts. It is currently thought that the 2-kb LAT is processed in nuclei like mRNA but plays a structural role in ribosomes like rRNA (2).
LAT contains small open reading frames (ORFs) (Fig. 2C), and there is a report of a latency-associated antigen (52). An independent study concluded that ORFs within the 2- kb LAT can be expressed in transient-transfection assays, but LAT protein expression was not detected in infected cells (157). ORF2 apparently stimulates productive infection in neuronal cells when overexpressed, suggesting that it has functional significance during reactivation from latency because it can substitute for ICP0 functions (254). A plasmid containing ORF2 fused to an epitope tag expresses a protein that localizes to punctate structures in the nucleus, and overexpression of ORF2 stimulates viral gene expression (255). Although ORF2 may play a role in the latency-reactivation cycle, there is no evidence that it is expressed during this cycle. Furthermore, ORF2 is not present within the 1.5-kb LAT fragment that is required for spontaneous reactivation in the rabbit eye model (200, 201). Similarity exists between the C terminus of HSV-1 ORF2 and HSV-2 ORF3 (128) because this region overlaps ICP0. In contrast, small ORFs within the 1.5-kb LAT that promote spontaneous reactivation do not have a high degree of amino acid similarity between strains of HSV-1 (53), suggesting that expression of a protein does not play an important role in the latency- reactivation cycle. Additional studies are obviously required to determine whether the LAT ORFs express proteins during the latency-reactivation cycle and whether these proteins play a role in regulating latency.
If LAT does not encode a protein, then how does a non-protein-coding RNA regulate latency? There are numerous examples of transcripts that do not apparently encode a protein but have important functions. For example, a cellular RNA molecule that does not encode a protein prevents the proliferation of cancer cells (85). Furthermore, a small poly(A)− transcript (166 and 172 nucleotides) expressed by Epstein-Barr/Virus in latently infected B cells supports Burkitt's lymphoma growth by inducing interleukin-10 expression (123). Additional support for the notion that LAT is a regulatory RNA (250) comes from the finding that LAT inhibits the ability of ICP0 to transactivate a promoter in transiently transfected cells (59). Thus, LAT RNA sequences could have several important functions while not encoding a protein.
LAT Regulates the Latency-Reactivation Cycle
As discussed above, the latency-reactivation cycle of HSV-1 can be operationally defined in three steps: establishment of latency, maintenance of latency, and reactivation from latency (summarized in Table 1). In a human, latency is maintained for the life of the host, indicating that a well-conceived strategy exists that allows for periodic reactivation while maintaining the viral genome in sensory neurons.
Numerous HSV-1 mutants that do not express detectable levels of LAT have been constructed and tested in animal models (116, 263). Although a couple of studies have suggested that LAT plays no role in a latent infection (14, 100), most have concluded that LAT is important but not required. LAT enhances the establishment of latency in mice (224, 257), because certain LAT− mutants contain lower levels of viral DNA in murine TG than does wild-type virus (44, 166). Furthermore, LAT enhances the establishment of latency in the rabbit eye model and consequently reduces reactivation from latency (204). The finding that LAT represses productive viral gene expression in TG of mice during acute infection (24, 65) supports the studies concluding that LAT facilitates the establishment of latency. When considering the role that LAT plays in reactivation from latency, its role in establishing latency has to be taken into consideration.
LAT is also important for in vivo reactivation by using two different rabbit eye infection models. The McKrae strain of HSV-1 is frequently shed in the tears of infected rabbits as a result of spontaneous reactivation (199-203). In contrast, spontaneous reactivation is severely impaired if the LAT gene is deleted. However, these same LAT− mutants grow with the same efficiency as wild-type virus in cultured cells and in ocular tissue of infected rabbits. The first 1.5 kb of the gene encoding LAT is sufficient for spontaneous reactivation from latency (201). Since this region does not overlap ICP0, antisense repression of ICP0 expression by LAT is not required for spontaneous reactivation in the rabbit model. HSV-1 17syn+ strains that have deletions in the LAT promoter and 5′ region of the gene encoding LAT (approximately 1,200 bp) also do not reactivate efficiently in a rabbit eye model (98, 259). Mutagenesis of LAT ORF-2 does not reduce reactivation kinetics (spontaneous or induced) in the rabbit eye model (60). Although the gene encoding LAT is not required for latency in small animal models, it greatly enhances reactivation in the rabbit. It would not be surprising to find that the importance of LAT is underestimated using small animal models and measuring latency in terms of weeks or months, not decades.
REGULATION OF APOPTOSIS BY HSV-1 AND LAT
HSV-1 Contains Several Genes That Regulate Apoptosis
Many viruses induce apoptosis in cultured cells (86, 210, 235, 252). Killing of infected cells by apoptosis in vivo can reduce inflammation, alter immune recognition, reduce burst size, and thus prevent virus spread. Members of the Alphaherpesvirinae subfamily induce apoptosis after infection of cultured cells (46, 63, 64, 219). HSV-1 can induce or inhibit apoptosis in a cell-type-dependent manner after infection of cultured cells (4, 5, 63, 64, 143). Several antiapoptotic genes within the HSV-1 genome have been identified (1, 4, 5, 13, 63, 107, 110, 111, 113, 185, 186, 197, 198). These viral genes include ICP27, US3, US5, gJ, gD, and LAT. US3 is a protein kinase that, in the absence of other HSV-1 proteins, inhibits the cleavage of BAD and the formation of the proapoptotic form of BAD (186). US3 is the only viral protein required for preventing caspase-3 activation, which is considered to be the “point of no return” in the apoptotic pathway (Fig. 3). The presence of several HSV-1 antiapoptotic genes suggests that they play specific roles following infection of humans.
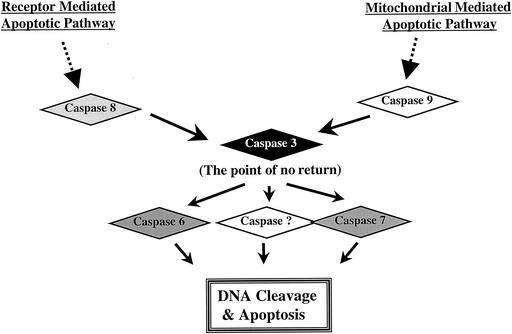
FIG. 3.
Schematic of the major apoptotic pathways in mammals. Two major apoptotic pathways, the receptor-mediated apoptotic pathway and the mitochondrial apoptotic pathway, exist in mammals (132, 230, 266). The receptor-mediated apoptotic pathway activates caspase-8, which induces a caspase cascade, including caspase-3. Activation of the mitochondrial apoptotic pathway results in caspase-9 activation, which culminates in activation of the effector caspases (including caspase-3). Activation of the effector caspases, in particular caspase- 3, leads to the morphological hallmarks of apoptosis. These pathways are not linear or exclusive of each other. For example, caspase-3 can activate caspase-9 and the receptor- mediated apoptotic pathway can lead to the activation of the mitochondrial apoptotic pathway (135, 144).
HSV infection has the potential to induce apoptosis by several distinct mechanisms. For example, HSV induces DNA damage even in the absence of productive infection (27, 56, 93, 206, 229). DNA damage is a potent stimulus for apoptosis (246). When expressed from baculovirus expression vectors, US1.5 and UL13 have the potential to induce caspase-3 (80). It is not clear how these viral proteins activate caspase-3. As expected, US3 can inhibit the proapoptotic activity of US1.5 and UL13 by virtue of its ability to interfere with caspase-3 activation.
LAT Protects Cells from Apoptosis
Three studies have demonstrated that LAT interferes with apoptosis in transiently transfected cells (1, 107, 198). The ability of LAT to interfere with apoptosis correlates with its ability to promote spontaneous reactivation (107), suggesting that the antiapoptotic activity of LAT is important during the latency-reactivation cycle. This hypothesis is supported by the finding that LAT promotes neuronal survival in TG of infected rabbits (198). In acutely infected mice, a LAT− mutant also induces extensive apoptosis compared to the LAT+ virus (1). Another independent study concluded that LAT protects neurons from cell death (256) but was unable to demonstrate changes in apoptotic frequencies in mice. In summary, there is evidence that LAT enhances neuronal survival because it has antiapoptotic activity.
How does LAT inhibit apoptosis? There are two major apoptotic pathways: the death receptor-mediated pathway (Fas or TNF receptor, for example) and the mitochondrial pathway (132, 230, 266) (Fig. 3 gives a summary of these pathways). The death receptor-mediated death pathway activates caspase-8, which induces a caspase cascade including caspase-3. Activation of the mitochondrial pathway results in the release of several important proapoptotic molecules, including cytochrome c and Smac/Diablo (266). Released cytochrome c associates with Apaf-1, leading to caspase-9 activation, which culminates in activation of the effector caspases (including caspase-3). Regardless of the pathway, activation of the effector caspases, in particular caspase-3, leads to the morphological hallmarks of apoptosis. These pathways are not linear or exclusive of each other. For example, caspase-3 can activate caspase-9 and the death receptor- mediated apoptosis, leading to the activation of the mitochondrial pathway by BID cleavage and cytochrome c release, thus activating caspase-9 (135, 144). In transiently transfected HeLa cells (human epithelial cells), LAT interferes with caspase-8-induced apoptosis (1). Cultures of mouse neuroblastoma cells (neuro-2A) infected with a LAT− mutant contained more cleaved (activated) caspase-9 at late times after infection as did neuro-2A cells infected with a wild-type LAT+ HSV-1 strain (94). Subtle differences were also detected in caspase-8 cleavage but not caspase-3 cleavage. Although these studies suggest that LAT interferes with proximal steps in the caspase cascade, additional studies are necessary for understanding the mechanism by which LAT inhibits apoptosis. For example, do LAT RNA coding sequences directly interfere with apoptosis or is a LAT protein involved? Second, which steps of apoptosis are blocked? Third, does LAT have other functional properties, in addition to its antiapoptotic properties, that are required for the latency-reactivation cycle?
Working Model for the Antiapoptotic Properties of LAT in the Latency-Reactivation Cycle
A working model has been devised to explain the findings that LAT inhibits apoptosis (1, 107, 198) (Fig. 4). This model takes into account previous findings that are consistent with those of other investigators studying LAT and latency. It is not possible to include every finding or reference that has been published. Furthermore, the model does not take into account any findings that appear to be specific for one HSV-1 strain or a specific strain of mouse.
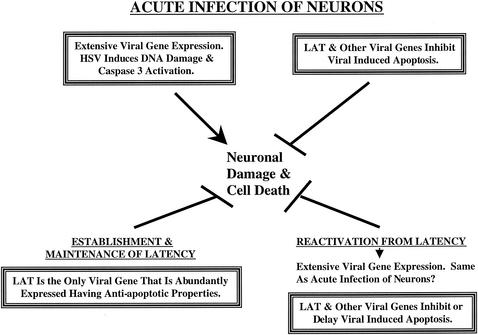
FIG. 4.
Model summarizing the role of LAT in enhancing neuronal survival in the context of the latency-reactivation cycle. See the text for details.
During acute infection of TG (1 to 4 days postinfection [p.i.]), extensive viral gene expression occurs (124, 125, 248). The toxic effects of HSV-1 infection, in particular ICP0 (220), US1.5 (80), and UL13 (80) make neurons vulnerable to damage and death. The ability of HSV to induce DNA damage (27, 93, 206, 229) would also stimulate the mitochondrial pathway of apoptosis (246). The antiapoptotic properties of US3, US5, gD, gJ, ICP27, and LAT would promote neuronal survival during acute infection (4, 5, 63, 64, 111). Deletion of LAT might not have a dramatic effect on apoptosis frequency during the early stages of acute infection because the other antiapoptotic viral genes are expressed. During transition from acute infection to latency (establishment of latency), viral gene expression is extinguished, including that of US3, US5, gD, gJ, and ICP27. At this time, LAT would be the only antiapoptotic viral gene that is abundantly expressed. Consequently, neurons in which extensive viral gene expression had occurred during acute infection (“permissive neurons”) would be vulnerable to apoptosis in the absence of LAT expression. Nonpermissive neurons that harbor viral genomes would have suffered low levels of viral induced damage and thus would have a higher probability of survival in the absence of LAT. In mice, subsets of neurons have been identified in TG and the ability of HSV-1 to infect these neurons is different (273), supporting the concept that permissive and nonpermissive neurons exist. This model is in agreement with the studies that have concluded that LAT facilitates establishment of latency (24, 44, 59, 65, 164, 166, 200, 223, 224, 257). The model also predicts that LAT protects neurons from apoptotic stimuli during the maintenance of latency because it is the only viral gene that is abundantly expressed.
Apoptosis occurs in neurons during neurodegenerative disorders, trauma, or imbalances of growth factors or cytokines (19, 68, 69, 104, 142, 195, 241, 242). Withdrawal of survival factors leads to activation of signal transduction pathways that initiate neuronal apoptosis (138). The response of the central or peripheral nervous system to trauma, stress, or immunosuppression plays an important role during reactivation from latency. Stress leads to elevated corticosteriod levels, which have rapid effects on neural activity (115, 170). Dexamethasone, a synthetic corticosteriod, induces viral gene expression (83), stimulates an HSV-1 origin of replication (Ori-L) in neuronal cells (87), and alters splicing patterns in the absence of protein synthesis (33). Repeated injections of dexamethasone also induce immunosuppression. Finally, corticosteriods induce apoptosis in lymphocytes (48) and neurons (177). Thus, LAT expression is likely to play an important role in prolonging or inhibiting neuronal death during reactivation. Since reactivation induces productive gene expression, the other HSV-1 antiapoptotic genes would also prolong neuronal survival, thus enhancing virus production.
COMPARISON OF THE BHV-1 LR GENE TO LAT
The LR Gene Restores Efficient Spontaneous Reactivation to a LAT− Mutant of HSV-1
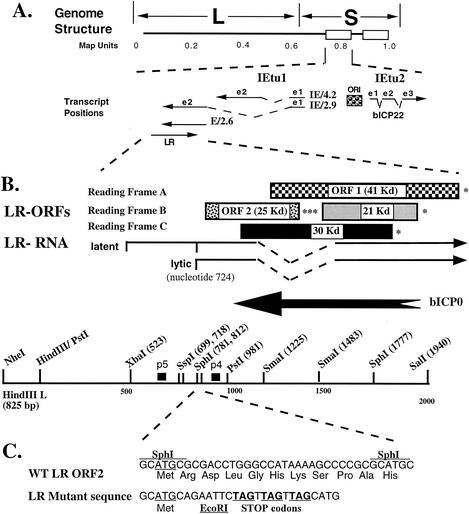
The BHV-1 LR gene, like HSV-1 LAT, is transcribed in an antisense direction of an ICP0 homologue (bICP0) (Fig. 5). The LR gene, like LAT, is abundantly expressed during latency of cattle or rabbits (45, 102, 134, 214, 226, 269). Interestingly, the BHV-1 LR gene also inhibits apoptosis in transiently transfected cells (31). Thus, the BHV-1 LR gene and HSV-1 LAT share certain structural and functional properties.
FIG. 5.
Schematic of the BHV-1 LR gene. (A) Positions of IE transcripts (62, 270-272) and the LR transcript (214, 217) are shown. IE/4.2 is the IE transcript that encodes bICP4. IE/2.9 is the IE transcript that encodes the bICP0 protein (95 kd). One IE promoter activates the expression of IE/4.2 and IE/2.9, and this IE transcription unit is designated IEtu1. E/2.6 is the early transcript that encodes bICP0. Exon 2 (e2) of bICP0 contains all of the protein-coding sequences of bICP0. The origin of replication (ORI) separates IEtu1 from IEtu2. IEtu2 encodes a protein named bICP22, which has a predicted molecular mass of 55 kd. Solid lines in the transcript position map represent exons (e1, e2, or e3). (B) Partial restriction map, location of LR-RNA, organization of the LR ORF, and the 3′ terminus of bICP0. The start sites for LR transcription during latency and productive infection were described previously (45, 102). Reading frame C contains an ORF but lacks an initiating Met. The asterisks denote the positions of stop codons that are in frame with the respective ORF. The approximate sizes of the ORFs and reading frames without an ATG are given. (C) DNA sequence of the SphI fragment and the mutant oligonucleotide. The first ATG in the wild-type (WT) sequence is the first in-frame ATG for ORF2 and is underlined. Stop codons in the mutant oligonucleotide are in all three reading frames (bold and underlined). The EcoRI restriction enzyme site (GAATTC) was incorporated into the mutant oligonucleotide to facilitate screening.
To test whether the LR gene can restore spontaneous reactivation to HSV-1, a 2-kb fragment containing the LR promoter and LR coding sequences (HindIII-SalI [Fig. 5B]) was inserted into a LAT− mutant and the recombinant virus was designated CJLAT (197). Insertion of the LR gene into the HSV-1 LAT locus restores high levels of spontaneous reactivation in the rabbit eye model and in explant-induced reactivation. In fact, the data strongly suggested that CJLAT induced higher levels of reactivation than did the wild- type HSV-1 or the LAT− mutant. CJLAT also induced higher levels of recurrent eye disease (stromal scarring and detached retinas) in rabbit eyes and was more lethal in mice than were the LAT+ and LAT− strains of HSV-1. This result indicated that LAT functions, at least with respect to enhancing reactivation from latency, can be replaced by the BHV- 1 LAT homologue.
Summary of LR Gene Functions
The LR RNA is the only abundant viral transcript detected in latently infected neurons (134, 213, 214). A fraction of LR-RNA is polyadenylated and alternatively spliced in TG, suggesting that this RNA is translated into more than one LR protein (45, 102). LR gene products inhibit S-phase entry, and an LR protein is associated with cyclin- dependent kinase 2 (cdk2)-cyclin complexes (102, 114). As discussed above, LR gene products promote cell survival following the induction of apoptosis in transiently transfected cells (31).
An LR mutant BHV-1 strain that contains three stop codons near the beginning of the LR RNA (Fig. 5C) was recently constructed to test whether LR gene products, in particular LR proteins, play a role in the ability of BHV-1 to replicate in cattle (106). Calves infected with the LR mutant consistently exhibited diminished clinical symptoms and ocular shedding of the virus compared to calves infected with wild-type virus or the LR- rescued virus. Conversely, the LR mutant had similar growth properties in productively infected bovine kidney cells and the nasal cavity of calves during acute infection. Diminished levels of virus were also detected in TG of calves acutely infected with the LR mutant compared to those infected with wild-type virus or the LR-rescued virus (105). Since cells in TG and the eye are highly differentiated whereas certain cells that comprise the mucosal epithelium are actively growing cells, we hypothesize that LR gene products promote viral growth in specific cell types or in cell types that are highly differentiated. The ability of the LR gene to inhibit cell growth (225) and the of LR protein to interact with cdk2-cyclin complexes (114) supports the concept that LR gene products regulate productive infection by altering cell cycle-regulatory components.
Although LR-RNA was detected by PCR in TG of calves infected with the LR mutant or with wild-type BHV-1, reduced levels of viral DNA were present in TG of calves latently infected with the LR mutant compared with those latently infected with wild-type BHV-1 (105). The LR mutant virus was not reactivated from latently infected calves following treatment with dexamethasone. In contrast, calves infected with wild-type virus or the LR-rescued virus reactivated efficiently following the same dexamethasone treatment. In summary, wild-type expression of LR gene products is crucial for efficient viral growth in ocular tissue and for the latency-reactivation cycle in cattle.
Comparison of the HSV-1 and BHV-1 LAT Regions
In general, HSV-1 LAT is important, but not required, for the latency-reactivation cycle in rabbits and mouse models (116, 263). LR gene products are required for the latency- reactivation cycle in calves when reactivation is initiated by dexamethasone (105). In addition, the LR gene is important for virus shedding from the eyes during acute infection (106), whereas no published study has demonstrated that LAT plays a role in virus growth in mice or rabbits. The finding that the BHV-1 LR gene enhances pathogenesis in the context of the HSV-1 genome implies that the LR gene has additional functions compared to HSV-1 LAT. The most obvious difference between LAT and the LR gene is the genetic evidence that a protein encoded by the LR gene plays a role in pathogenesis and the latency-reactivation cycle (105, 106). BHV-1 also lacks several genes contained in the HSV-1 repeats that mediate pathogenesis and/or latency, 34.5, ORFO, and ORFP, for example (Fig. 2 and 5). The HSV-1 34.5 gene plays a crucial role in neurovirulence by inhibiting antiviral functions of the interferon (IFN)-inducible double-stranded RNA- dependent protein kinase R (PKR) (28, 140). HSV-1 34.5 null mutants have reduced pathogenesis in rabbits and mice, in large part because of poor growth properties in the eyes and TG (205). Although the present studies point to added functions of the LR gene in the life cycle of BHV-1, the importance of HSV-1 LAT in the latency-reactivation cycle may be underestimated because its role in the natural host cannot be analyzed.
REGULATION OF LATENCY BY THE IMMUNE SYSTEM
Infiltration of Lymphocytes in TG during Acute Infection
Several independent studies have demonstrated that T cells, CD8+ T lymphocytes in particular, are crucial for controlling HSV infection in sensory ganglia (188, 243, 244). Infiltration of lymphoid cells in TG was examined following ocular infection of A/J mice with the RE strain of HSV-1 (154, 237). After corneal infection, transient epithelial lesions are present between 2 and 4 days p.i. (95, 96, 191). Most infected mice also develop corneal inflammation and periocular disease 1 to 2 weeks p.i. During acute infection, HSV antigen expression increases until 3 days p.i. in TG but is undetectable at 7 days p.i. (154). Coincident with a decline in the level of HSV antigen in TG, there is an increase in the levels of Mac-1+ cells, macrophages, NK cells, and certain CD8+ cells. No cells with characteristic lymphoid cell morphology can be detected in uninfected TG. After 5 days p.i., the number of CD8+ T cells, F4/80+ cells (macrophages), and γδ T cells increase dramatically. At 3 days p.i., TG neurons that are viral antigen positive can be detected that are surrounded by nonneural cells expressing TNF-α, interleukin-6 (IL-6), or IFN-γ (239). Cells that express IL-2 or IL-4 are detected later after infection, when viral antigens are difficult to detect. The number of cells producing IFN-γ and IL-4 increases between 3 and 7 days p.i., but the same cells do not appear to produce both factors (154). At 7 days p.i., transcripts encoding IL-2, IL-10, IFN-γ, TNF-α, or RANTES (regulated upon activation, normal T cell expressed and secreted [mRNA]) are detected by RT-PCR (84). By enzyme-linked immunosorbent assay IL-2, IL-6, IL-10, and IFN-γ are detected at the same time, confirming the RT-PCR results. The same cellular antigens are not detected in TG from uninfected mice, indicating that these changes are induced by infection. In summary, several studies have indicated that immunological factors play a role in repressing infection. Furthermore, these immunological factors may prevent neuronal death or promote repair of damaged neurons after infection.
Persistence of Lymphocytes in the Peripheral Nervous System
If true latency of HSV is established, it is reasonable to predict that cytokine expression in TG would not be detected. However, several recent studies have concluded that a persistent cell-mediated immune response occurs in TG of latently infected mice (21, 84, 154, 238-240). Immunohistochemical studies have detected IFN-γ-positive cells 6 months p.i. (21). CD4+, CD8+, γδ T cells, and macrophages are present in latently infected TG from 5 to 92 days p.i. By RT-PCR, IL-10, IFN-γ, RANTES, and TNF-α mRNA expression correlate very well with the expression of LAT in TG of latently infected mice (24 to 60 days p.i.). In contrast, the expression of IL-2 is variable. Although ICP27 is readily detected at 5 days p.i. in TG, it is not detected at later time points, confirming that latency is established. Another obvious change in TG after infection is long-term expression of TNF-α by satellite cells, Schwann cells, and infiltrating cells (239). Finally, levels of serum antibodies directed against HSV-1 remained elevated 125 days p.i. Collectively, these studies demonstrate that immune effector cells persist at the site of a latent infection.
The obvious explanation for the persistence of immune effector cells in TG is that low levels of viral proteins are expressed and the immune system is responding. This point is supported by two findings: (i) an ICP4-specific antibody reacts with latently infected rabbit TG (73), and (ii) low levels of TK or ICP4 mRNA are detected in latently infected mouse TG (126). Acute infection with HSV-1 induces H2-encoded heavy chains (alphaCs) and their associated light chain, β2-microglobulin, in sensory neurons (196), suggesting that reactivating neurons are recognized by CD8+ T cells via the class I major histocompatibility complex.
A careful examination of TG neurons for viral gene expression in latently infected mice (37 to 47 days p.i.) demonstrated that abundant viral transcripts, viral protein, and viral DNA replication occur in approximately 1 neuron per each 10 TG (61). The viral transcripts examined were ICP4, TK, glycoprotein C, and LAT. The same neurons that were expressing high levels of transcripts were invariably surrounded by foci of infiltrating white blood cells. Productive viral gene expression during latency is probably due to incomplete reactivation events or is the result of virus-producing neurons that are quickly recognized by the immune systems. In the absence of detectable infectious virus in TG, the term “spontaneous molecular reactivation” has been coined to describe this process (61). Considering that distinct subsets of TG neurons exhibit differences with respect to HSV-1 infection patterns (273), it would be interesting to know whether specific populations of neurons are prone to spontaneous molecular reactivation.
Calves latently infected with BHV-1 also have rare cells that express abundant viral proteins or transcripts in addition to the LR gene (268). This implies that BHV-1 and HSV-1 latency does not necessarily mean true quiescence. It seems obvious that certain latently infected neurons receive stimuli or express cellular factors that lead to spontaneous molecular reactivation and that this maintains the persistent cell-mediated immune response in TG. It is also tempting to speculate that neurons undergoing spontaneous molecular reactivation do not receive all of the signals that are necessary for virus production and spread. It is also not clear whether neurons undergoing reactivation (spontaneous molecular reactivation or reactivation that leads to the production of infectious virus) are always killed by infiltrating cells or if some of these neurons can survive and then resume latency.
Interferon Can Inhibit Reactivation from Latency
The presence of the immune system in TG after the establishment of latency may play an important role in maintaining latency. Recent studies have demonstrated that the ability of CD8+ T cells to produce IFN-γ plays an important role in preventing reactivation from latency in sensory neurons (152, 153). An independent study has also concluded that IFN-α and IFN-γ contribute to the control of recurrent herpetic lesions (36, 176).
HSV-1 encodes three proteins, ICP0 (55, 182-184), ICP34.5 (90-92, 139, 140), and US11, that counteract distinct IFN-induced restrictions to virus replication. ICP34.5 binds to cellular protein phosphatase 1 and acts to reverse the activity of PKR. US11 is an RNA-binding protein that prevents PKR activation. PKR is an IFN-inducible kinase that inhibits protein synthesis, primarily by phosporylating eukaryotic initiation factor 2 (eIF-2α). ICP0 has the ability to inhibit the induction of IFN-induced genes by an unknown mechanism. A delicate balance must exist between IFN and other unknown immunomodulatory cytokines with respect to maintaining latency, preventing reactivation from latency, and being neurotoxic. In summary, reactivation from latency that leads to detectable levels of infectious virus requires stimuli that induce viral gene expression and viral DNA replication and suppress immune functions.
CONCLUSIONS
Latency of HSV-1 is a complicated virus-host interaction that plays a crucial role in the pathogenic potential of this virus. Numerous studies have indicated that sensory neurons are the primary sites of HSV-1 and BHV-1 latency. The ability of these viruses to reactivate from latency is responsible for recurrent disease and virus transmission. Since LAT and the LR gene are the only known viral transcripts that are abundantly transcribed in latently infected neurons, it is reasonable to hypothesize that they regulate latency. Genetic analysis has demonstrated that LAT and the LR gene are important. Although the expression of a protein encoded by the BHV-1 LR gene appears to be required for the latency-reactivation cycle in cattle, it is currently not clear whether expression of a LAT protein is important. If a LAT protein is expressed, its expression is tightly regulated and may occur only at specific times during latency to prevent immune recognition. Recent studies demonstrating that the genes encoding LAT and LR-RNA have antiapoptotic properties strongly suggest that this function plays a crucial role in promoting neuronal survival and thus latency. It will be of interest to determine the mechanism by which these genes inhibit apoptosis and to find whether the ability to inhibit apoptosis is required for regulation of the latency-reactivation cycle.
Acknowledgments
My laboratory is supported by grants from the USDA (2000-02060) and NIH (P20RR15635).
I thank Melissa Inman for reviewing the manuscript.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosisin vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., and N. W. Fraser. 2001. Herpes simplex virus type 1 2-kilobase latency-associated transcript intron associates with ribosomal proteins and splicing factors. J. Virol. 75:12070-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, J. L., R. Everett, I. Brierley, and S. Efstathiou. 1998. Disruption of the 5′ and 3′ splice sites flanking the major latency-associated transcripts of herpes simplex virus type 1: evidence for alternate splicing in lytic and latent infections. J. Gen. Virol. 79:107-116. [DOI] [PubMed] [Google Scholar]
- 4.Asano, S., T. Honda, F. Goshima, D. Watanabe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51-56. [DOI] [PubMed] [Google Scholar]
- 5.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baringer, J. R., and P. Pisani. 1994. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann. Neurol. 36:823-829. [DOI] [PubMed] [Google Scholar]
- 7.Baringer, J. R., and P. Swoveland. 1973. Recovery of herpes-simplex virus from human trigeminal ganglions. N. Engl. J. Med. 288:648-650. [DOI] [PubMed] [Google Scholar]
- 8.Bastian, F. O., A. S. Rabson, C. L. Yee, and T. S. Tralka. 1972. Herpesvirus hominis: isolation from human trigeminal ganglion. Science 178:306-307. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor, A. H., and P. O'Hare. 1992. Localization of cis-acting sequence requirements in the promoter of the latency-associated transcript of herpes simplex virus type 1 required for cell-type-specific activity. J. Virol. 66:3573-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batchelor, A. H., and P. O'Hare. 1990. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J. Virol. 64:3269-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelor, A. H., K. W. Wilcox, and P. O'Hare. 1994. Binding and repression of the latency-associated promoter of herpes simplex virus by the immediate early 175K protein. J. Gen. Virol. 75:753-767. [DOI] [PubMed] [Google Scholar]
- 12.Berthomme, H., J. Lokensgard, L. Yang, T. Margolis, and L. T. Feldman. 2000. Evidence for a bidirectional element located downstream from the herpes simplex virus type 1 latency-associated promoter that increases its activity during latency. J. Virol. 74:3613-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaho, M. A., and M. Aubert. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Block, T. M., J. G. Spivack, I. Steiner, S. Deshmane, M. T. McIntosh, R. P. Lirette, and N. W. Fraser. 1990. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J. Virol. 64:3417-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom, D. C., J. G. Stevens, J. M. Hill, and R. K. Tran. 1997. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology 236:202-207. [DOI] [PubMed] [Google Scholar]
- 16.Bohenzky, R. A., M. Lagunoff, B. Roizman, E. K. Wagner, and S. Silverstein. 1995. Two overlapping transcription units which extend across the L-S junction of herpes simplex virus type 1. J. Virol. 69:2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohenzky, R. A., A. G. Papavassiliou, I. H. Gelman, and S. Silverstein. 1993. Identification of a promoter mapping within the reiterated sequences that flank the herpes simplex virus type 1 UL region. J. Virol. 67:632-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowland, S. L., and P. E. Shewen. 2000. Bovine respiratory disease: commercial vaccines currently available in Canada. Can. Vet. J. 41:33-48. [PMC free article] [PubMed] [Google Scholar]
- 19.Busser, J., D. S. Geldmacher, and K. Herrup. 1998. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J. Neurosci. 18:2801-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICPO, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrozza, M. J., and N. A. DeLuca. 1996. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol. Cell. Biol. 16:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter, J. J., A. D. Weinberg, A. Pollard, R. Reeves, J. A. Magnuson, and N. S. Magnuson. 1989. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J. Virol. 63:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, S. H., M. F. Kramer, P. A. Schaffer, and D. M. Coen. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, X., J. Li, M. Mata, J. Goss, D. Wolfe, J. C. Glorioso, and D. J. Fink. 2000. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J. Virol. 74:10132-10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, X., M. C. Schmidt, W. F. Goins, and J. C. Glorioso. 1995. Two herpes simplex virus type 1 latency-active promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections. J. Virol. 69:7899-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chenet-Monte, C., F. Mohammad, C. M. Celluzzi, P. A. Schaffer, and F. E. Farber. 1986. Herpes simplex virus gene products involved in the induction ofchromosomal aberrations. Virus Res. 6:245-260. [DOI] [PubMed] [Google Scholar]
- 28.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r)90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhancedphosphorylation of translation initiation factor eIF-2 alpha and premature shutoff ofprotein synthesis after infection with gamma 134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpessimplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth inculture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 30.Chou, J., and B. Roizman. 1990. The herpes simplex virus 1 gene for ICP34.5,which maps in inverted repeats, is conserved in several limited-passage isolates but not instrain 17syn+. J. Virol. 64:1014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciacci-Zanella, J., M. Stone, G. Henderson, and C. Jones. 1999. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J. Virol. 73:9734-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P.A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpessimplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collett, J. W., and R. E. Steele. 1993. Alternative splicing of a neural-specific Src mRNA (Src+) is a rapid and protein synthesis-independent response to neural induction in Xenopus laevis. Dev. Biol. 158:487-495. [DOI] [PubMed] [Google Scholar]
- 34.Crnic, L. S., and L. I. Pizer. 1988. Behavioral effects of neonatal herpes simplex type 1 infection of mice. Neurotoxicol. Teratol. 10:381-386. [DOI] [PubMed] [Google Scholar]
- 35.Croen, K. D., J. M. Ostrove, L. J. Dragovic, J. E. Smialek, and S. E. Straus.1987. Latent herpes simplex virus in human trigeminal ganglia. Detection of animmediate early gene “anti-sense” transcript by in situ hybridization. N. Engl. J. Med. 317:1427-1432. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham, A. L., and Z. Mikloska. 2001. The Holy Grail: immune control of human herpes simplex virus infection and disease. Herpes 8(Suppl. 1):6A-10A. [PubMed] [Google Scholar]
- 37.Deatly, A. M., J. G. Spivack, E. Lavi, and N. W. Fraser. 1987. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc. Natl. Acad. Sci. USA 84:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deatly, A. M., J. G. Spivack, E. Lavi, D. R. O'Boyle, Jr., and N. W. Fraser. 1988. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J. Virol. 62:749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLuca, N. A., and P. A. Schaffer. 1985. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 5:1997-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dent, C. L., K. A. Lillycrop, J. K. Estridge, N. S. Thomas, and D. S. Latchman. 1991. The B-cell and neuronal forms of the octamer-binding protein Oct-2 differ in DNA-binding specificity and functional activity. Mol. Cell. Biol. 11:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deshmane, S. L., and N. W. Fraser. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 63:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshmane, S. L., M. Nicosia, T. Valyi-Nagy, L. T. Feldman, A. Dillner, and N. W. Fraser. 1993. An HSV-1 mutant lacking the LAT TATA element reactivates normally in explant cocultivation. Virology 196:868-872. [DOI] [PubMed] [Google Scholar]
- 44.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 68:1271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devireddy, L. R., and C. Jones. 1998. Alternative splicing of the latency-related transcript of bovine herpesvirus 1 yields RNAs containing unique open reading frames. J. Virol. 72:7294-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devireddy, L. R., and C. J. Jones. 1999. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J. Virol. 73:3778-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devireddy, L. R., and C. J. Jones. 2000. Olf-1, a neuron-specific transcription factor, can activate the herpes simplex virus type 1-infected cell protein 0 promoter. J. Biol. Chem. 275:77-81. [DOI] [PubMed] [Google Scholar]
- 48.Dieken, E. S., and R. L. Miesfeld. 1992. Transcriptional transactivation functions localized to the glucocorticoid receptor N terminus are necessary for steroid induction of lymphocyte apoptosis. Mol. Cell. Biol. 12:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon, R. A., and P. A. Schaffer. 1980. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J. Virol. 36:189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobson, A. T., F. Sederati, G. Devi-Rao, W. M. Flanagan, M. J. Farrell, J. G. Stevens, E. K. Wagner, and L. T. Feldman. 1989. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J. Virol. 63:3844-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobson, A. T., F. Sederati, G. Devi-Rao, W. M. Flanagan, M. J. Farrell, J. G. Stevens, E. K. Wagner, and L. T. Feldman. 1989. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J. Virol. 63:3844-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doerig, C., L. I. Pizer, and C. L. Wilcox. 1991. An antigen encoded by the latency-associated transcript in neuronal cell cultures latently infected with herpes simplex virus type 1. J. Virol. 65:2724-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drolet, B. S., G. C. Perng, J. Cohen, S. M. Slanina, A. Yukht, A. B. Nesburn, and S. L. Wechsler. 1998. The region of the herpes simplex virus type 1 LAT gene involved in spontaneous reactivation does not encode a functional protein. Virology 242:221-232. [DOI] [PubMed] [Google Scholar]
- 54.Efstathiou, S., S. Kemp, G. Darby, and A. C. Minson. 1989. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J. Gen. Virol. 70:869-879. [DOI] [PubMed] [Google Scholar]
- 55.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellison, S. A., and B. Hampar. 1961. Chromosomal aberrations induced by an animal virus. Nature (London) 192:145-147. [DOI] [PubMed] [Google Scholar]
- 57.Erkman, L., R. J. McEvilly, L. Luo, A. K. Ryan, F. Hooshmand, S. M. O'Connell, E. M. Keithley, D. H. Rapaport, A. F. Ryan, and M. G. Rosenfeld. 1996. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381:603-606. [DOI] [PubMed] [Google Scholar]
- 58.Estridge, J. K., L. M. Kemp, and D. S. Latchman. 1990. The herpes simplex virus protein Vmw65 can trans-activate both viral and cellular promoters in neuronal cells. Biochem. J. 271:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrell, M. J., A. T. Dobson, and L. T. Feldman. 1991. Herpes simplex virus latency-associated transcript is a stable intron. Proc. Natl. Acad. Sci. USA 88:790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farrell, M. J., T. P. Margolis, W. A. Gomes, and L. T. Feldman. 1994. Effect of the transcription start region of the herpes simplex virus type 1 latency-associated transcript promoter on expression of productively infected neurons in vivo. J. Virol. 68:5337-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraefel, C., J. Zeng, Y. Choffat, M. Engels, M. Schwyzer, and M. Ackermann. 1994. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICPO. J. Virol. 68:3154-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garber, D. A., P. A. Schaffer, and D. M. Knipe. 1997. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J. Virol. 71:5885-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 67.Gesser, R. M., and S. C. Koo. 1997. Latent herpes simplex virus type 1 gene expression in ganglia innervating the human gastrointestinal tract. J. Virol. 71:4103-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gill, J. S., and A. J. Windebank. 1998. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J. Clin. Investing. 101:2842-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gobbel, G. T., M. Bellinzona, A. R. Vogt, N. Gupta, J. R. Fike, and P. H. Chan. 1998. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J. Neurosci. 18:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goins, W. F., L. R. Sternberg, K. D. Croen, P. R. Krause, R. L. Hendricks, D. J. Fink, S. E. Straus, M. Levine, and J. C. Glorioso. 1994. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J. Virol. 68:2239-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldenberg, D., N. Mador, M. J. Ball, A. Panet, and I. Steiner. 1997. The abundant latency-associated transcripts of herpes simplex virus type 1 are bound to polyribosomes in cultured neuronal cells and during latent infection in mouse trigeminal ganglia. J. Virol. 71:2897-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldsmith, K., W. Chen, D. C. Johnson, and R. L. Hendricks. 1998. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 187:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Green, M. T., R. J. Courtney, and E. C. Dunkel. 1981. Detection of an immediate early herpes simplex virus type 1 polypeptide in trigeminal ganglia from latently infected animals. Infect. Immun. 34:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griebel, P. J., H. B. Ohmann, M. J. Lawman, and L. A. Babiuk. 1990. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J. Gen. Virol. 71:369-377. [DOI] [PubMed] [Google Scholar]
- 75.Griebel, P. J., L. Qualtiere, W. C. Davis, A. Gee, H. Bielefeldt Ohmann, M. J. Lawman, and L. A. Babiuk. 1987. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1:287-304. [DOI] [PubMed] [Google Scholar]
- 76.Griebel, P. J., L. Qualtiere, W. C. Davis, M. J. Lawman, and L. A. Babiuk. 1987. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1:267-286. [DOI] [PubMed] [Google Scholar]
- 77.Gruber, C. A., J. M. Rhee, A. Gleiberman, and E. E. Turner. 1997. POU domain factors of the Brn-3 class recognize functional DNA elements which are distinctive, symmetrical, and highly conserved in evolution. Mol. Cell. Biol. 17:2391-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu, B., and N. DeLuca. 1994. Requirements for activation of the herpes simplex virus glycoprotein C promoter in vitro by the viral regulatory protein ICP4. J. Virol. 68:7953-7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu, B., R. Rivera-Gonzalez, C. A. Smith, and N. A. DeLuca. 1993. Herpes simplex virus infected cell polypeptide 4 preferentially represses Sp1-activated over basal transcription from its own promoter. Proc. Natl. Acad. Sci. USA 90:9528-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagglund, R., J. Munger, A. P. Poon, and B. Roizman. 2002. U(S)3 protein kinase of herpes simplex virus 1 blocks caspase 3 activation induced by the products of U(S)1.5 and U(L)13 genes and modulates expression of transduced U(S)1.5 open reading frame in a cell type-specific manner. J. Virol. 76:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagman, J., C. Belanger, A. Travis, C. W. Turck, and R. Grosschedl. 1993. Cloning and functional characterization of early B cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 11:760-773. [DOI] [PubMed] [Google Scholar]
- 82.Hagmann, M., O. Georgiev, W. Schaffner, and P. Douville. 1995. Transcription factors interacting with herpes simplex virus alpha gene promoters in sensory neurons. Nucleic Acids Res. 23:4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Mechanisms of herpes simplex virus type 1 reactivation. J. Virol. 70:5051-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542-3549. [PubMed] [Google Scholar]
- 85.Hao, Y., T. Crenshaw, T. Moulton, E. Newcomb, and B. Tycko. 1993. Tumour-suppressor activity of H19 RNA. Nature 365:764-767. [DOI] [PubMed] [Google Scholar]
- 86.Hardwick, J. M. 1998. Viral interference with apoptosis. Semin. Cell. Dev. Biol. 9:339-349. [DOI] [PubMed] [Google Scholar]
- 87.Hardwicke, M. A., and P. A. Schaffer. 1997. Differential effects of nerve growth factor and dexamethasone on herpes simplex virus type 1 oriL- and oriS-dependent DNA replication in PC12 cells. J. Virol. 71:3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hariharan, M. J., C. Nataraj, and S. Srikumaran. 1993. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 6:273-284. [DOI] [PubMed] [Google Scholar]
- 90.He, B., J. Chou, R. Brandimarti, I. Mohr, Y. Gluzman, and B. Roizman. 1997. Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J. Virol. 71:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He, B., M. Gross, and B. Roizman. 1998. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 92.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heilbronn, R., and H. zur Hausen. 1989. A subset of herpes simplex virus replication genes induces DNA amplification within the host cell genome. J. Virol. 63:3683-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henderson, G. W., L. Peng, L. Jin, G.-C. Perng, A. B. Nesburn, S. L. Wechsler, and C. Jones. Regulation of caspase 8 and caspase 9 induced apoptosis by the herpes simplex virus type 1 (HSV-1) latency associated transcript (LAT). J. Neurovirol., in press. [DOI] [PubMed]
- 95.Hendricks, R. L., and T. M. Tumpey. 1991. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr. Eye Res. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 96.Hendricks, R. L., T. M. Tumpey, and A. Finnegan. 1992. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 149:3023-3028. [PubMed] [Google Scholar]
- 97.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 98.Hill, J. M., F. Sedarati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117-125. [DOI] [PubMed] [Google Scholar]
- 99.Hinkley, S., A. B. Hill, and S. Srikumaran. 1998. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 53:91-96. [DOI] [PubMed] [Google Scholar]
- 100.Ho, D. Y., and E. S. Mocarski. 1988. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology 167:279-283. [DOI] [PubMed] [Google Scholar]
- 101.Honess, R. W., and B. Roizman. 1974. Regulation of herpes virus macromalecular synthesis: cascade regulation of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hossain, A., L. M. Schang, and C. Jones. 1995. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 69:5345-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu, W. L., and R. D. Everett. 2001. Human neuron-committed teratocarcinoma NT2 cell line has abnormal ND10 structures and is poorly infected by herpes simplex virus type 1. J. Virol. 75:3819-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu, S., P. K. Peterson, and C. C. Chao. 1997. Cytokine-mediated neuronal apoptosis. Neurochem. Int. 30:427-431. [DOI] [PubMed] [Google Scholar]
- 105.Inman, M., L. Lovato, A. Doster, and C. Jones. 2002. A mutation in the latency-related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J. Virol. 76:6771-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inman, M., L. Lovato, A. Doster, and C. Jones. 2001. A mutation in the latency-related gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves. J. Virol. 75:8507-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inman, M., G. C. Perng, G. Henderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Itzhaki, R. F., W. R. Lin, D. Shang, G. K. Wilcock, B. Faragher, and G. A. Jamieson. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241-244. [DOI] [PubMed] [Google Scholar]
- 109.Reference deleted.
- 110.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 111.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reference deleted.
- 113.Jerome, K. R., J. F. Tait, D. M. Koelle, and L. Corey. 1998. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J. Virol. 72:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang, Y., A. Hossain, M. T. Winkler, T. Holt, A. Doster, and C. Jones. 1998. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 72:8133-8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joels, M., and E. R. de Kloet. 1992. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 15:25-30. [DOI] [PubMed] [Google Scholar]
- 116.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 117.Jordan, R., and P. A. Schaffer. 1997. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J. Virol. 71:6850-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katz, J. P., E. T. Bodin, and D. M. Coen. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 alpha regulatory protein ICPO with elongation factor 1 delta: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICPO interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kemp, L. M., J. K. Estridge, A. Brennan, D. R. Katz, and D. S. Latchman. 1990. Mononuclear phagocytes and HSV-1 infection: increased permissivity in differentiated U937 cells is mediated by post-transcriptional regulation of viral immediate-early gene expression. J. Leukoc. Biol. 47:483-489. [DOI] [PubMed] [Google Scholar]
- 122.Kenny, J. J., F. C. Krebs, H. T. Hartle, A. E. Gartner, B. Chatton, J. M. Leiden, J. P. Hoeffler, P. C. Weber, and B. Wigdahl. 1994. Identification of a second ATF/CREB-like element in the herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) promoter. Virology 200:220-235. [DOI] [PubMed] [Google Scholar]
- 123.Kitagawa, N., M. Goto, K. Kurozumi, S. Maruo, M. Fukayama, T. Naoe, M. Yasukawa, K. Hino, T. Suzuki, S. Todo, and K. Takada. 2000. Epstein-Barr virus-encoded poly(A)(−) RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J. 19:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knotts, F. B., M. L. Cook, and J. G. Stevens. 1974. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J. Infect. Dis. 130:16-27. [DOI] [PubMed] [Google Scholar]
- 125.Kramer, M. F., S. H. Chen, D. M. Knipe, and D. M. Coen. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J. Virol. 72:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krause, P. R., K. D. Croen, S. E. Straus, and J. M. Ostrove. 1988. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J. Virol. 62:4819-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krause, P. R., J. M. Ostrove, and S. E. Straus. 1991. The nucleotide sequence, 5′ end, promoter domain, and kinetics of expression of the gene encoding the herpes simplex virus type 2 latency-associated transcript. J. Virol. 65:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kristie, T. M., J. L. Pomerantz, T. C. Twomey, S. A. Parent, and P. A. Sharp. 1995. The cellular C1 factor of the herpes simplex virus enhancer complex is a family of polypeptides. J. Biol. Chem. 270:4387-4394. [DOI] [PubMed] [Google Scholar]
- 130.Kristie, T. M., and B. Roizman. 1988. Differentiation and DNA contact points of host proteins binding at the cis site for virion-mediated induction of alpha genes of herpes simplex virus 1. J. Virol. 62:1145-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kristie, T. M., J. L. Vogel, and A. E. Sears. 1999. Nuclear localization of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc. Natl. Acad. Sci. USA 96:1229-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kruegger, A., S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krummenacher, C., J. M. Zabolotny, and N. W. Fraser. 1997. Selection of a nonconsensus branch point is influenced by an RNA stem-loop structure and is important to confer stability to the herpes simplex virus 2-kilobase latency-associated transcript. J. Virol. 71:5849-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kutish, G., T. Mainprize, and D. Rock. 1990. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 64:5730-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuwana, T., J. J. Smith, M. Muzio, V. Dixit, D. D. Newmeyer, and S. Kornbluth. 1998. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J. Biol. Chem. 273:16589-16594. [DOI] [PubMed] [Google Scholar]
- 136.Lagunoff, M., and B. Roizman. 1994. Expression of a herpes simplex virus 1 open reading frame antisense to the gamma(1)34.5 gene and transcribed by an RNA 3′ coterminal with the unspliced latency-associated transcript. J. Virol. 68:6021-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Latchman, D. S., J. F. Partidge, J. K. Estridge, and L. M. Kemp. 1989. The different competitive abilities of viral TAATGARAT elements and cellular octamer motifs, mediate the induction of viral immediate-early genes and the repression of the histone H2B gene in herpes simplex virus infected cells. Nucleic Acids Res. 17:8533-8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leib, D. A., K. C. Nadeau, S. A. Rundle, and P. A. Schaffer. 1991. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc. Natl. Acad. Sci. USA 88:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Le-Niculescu, H., E. Bonfoco, Y. Kasuya, F. X. Claret, D. R. Green, and M. Karin. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell. Biol. 19:751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 145.Liedtke, W., B. Opalka, C. W. Zimmermann, and E. Lignitz. 1993. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J. Neurol. Sci. 116:6-11. [DOI] [PubMed] [Google Scholar]
- 146.Lillycrop, K. A., V. S. Budrahan, N. D. Lakin, G. Terrenghi, J. N. Wood, J. M. Polak, and D. S. Latchman. 1992. A novel POU family transcription factor is closely related to Brn-3 but has a distinct expression pattern in neuronal cells. Nucleic Acids Res. 20:5093-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lillycrop, K. A., S. J. Dawson, J. K. Estridge, T. Gerster, P. Matthias, and D. S. Latchman. 1994. Repression of a herpes simplex virus immediate-early promoter by the Oct-2 transcription factor is dependent on an inhibitory region at the N terminus of the protein. Mol. Cell. Biol. 14:7633-7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lillycrop, K. A., J. K. Estridge, and D. S. Latchman. 1994. Functional interaction between different isoforms of the Oct-2 transcription factor expressed in neuronal cells. Biochem. J. 298:245-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lillycrop, K. A., J. K. Estridge, and D. S. Latchman. 1993. The octamer binding protein Oct-2 inhibits transactivation of the herpes simplex virus immediate-early genes by the virion protein Vmw65. Virology 196:888-891. [DOI] [PubMed] [Google Scholar]
- 150.Lillycrop, K. A., M. K. Howard, J. K. Estridge, and D. S. Latchman. 1994. Inhibition of herpes simplex virus infection by ectopic expression of neuronal splice variants of the Oct-2 transcription factor. Nucleic Acids Res. 22:815-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin, W. R., J. Graham, S. M. MacGowan, G. K. Wilcock, and R. F. Itzhaki. 1998. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Alzheimer's Rep. 1:173-178. [Google Scholar]
- 152.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu, T., Q. Tang, and R. L. Hendricks. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lium, E. K., C. A. Panagiotidis, X. Wen, and S. J. Silverstein. 1998. The NH2 terminus of the herpes simplex virus type 1 regulatory protein ICP0 contains a promoter-specific transcription activation domain. J. Virol. 72:7785-7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lium, E. K., and S. Silverstein. 1997. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 71:8602-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lock, M., C. Miller, and N. W. Fraser. 2001. Analysis of protein expression from within the region encoding the 2.0-kilobase latency-associated transcript of herpes simplex virus type 1. J. Virol. 75:3413-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lohr, J. M., J. A. Nelson, and M. B. Oldstone. 1990. Is herpes simplex virus associated with peptic ulcer disease? J. Virol. 64:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loiacono, C. M., R. Myers, and W. J. Mitchell. 2002. Neurons differentially activate the herpes simplex virus type 1 immediate-early gene ICP0 and ICP27 promoters in transgenic mice. J. Virol. 76:2449-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lokensgard, J. R., H. Berthomme, and L. T. Feldman. 1997. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J. Virol. 71:6714-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lokensgard, J. R., D. C. Bloom, A. T. Dobson, and L. T. Feldman. 1994.Long-term promoter activity during herpes simplex virus latency. J. Virol. 68:7148-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu, R., and V. Misra. 2000. Potential role for luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J. Virol. 74:934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu, R., and V. Misra. 2000. Zhangfei: a second cellular protein interacts with herpes simplex virus accessory factor HCF in a manner similar to Luman and VP16. Nucleic Acids Res. 28:2446-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mador, N., D. Goldenberg, O. Cohen, A. Panet, and I. Steiner. 1998. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J. Virol. 72:5067-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mador, N., A. Panet, D. Latchman, and I. Steiner. 1995. Expression and splicing of the latency-associated transcripts of herpes simplex virus type 1 in neuronal and non-neuronal cell lines. J. Biochem. (Tokyo) 117:1288-1297. [DOI] [PubMed] [Google Scholar]
- 166.Maggioncalda, J., A. Mehta, Y. H. Su, N. W. Fraser, and T. M. Block. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72-81. [DOI] [PubMed] [Google Scholar]
- 167.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150-160. [DOI] [PubMed] [Google Scholar]
- 168.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 63:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McEvilly, R. J., L. Erkman, L. Luo, P. E. Sawchenko, A. F. Ryan, and M. G. Rosenfeld. 1996. Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature 384:574-577. [DOI] [PubMed] [Google Scholar]
- 170.McEwen, B. S. 1991. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol. Sci. 12:141-147. [DOI] [PubMed] [Google Scholar]
- 171.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 64:3471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mehta, A., J. Maggioncalda, O. Bagasra, S. Thikkavarapu, P. Saikumari, T. Valyi-Nagy, N. W. Fraser, and T. M. Block. 1995. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology 206:633-640. [DOI] [PubMed] [Google Scholar]
- 173.Meredith, M., A. Orr, M. Elliott, and R. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 174.Meredith, M., A. Orr, and R. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 175.Michael, N., and B. Roizman. 1993. Repression of the herpes simplex virus 1 alpha 4 gene by its gene product occurs within the context of the viral genome and is associated with all three identified cognate sites. Proc. Natl. Acad. Sci. USA 90:2286-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mikloska, Z., and A. L. Cunningham. 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 75:11821-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mitchell, I. J., A. J. Cooper, M. R. Griffiths, and D. J. Barber. 1998. Phencyclidine and corticosteroids induce apoptosis of a subpopulation of striatal neurons: a neural substrate for psychosis? Neuroscience 84:489-501. [DOI] [PubMed] [Google Scholar]
- 178.Mitchell, W. J., R. P. Lirette, and N. W. Fraser. 1990. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Gen. Virol. 71:125-132. [DOI] [PubMed] [Google Scholar]
- 179.Mitchell, W. J., I. Steiner, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1990. A herpes simplex virus type 1 variant, deleted in the promoter region of the latency-associated transcripts, does not produce any detectable minor RNA species during latency in the mouse trigeminal ganglion. J. Gen. Virol. 71:953-957. [DOI] [PubMed] [Google Scholar]
- 180.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 181.Morris, P. J., T. Theil, C. J. Ring, K. A. Lillycrop, T. Moroy, and D. S. Latchman. 1994. The opposite and antagonistic effects of the closely related POU family transcription factors Brn-3a and Brn-3b on the activity of a target promoter are dependent on differences in the POU domain. Mol. Cell. Biol. 14:6907-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon- induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Munger, J., A. V. Chee, and B. Roizman. 2001. The U(S)3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nahmias, A. J., and B. Roizman. 1973. Infection with herpes-simplex viruses 1 and 2. 3. N. Engl. J. Med. 289:781-789. [DOI] [PubMed] [Google Scholar]
- 188.Nash, A. A., A. Jayasuriya, J. Phelan, S. P. Cobbold, H. Waldmann, and T. Prospero. 1987. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen. Virol. 68:825-833. [DOI] [PubMed] [Google Scholar]
- 189.Nataraj, C., S. Eidmann, M. J. Hariharan, J. H. Sur, G. A. Perry, and S. Srikumaran. 1997. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 10:21-34. [DOI] [PubMed] [Google Scholar]
- 190.Nesburn, A. B. 1983. Report of the corneal disease panel: vision research—a national plan 1983-1987. The C.V. Mosby Co., St. Louis, Mo.
- 191.Newell, C. K., D. Sendele, and B. T. Rouse. 1989. Effects of CD4+ and CD8+ T-lymphocyte depletion on the induction and expression of herpes simplex stromal keratitis. Reg. Immunol. 2:366-369. [PubMed] [Google Scholar]
- 192.Nicosia, M., J. M. Zabolotny, R. P. Lirette, and N. W. Fraser. 1994. The HSV- 1 2-kb latency-associated transcript is found in the cytoplasm comigrating with ribosomal subunits during productive infection. Virology 204:717-728. [DOI] [PubMed] [Google Scholar]
- 193.O'Hare, P. 1993. The virion transactivator of herpes simplex virus. Semin. Virol. 4:145-155. [Google Scholar]
- 194.O'Hare, P., and G. S. Hayward. 1985. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J. Virol. 56:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park, D. S., E. J. Morris, L. Stefanis, C. M. Troy, M. L. Shelanski, H. M. Geller, and L. A. Greene. 1998. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J. Neurosci. 18:830-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pereira, R. A., and A. Simmons. 1999. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J. Virol. 73:6484-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Perng, G.-C., B. Maguen, L. Jin, K. R. Mott, N. Osorio, S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, M. Inman, G. Henderson, C. Jones, and S. L. Wechsler. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hoffman, H. Ghiasi, A. B. Nesburn, and S. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript (LAT). Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 199.Perng, G. C., K. Chokephaibulkit, R. L. Thompson, N. M. Sawtell, S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J. Virol. 70:282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perng, G. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3- kilobase primary transcript. J. Virol. 70:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Perng, G. C., S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J. Virol. 70:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perng, G. C., S. M. Slanina, A. Yukht, B. S. Drolet, W. Keleher, Jr., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1999. A herpes simplex virus type 1 latency-associated transcript mutant with increased virulence and reduced spontaneous reactivation. J. Virol. 73:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Perng, G. C., S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 74:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perng, G. C., R. L. Thompson, N. M. Sawtell, W. E. Taylor, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1995. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J. Virol. 69:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pilon, L., Y. Langelier, and A. Royal. 1986. Herpes simplex virus type 2 mutagenesis: characterization of mutants induced at the hprt locus of nonpermissive XC cells. Mol. Cell. Biol. 6:2977-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rader, K. A., C. E. Ackland-Berglund, J. K. Miller, J. S. Pepose, and D. A. Leib. 1993. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74:1859-1869. [DOI] [PubMed] [Google Scholar]
- 208.Ramakrishnan, R., D. J. Fink, G. Jiang, P. Desai, J. C. Glorioso, and M. Levine. 1994. Competitive quantitative PCR analysis of herpes simplex virus type 1 DNA and latency-associated transcript RNA in latently infected cells of the rat brain. J. Virol. 68:1864-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ramakrishnan, R., M. Levine, and D. J. Fink. 1994. PCR-based analysis of herpes simplex virus type 1 latency in the rat trigeminal ganglion established with a ribonucleotide reductase-deficient mutant. J. Virol. 68:7083-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Razvi, E. S., and R. M. Welsh. 1995. Apoptosis in viral infections. Adv. Virus Res. 45:1-60. [DOI] [PubMed] [Google Scholar]
- 211.Rice, S. A., M. C. Long, V. Lam, P. A. Schaffer, and C. A. Spencer. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 69:5550-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roberts, M. S., A. Boundy, P. O'Hare, M. C. Pizzorno, D. M. Ciufo, and G. S. Hayward. 1988. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (alpha 4) promoter and a specific binding site for the IE175 (ICP4) protein. J. Virol. 62:4307-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rock, D., J. Lokensgard, T. Lewis, and G. Kutish. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 66:2484-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rock, D. L., S. L. Beam, and J. E. Mayfield. 1987. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 61:3827-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rock, D. L., and N. W. Fraser. 1983. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature 302:523-525. [DOI] [PubMed] [Google Scholar]
- 216.Rock, D. L., and N. W. Fraser. 1985. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J. Virol. 55:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sadzot-Delvaux, C., P. Thonard, S. Schoonbroodt, J. Piette, and B. Rentier. 1995. Varicella-zoster virus induces apoptosis in cell culture. J. Gen. Virol. 76:2875-2879. [DOI] [PubMed] [Google Scholar]
- 220.Samaniego, L. A., N. Wu, and N. A. DeLuca. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 71:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sandri-Goldin, R. M., M. K. Hibbard, and M. A. Hardwicke. 1995. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J. Virol. 69:6063-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 223.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishmentand reactivation from latency. J. Virol. 66:2157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schang, L. M., A. Hossain, and C. Jones. 1996. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J. Virol. 70:3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schang, L. M., and C. Jones. 1997. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J. Virol. 71:6786-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin- dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schlehofer, J. R., and J. Z. Hausen. 1982. Induction of mutations within the host cell genome by partially inactivated herpes simplex virus type 1. Virology 122:471-475. [DOI] [PubMed] [Google Scholar]
- 230.Schmitz, I., S. Kirchhoff, and P. H. Krammer. 2000. Regulation of death receptor-mediated apoptosis pathways. Int. J. Biochem. Cell. Biol. 32:1123-1136. [DOI] [PubMed] [Google Scholar]
- 231.Schonemann, M. D., A. K. Ryan, L. Erkman, R. J. McEvilly, J. Bermingham, and M. G. Rosenfeld. 1998. POU domain factors in neural development. Adv. Exp.Med. Biol. 449:39-53. [DOI] [PubMed] [Google Scholar]
- 232.Sears, A. E., I. W. Halliburton, B. Meignier, S. Silver, and B. Roizman. 1985.Herpes simplex virus 1 mutant deleted in the alpha 22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J. Virol. 55:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sears, A. E., V. Hukkanen, M. A. Labow, A. J. Levine, and B. Roizman. 1991. Expression of the herpes simplex virus 1 alpha transinducing factor (VP16) does not induce reactivation of latent virus or prevent the establishment of latency in mice. J. Virol. 65:2929-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sedarati, F., T. P. Margolis, and J. G. Stevens. 1993. Latent infection can be established with drastically restricted transcription and replication of the HSV-1 genome. Virology 192:687-691. [DOI] [PubMed] [Google Scholar]
- 235.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5:105-111. [DOI] [PubMed] [Google Scholar]
- 236.Shimeld, C., T. J. Hill, W. A. Blyth, and D. L. Easty. 1990. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J. Gen. Virol. 71:397-404. [DOI] [PubMed] [Google Scholar]
- 237.Shimeld, C., J. L. Whiteland, S. M. Nicholls, D. L. Easty, and T. J. Hill. 1996. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J. Gen. Virol. 77:977-985. [DOI] [PubMed] [Google Scholar]
- 238.Shimeld, C., J. L. Whiteland, S. M. Nicholls, E. Grinfeld, D. L. Easty, H. Gao, and T. J. Hill. 1995. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J. Neuroimmunol. 61:7-16. [DOI] [PubMed] [Google Scholar]
- 239.Shimeld, C., J. L. Whiteland, N. A. Williams, D. L. Easty, and T. J. Hill. 1997. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J. Gen. Virol. 78:3317-3325. [DOI] [PubMed] [Google Scholar]
- 240.Shimeld, C., J. L. Whiteland, N. A. Williams, D. L. Easty, and T. J. Hill. 1996. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J. Gen. Virol. 77:2583-2590. [DOI] [PubMed] [Google Scholar]
- 241.Shirvan, A., I. Ziv, A. Barzilai, R. Djaldeti, R. Zilkh-Falb, T. Michlin, and E. Melamed. 1997. Induction of mitosis-related genes during dopamine-triggered apoptosis in sympathetic neurons. J. Neural Transm. Suppl. 50:67-78. [DOI] [PubMed] [Google Scholar]
- 242.Shirvan, A., I. Ziv, T. Machlin, R. Zilkha-Falb, E. Melamed, and A. Barzilai. 1997. Two waves of cyclin B and proliferating cell nuclear antigen expression during dopamine-triggered neuronal apoptosis. J. Neurochem. 69:539-549. [DOI] [PubMed] [Google Scholar]
- 243.Simmons, A., D. Tscharke, and P. Speck. 1992. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr. Top. Microbiol. Immunol. 179:31-56. [DOI] [PubMed] [Google Scholar]
- 244.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Smith, C. A., P. Bates, R. Rivera-Gonzalez, B. Gu, and N. A. DeLuca. 1993. ICP4, the major transcriptional regulatory protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J. Virol. 67:4676-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Soengas, M. S., R. M. Alarcon, H. Yoshida, A. J. Giaccia, R. Hakem, T. W. Mak, and S. W. Lowe. 1999. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156-159. [DOI] [PubMed] [Google Scholar]
- 247.Spear, P. G. 1993. Entry of alphaherpesviruses into cells. Semin. Virol. 4:167-180. [Google Scholar]
- 248.Speck, P. G., and A. Simmons. 1991. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J. Virol. 65:4001-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Steiner, I., J. G. Spivack, S. L. Deshmane, C. I. Ace, C. M. Preston, and N. W. Fraser. 1990. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol. 64:1630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 251.Taus, N. S., and W. J. Mitchell. 2001. The transgenic ICP4 promoter is activated in Schwann cells in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 75:10401-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Thomas, D. L., M. Lock, J. M. Zabolotny, B. R. Mohan, and N. W. Fraser. 2002. The 2-kilobase intron of the herpes simplex virus type 1 latency-associated transcript has a half-life of approximately 24 hours in SY5Y and COS-1 cells. J. Virol. 76:532-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Thomas, S. K., G. Gough, D. S. Latchman, and R. S. Coffin. 1999. Herpes simplex virus latency-associated transcript encodes a protein which greatly enhances virus growth, can compensate for deficiencies in immediate-early gene expression, and is likely to function during reactivation from virus latency. J. Virol. 73:6618-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Thomas, S. K., C. E. Lilley, D. S. Latchman, and R. S. Coffin. 2002. A protein encoded by the herpes simplex virus (HSV) type 1 2-kilobase latency-associated transcript is phosphorylated, localized to the nucleus, and overcomes the repression of expression from exogenous promoters when inserted into the quiescent HSV genome. J. Virol. 76:4056-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Thompson, R. L., and N. M. Sawtell. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tikoo, S. K., M. Campos, and L. A. Babiuk. 1995. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv. Virus Res. 45:191-223. [DOI] [PubMed] [Google Scholar]
- 259.Trousdale, M. D., I. Steiner, J. G. Spivack, S. L. Deshmane, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J. Virol. 65:6989-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Turner, E. E., N. Fedtsova, and M. G. Rosenfeld. 1996. POU-domain factor expression in the trigeminal ganglion and implications for herpes virus regulation. Neuroreport 7:2829-2832. [DOI] [PubMed] [Google Scholar]
- 261.Turner, E. E., J. M. Rhee, and L. T. Feldman. 1997. The POU-domain factor Brn-3.0 recognizes characteristic sites in the herpes simplex virus genome. Nucleic Acids Res. 25:2589-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Valyi-Nagy, T., S. L. Deshmane, B. Raengsakulrach, M. Nicosia, R. M. Gesser, M. Wysocka, A. Dillner, and N. W. Fraser. 1992. Herpes simplex virus type 1 mutant strain in 1814 establishes a unique, slowly progressing infection in SCID mice. J. Virol. 66:7336-7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wagner, E. K., G. Devi-Rao, L. T. Feldman, A. T. Dobson, Y. F. Zhang, W. M. Flanagan, and J. G. Stevens. 1988. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J. Virol. 62:1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wagner, E. K., W. M. Flanagan, G. Devi-Rao, Y. F. Zhang, J. M. Hill, K. P. Anderson, and J. G. Stevens. 1988. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J. Virol. 62:4577-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 267.Winkler, M. T., A. Doster, and C. Jones. 1999. Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 73:8657-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Winkler, M. T., A. Doster, J. H. Sur, and C. Jones. 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet. Microbiol. 86:139-155. [DOI] [PubMed] [Google Scholar]
- 269.Winkler, M. T. C., A. Doster, and C. Jones. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J. Virol. 74:5337-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wirth, U. V., C. Fraefel, B. Vogt, C. Vlcek, V. Paces, and M. Schwyzer. 1992. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3′ coterminal and encode a putative zinc finger transactivator protein. J. Virol. 66:2763-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wirth, U. V., K. Gunkel, M. Engels, and M. Schwyzer. 1989. Spatial and temporal distribution of bovine herpesvirus 1 transcripts. J. Virol. 63:4882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wirth, U. V., B. Vogt, and M. Schwyzer. 1991. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J. Virol. 65:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang, L., C. C. Voytek, and T. P. Margolis. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yeh, L., and P. A. Schaffer. 1993. A novel class of transcripts expressed with late kinetics in the absence of ICP4 spans the junction between the long and short segments of the herpes simplex virus type 1 genome. J. Virol. 67:7373-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhao, Z. S., F. Granucci, L. Yeh, P. A. Schaffer, and H. Cantor. 1998. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 279:1344-1347. [DOI] [PubMed] [Google Scholar]
- 276.Zwaagstra, J. C., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1991. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1). Virology 182:287-297. [DOI] [PubMed] [Google Scholar]
- 277.Zwaagstra, J. C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, S. C. Wheatley, K. Lillycrop, J. Wood, D. S. Latchman, K. Patel, and S. L. Wechsler. 1990. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J. Virol. 64:5019-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]