Abstract
Fission yeast heterochromatin is formed at centromeres, telomeres, and in the mating-type region where it mediates the transcriptional silencing of the mat2-P and mat3-M donor loci and the directionality of mating-type switching. We conducted a genetic screen for directionality mutants. This screen revealed the essential role of two previously uncharacterized factors, Clr7 and Clr8, in heterochromatin formation. Clr7 and Clr8 are required for localization of the Swi6 chromodomain protein and for histone H3 lysine 9 methylation, thereby influencing not only mating-type switching but also transcriptional silencing in all previously characterized heterochromatic regions, chromosome segregation, and meiotic recombination in the mating-type region. We present evidence for physical interactions between Clr7 and the mating-type region and between Clr7 and the S. pombe cullin Pcu4, indicating that a complex containing these proteins mediates an early step in heterochromatin formation and implying a role for ubiquitination at this early stage prior to the action of the Clr4 histone methyl-transferase. Like Clr7 and Clr8, Pcu4 is required for histone H3 lysine 9 methylation, and bidirectional centromeric transcripts that are normally processed into siRNA by the RNAi machinery in wild-type cells are easily detected in cells lacking Clr7, Clr8, or Pcu4. Another physical interaction, between the nucleoporin Nup189 and Clr8, suggests that Clr8 might be involved in tethering heterochromatic regions to the nuclear envelope by association with the nuclear-pore complex.
THE mating type of fission yeast is determined by the allele present at the mat1 locus, mat1-M in M cells or mat1-P in P cells. Homothallic strains are able to switch their mating type, giving rise to homogenous mixtures of P and M cells within individual colonies (reviewed by Arcangioli and Thon 2004). The switches in mating type result from gene conversions of mat1 by the transcriptionally silent cassettes mat2-P or mat3-M. mat2-P and mat3-M take turns in providing genetic information to mat1, mat2-P being a preferred donor in M cells while mat3-M is preferred in P cells. The ability of a cell to distinguish between the two donors and use one preferentially is called directionality of switching.
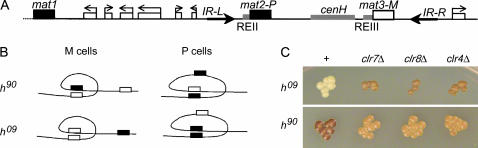
Directionality of switching depends on the chromosomal location of the silent mating-type information (Thon and Klar 1993). mat1, mat2-P, and mat3-M are linked in an ∼30-kb region of chromosome 2 (Figure 1). The wild-type mating-type region is denoted as h90. Cells in which the contents of the silent cassettes are experimentally swapped to mat2-M mat3-P (h09 genotype) switch their mating type inefficiently in contrast to h90 cells, indicating that the location of the silent cassettes, rather than their content, determines donor choice (Thon and Klar 1993). The differential switching ability of h90 and h09 cells suggests that mat1-mat2 interactions are favored in M cells and mat1-mat3 interactions are favored in P cells perhaps due to alternative chromosome foldings taking place in the two cell types or to alternative chromatin structures facilitating recombination with one of the silent donors or the other.
Figure 1.
Genetic screen for directionality mutants. (A) S. pombe mating-type region. The mating-type region spans ∼30 kb in the right arm of chromosome 2. It includes an ∼17-kb heterochromatic region containing the mat2-P and mat3-M silent cassettes. The heterochromatic region is flanked by 2.1-kb inverted repeats, IR-L and IR-R. REII and REIII are silencing elements operating, respectively, on mat2-P and mat3-M. cenH is an ∼4.3-kb region homologous to centromeric repeats. The wild-type h90 configuration is represented. The contents of the mat2-P and mat3-M cassettes are swapped in the h09 configuration, which is otherwise identical to h90. (B) Model for the directionality of mating-type switching. The drawings represent interactions occurring between mat1 and the silent cassettes in h90 and h09. Identical foldings would lead to efficient heterologous switching in h90 cells and inefficient switching in h09 cells. Mutations affecting these interactions would decrease heterologous switching in h90 cells and increase heterologous switching in h09cells. (C) Iodine-staining phenotypes of directionality mutants. Colonies grown on sporulation plates were stained with iodine vapors and photographed. Dark iodine staining is indicative of efficient mating-type switching while light staining reflects inefficient switching.
mat2-P and mat3-M are embedded in heterochromatin. Nucleosomal histones are hypoacetylated and histone H3 is methylated at lysine 9 rather than at lysine 4 in a 17-kb silenced region that includes the two cassettes (Noma et al. 2001), a pattern similar to the histone modifications found in heterochromatic regions of higher eukaryotes. Also similar to the situation in higher eukaryotes, the mat2-mat3 region is physically associated with chromodomain proteins (Ekwall et al. 1995; Sadaie et al. 2004). Two of those, Swi6 and Chp2, are members of the HP1 family. The association of chromodomain proteins with the mating-type region or other heterochromatic regions of Schizosaccharomyces pombe occurs via an interaction between their chromodomain and histone H3 methylated at lysine 9 (Bannister et al. 2001; Nakayama et al. 2001; Partridge et al. 2002; Sadaie et al. 2004). This mode of interaction has been conserved in evolution as well as in the enzymes responsible for the methylation of histone H3 lysine 9: cryptic loci regulator 4 (Clr4) in S. pombe or the SuVar39 proteins in flies or mammals (Rea et al. 2000). In S. pombe, in vivo methylation of chromosomal histones by Clr4 depends on Rik1, a protein with a β-propeller domain and with similarities to UV-damaged DNA-binding proteins (Neuwald and Poleksic 2000; Tuzon et al. 2004). In clr4 or rik1 mutants histone H3 is not methylated at lysine 9 and Swi6 is delocalized (Ekwall et al. 1996; Bannister et al. 2001; Nakayama et al. 2001). Although the mechanism by which Rik1 facilitates the action of Clr4 is not known, Clr4 and Rik1 copurify and associate with chromatin in an interdependent manner, suggesting that they are part of a complex (Sadaie et al. 2004).
Heterochromatin participates in different processes in the various chromosomal locations at which it is formed. In centromeric regions, heterochromatin promotes kinetochore assembly and cohesion (reviewed by Pidoux and Allshire 2004). Mutants lacking heterochromatin components display lagging chromosomes and increased rates of chromosome loss in mitosis most likely due to defects in their centromeres. In telomeric and subtelomeric regions, heterochromatin plays both structural and regulatory roles; it facilitates the association of telomeres with the spindle pole body in meiotic prophase (Tuzon et al. 2004) and represses the transcription of clusters of subtelomeric genes regulated by nitrogen starvation (Hansen et al. 2005). In the mating-type region, heterochromatin mediates the transcriptional silencing of the mat2-P and mat3-M cassettes. This is a crucial role since cells expressing mat2-P and mat3-M tend to undergo meiosis, a generally lethal event when initiated in the haploid stage. In addition, mutations in Swi6, Clr4, or Rik1 impair donor choice (Thon and Klar 1993; Ivanova et al. 1998; Tuzon et al. 2004). Consistent with the mating-type region attaining different structures in the two cell types, transcriptional silencing of reporter genes artificially introduced in the region is more stringent in M than in P cells (Ayoub et al. 1999; Thon et al. 1999) and the association of Swi6 with the mating-type region differs between the two cell types (Noma et al. 2001). Furthermore, the Swi6-interacting protein Swi2 associates with a DNA element centromere distal to the mat3-M cassette in both P and M cells in a Swi6-independent manner and spreads to the entire region in M cells in a Swi6-dependent manner (Jia et al. 2004b). This differential distribution and the switching defects of cells lacking Swi2 suggest that Swi2 has a role in donor selection. Unlike mutations in Rik1 or Clr4 that impair the directionality of switching by affecting heterochromatin formation, lack of Swi2 does not prevent heterochromatin formation, nor does it influence transcriptional silencing. Rather, Swi2 appears to play a role specific to mating-type switching possibly by recruiting the recombination protein Rhp51 to the mating-type region (Akamatsu et al. 2003).
Heterochromatin near centromeres, telomeres, or in the mating-type region is strickingly related in composition and properties. Several differences have been documented as well, ranging from the mode of heterochromatin establishment to the nature of physically associated factors. For example, the RNA interference (RNAi) pathway is crucial to centromeric heterochromatin formation (Volpe et al. 2002) while its role in the mating-type region is obscured by a parallel recruitment mechanism involving the transcription factor Atf1 (Thon and Verhein-Hansen 2000; Jia et al. 2004a; Kim et al. 2004) and by a silencing mechanism independent of Clr4 and Swi6 (Thon et al. 1994; Thon and Verhein-Hansen 2000). Similarly, the three chromodomain proteins Swi6, Chp1, and Chp2 are associated with the mating-type region and the centromeric and telomeric regions, but the requirements for their association with each of these regions differ as do their contributions to transcriptional silencing (Thon and Verhein-Hansen 2000; Sadaie et al. 2004; Petrie et al. 2005). Such differences are not surprising, given the various nuclear components with which heterochromatin can be expected to interact, and they indicate that characterizing heterochromatin-dependent processes will lead to the identification of core components acting at all known heterochromatic locations as well as components more specific to the particular region examined.
We conducted a genetic screen for factors affecting the directionality of mating-type switching. We present here the characterization of two factors that had not been identified in previous studies, Clr7 and Clr8. These factors act early in heterochromatin formation at all known heterochromatic regions of S. pombe.
MATERIALS AND METHODS
Strains and media:
The S. pombe strains used in this study and their genotypes are listed in Table 1. S. pombe media were as described previously (Thon and Friis 1997). SC drop-out media (Rose et al. 1990) lacking leucine and tryptophan (SC-leu-trp); leucine, tryptophan, and adenine (SC-leu-trp-ade); or leucine, tryptophan, and histidine (SC-leu-trp-his) were used to perform the two-hybrid screens. SC media lacking leucine, tryptophan, and histidine and containing 1.5 or 3 mm 3-aminotriazole (3-AT) were also used.
TABLE 1.
Strains and their genotypes
| Strain | Genotype |
|---|---|
| Hu52 | h90 mat3-M(EcoRV)∷ade6+ leu1-32 ura4-D18 ade6-DN/N |
| FY336 | h+ cnt1/TM1(NcoI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 |
| FY520 | h+ Ch16 m23∷ura4+-TEL[72] leu1-32 ura4-DS/E ade6-M210 (Ch16 ade6-M216) |
| FY648 | h+ otr1R(SphI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 |
| FY986 | h+ otr1L(dg1a/HindIII)∷ura4+ oriII leu1-32 ura4-DS/E ade6-M210 |
| PG445 | mat1-PΔ17∷LEU2 leu1-32 ura4-D18 ade6-M216 |
| PG1225 | h90 mat3-M(EcoRV)∷ura4+ leu1-32 ura4-D18 ade6-M210 clr8-109 |
| PG1403 | mat1-PΔ17∷LEU2 Δ(482)mat3-M leu1-32 ura4-D18 ade6-M216 |
| PG1785 | mat1-Msmt-0 Δ(BglII-BssHII)mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 |
| PG1789 | mat1-Msmt-0 mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M216 |
| PG1899 | h90 mat3-M(EcoRV)∷ura4+ leu1-32 ura4-DS/E ade6-M216 |
| PG3389 | h+ otr1R(SphI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr8Δ∷kanR |
| PG3393 | h90 clr8Δ∷kanR ura4-DS/E ade6-M216 |
| PG3397 | mat1-PΔ17∷LEU2 Δ(482)mat3-M leu1-32 ura4-DS/E ade6-M210 clr8Δ∷kanR |
| PG3399 | mat1-PΔ17∷LEU2 leu1-32 ura4-DS/E ade6-M216 clr8Δ∷kanR |
| PG3400 | h+ otr1L(dg1a/HindIII)∷ura4+ oriII leu1-32 ura4-DS/E ade6-M210 clr8Δ∷kanR |
| PG3402 | h− leu1-32 ura4-DS/E ade6-M216 ars1∷pREP42-EGFP-swi6+ clr8Δ∷kanR |
| PG3403 | mat1-Msmt-0 Δ(BglII-BssHII)mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr8Δ∷kanR |
| PG3404 | mat1-Msmt-0 mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr8Δ∷kanR |
| PG3406 | h− cnt1/TM1(NcoI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr8Δ∷kanR |
| PG3408 | h90 Ch16 m23∷ura4+-TEL[72] leu1-32 ura4-DS/E ade6-M210 (Ch16 ade6-M216) clr8Δ∷kanR |
| PG3409 | mat1-PΔ17∷LEU2 Δ(482)mat3-M leu1-32 ura4-D18 ade6-M216 clr4Δ∷LEU2 |
| PG3410 | mat1-PΔ17∷LEU2 leu1-32 ura4-D18 ade6-M216 clr4Δ∷LEU2 |
| PG3413 | h+ otr1L(dg1a/HindIII)∷ura4+ oriII leu1-32 ura4-DS/E ade6-M210 clr4Δ∷LEU2 |
| PG3416 | h− cnt1/TM1(NcoI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr4Δ∷LEU2 |
| PG3419 | h+ otr1R(SphI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr4Δ∷LEU2 |
| PG3420 | h Ch16 m23∷ura4+-TEL[72] leu1-32 ura4-DS/E ade6-M210 (Ch16 ade6-M216) clr4Δ∷LEU2 |
| PG3423 | mat1-Msmt-0 mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr4Δ∷LEU2 |
| PG3424 | mat1-Msmt-0 Δ(BglII-BssHII)mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr4Δ∷LEU2 |
| PG3426 | h90 mat3-M(EcoRV)∷ade6+ leu1-32 ura4-D18 ade6-DN/N clr4Δ∷LEU2 |
| PG3427 | h90 mat3-M(EcoRV)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr8Δ∷kanR |
| PG3428 | h90 mat3-M(EcoRV)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr4Δ∷LEU2 |
| PG3429 | h90 mat3-M(EcoRV)∷ade6+ leu1-32 ura4-DS/E ade6-DN/N clr8Δ∷kanR |
| PG3430 | h90 mat3-M(EcoRV)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr7Δ∷kanR |
| PG3435 | h90 ura4-D18 pcu4∷ura4+ |
| PI213 | h90 leu1-32 ura4-D18 ade6-M216 ars1∷pREP42-EGFP-swi6+ |
| SP837 | h90 leu1-32 ura4-D18 ade6-M216 |
| SP1726 | h09 mat3-P(EcoRV)∷ura4+ leu1-32 ura4-D18 ade6-M216 clr7-116 |
| SP2121 | h09 mat3-P(EcoRV)∷ura4+ leu1-32 ura4-D18 ade6-M216 clr7Δ∷kanR |
| SP2122 | h09 mat3-P(EcoRV)∷ura4+ leu1-32 ura4-D18 ade6-M216 clr7-myc |
| SPA9 | h+ otr1L(dg1a/HindIII)∷ura4+ oriII leu1-32 ura4-DS/E ade6-M216 clr7Δ∷kanR |
| SPA14 | h+ Ch16 m23∷ura4+-TEL[72] leu1-32 ura4-DS/E ade6-M210 (Ch16 ade6-M216) clr7Δ∷kanR |
| SPA15 | h− otr1R(SphI)∷ura4+ leu1-32 ura4-DS/E ade6-M210 clr7Δ∷kanR |
| SPA16 | h−- leu1-32 ura4-D18 ade6-M216 ars1∷pREP42-EGFP-swi6+ clr7Δ∷kanR |
| SPA17 | mat1-Msmt-0 mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr7Δ∷kanR |
| SPA20 | h90 mat3-M(EcoRV)∷ade6+ leu1-32 ura4-D18 ade6-DN/N clr7Δ∷kanR |
| SPA21 | mat1-PΔ17∷LEU2 leu1-32 ura4-D18 ade6-M216 clr7Δ∷kanR |
| SPA25 | mat1-PΔ17∷LEU2 Δ(482)mat3-M leu1-32 ura4-D18 ade6-M210 clr7Δ∷kanR |
| SPA27 | mat1-Msmt-0 Δ(BglII-BssHII)mat2-P(XbaI)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr7Δ∷kanR |
| SPA28 | h− cnt1/TM1(NcoI)∷ura4+ leu1-32 ura4-DS/E ade6-M216 clr7Δ∷kanR |
Cloning and deletion of clr7 and clr8:
Several clones complementing the sporulation and silencing defects of, respectively, SP1726 (h09 mat3-P(EcoRV)∷ura4+ clr7-116) and PG1225 (h90 mat3-M(EcoRV)∷ura4+clr8-109) were isolated from a partial HindIII library of S. pombe genomic DNA cloned in the vector pDW15 (Wright et al. 1986). FOA-resistant transformants were isolated and screened for light iodine staining in the case of the SP1726 strain or dark staining in the case of PG1225. Plasmids were rescued from transformants with these phenotypes and partially sequenced, which indicated that clr7-116 was complemented by the SPCC970.07c ORF and clr8-109 by SPCC613.12c. The pFA6A-kanMX6 plasmid (Bahler et al. 1998) was used to create precise deletions of SPCC970.07c and SPCC613.12c in diploid strains. Stable G418-resistant transformants were examined by Southern blots. Transformants with correct integrations were sporulated. The G418-resistant progeny were viable in both cases, revealing that neither SPCC970.07c nor SPCC613.12 is essential for viability. clr7 was identified as SPCC970.07c by measuring linkage between clr7-116 and SPCC970.07cΔ∷kanR. Similarly, clr8 was identified as SPCC613.12c.
Two-hybrid screens:
The clr7 and clr8 ORFs amplified by PCR with GTO-239 (GCGGATCCTTAATTTATGTTGTACATTATGTGTTCAAG) and GTO-240 (GAGGCCATGGAGGCCATGCCGCCCGTACGTGCTGAAAAAAAGC) in the case of clr7 or GTO-250 (GAGGCCATGGAGGCCATGACTAATAGTTCACCACGGGTG) and GTO-251 (GCGGATCCTCAGGTTAAAAGTCGATTTTCTATAATACG) in the case of clr8 were cloned between the SfiI and BamHI sites of pGBKT7 (CLONTECH, Palo Alto, CA), creating pSPA6 (clr7) and pGT402 (clr8). The inserts in pSPA6 and pGT402 were entirely sequenced to verify that the PCR had not introduced any nucleotide change altering the protein sequence. pSPA6 and pGT402 were transformed separately into the Saccharomyces cerevisiae strain PJ69-4A (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2∷GAL1-HIS3 GAL2-ADE2 met2∷GAL7-lacZ; James et al. 1996). Large-scale transformations were performed using an S. pombe cDNA library in the vector pGAD-GH (CLONTECH). Plasmids in this library express S. pombe ORFs fused to the S. cerevisiae GAL4-transcription-activating domain. Transformants expressing the HIS3 reporter gene were selected on SC-leu-trp-his containing either 1.5 or 3 mm 3-AT. Approximately 0.5 × 106 Leu+Trp+ transformants were plated for each bait. Among those, ∼500 formed colonies on the 3-AT-containing selective plates within 1 week at 30°. A total of 213 (clr7) or 99 (clr8) of these Leu+Trp+His+ transformants were streaked onto SC-leu-trp plates and replicated onto SC-leu-trp-ade plates. Five Ade+ transformants were obtained with the Clr7 bait and 78 were obtained in the case of Clr8. Plasmids were extracted from the His+Ade+ transformants as well as from 20 His+Ade− transformants obtained with the Clr7 bait. These were retransformed into PJ69-4A with either pSPA6 or pGT402. Leu+Trp+ transformants were selected. Most failed to express HIS3 or ADE2 with the exception of two clones in the case of Clr7, both encoding Pcu4 (SPAC3A11.08; O14122), and one in the case of Clr8, encoding a truncated version of Nup189 (SPAC1486.05; Q9UTK4) starting at amino acid 446. Two other plasmids extracted from Leu+Trp+His+Ade+ transformants could activate transcription of the reporter PJ69-4A genes in the absence of Clr7 and Clr8 and were discarded.
Chromosome-loss assay:
Mitotic chromosome loss was assayed as previously described (Allshire et al. 1994) using cells containing the ade6-M210 allele on chromosome 3 and the ade6-M216 allele on the nonessential minichromosome Ch16m23∷ura4+-Tel[72] (Nimmo et al. 1994). Cells with this genotype are phenotypically Ade+ due to the interallelic complementation between ade6-M210 and ade6-M216. They form white colonies on media containing low concentrations of adenine and red sectors or colonies following loss of Ch16m23∷ura4+-Tel[72]. FY520 (+), SPA14 (clr7Δ), PG3408 (clr8Δ), and PG3420 (clr4Δ) cultures were propagated in all additions (AA)-ade, plated on AA containing 15 mg/liter adenine, and incubated at 33°. White and sectored colonies were counted. The rate of minichromosome loss was determined as the number of colonies with a red sector equal to or greater than half the colony size (that is, the number of cells having lost their minichromosome at the first division after plating) divided by the number of white or sectored colonies (that is, the number of cells containing the minichromosome when plated).
Swi6 tagging and fluorescence microscopy:
The swi6+ ORF was amplified from pAL2 (Lorentz et al. 1994) with native Pfu polymerase (Stratagene, La Jolla, CA) using the two primers ACGCGTCGACAATGAAGAAAGGAGGTGTTCG and CGGGATCCTTATTCATTTTCACGGAAC. The amplification product was cloned between the SalI and BamHI sites of pREP42/enhanced green fluorescent protein (EGFP)-N (Craven et al. 1998) to create an N-terminal fusion with EGFP. pREP42-EGFP-Swi6 was linearized at a unique MluI site within ars1 and integrated at ars1 in SP837 to produce PI213. This construct is similar to the construct made by Pidoux et al. (2000). Cells were thinly patched on AA-thiamine medium and allowed to grow at 33° for 15 hr prior to microscopic examination. Localization images were obtained using a Zeiss Axio Imager fluorescence microscope equiped with a Hamamatsu Orca-ER digital camera and Volocity 3.5.1 software.
RT-PCR analyses:
RNA extractions and RT-PCR were performed as in Hansen et al. (2005). The oligonucleotides GTO-232 (CATTGGCTTACGACGGTCGTGG) and GTO-233 (CCACATATGGCCCGTAAGTGAGC) were used to amplify ade6+ and ade6-DN/N; GTO-265 (GCTATTCAGCTAGAGCTGAGGG) and GTO-266 (CTTCGACAACAGGATTACGACC) to amplify ura4+ and ura4-DS/E; GTO-223 (GAAAACACATCGTTGTCTTCAGAG) and GTO-226 (TCGTCTTGTAGCTGCATGTGA) to amplify RNA originating from centromeric repeats or mating-type region; OKR-40 (TCGTCTTGTAGCAGCATGTGA) and OKR-41 (GAGATGAACGTATCTCTATCGAC) to amplify telomeric sequences that are part of subtelomeric helicases (tlh) ORFs; OKR70 (GGCATCACACTTTCTACAACG) and OKR71(GAGTCCAAGACGATACCAGTG) to amplify actin mRNA. Strand-specific RT-PCR was achieved by using GTO-226 to prime reverse transcription on centromeric forward transcripts, GTO-223 on centromeric reverse transcripts, OKR-41 on forward tlh transcripts, and OKR-40 on reverse tlh transcripts.
Chromatin immunoprecipitations:
Chromatin immunoprecipitations (ChIP) were performed as described (Ekwall and Partridge 1999). Yeast cells were grown in rich medium to midlog phase and fixed in 2% formaldehyde for 30 min. Crosslinked chromatin was sonicated to an average size of 600 bp and immunoprecipitated with antibodies directed against dimethyl histone H3 lysine 9 (Upstate, Lake Placid, NY), dimethyl histone H3 lysine 4 (Upstate), or the c-myc epitope (Roche). The immunoprecipitated DNA was analyzed by PCR. PCR products were labeled by including [α-32P]dCTP (Amersham Pharmacia) as a tracer in the reactions. The primer set used for amplifying the K-region was GTATGTGGAACAAGAGAAG and CTCGCCTGCTTACATTTTAAGG, and for act1 GAAGTACCCCATTGAGCACGG and CAATTTCACGTTCGGCGGTAG. Amplified products were resolved on a 6% nondenaturing polyacrylamide gel and quantified using a phosphorimager (Typhoon 8600, Amersham Pharmacia).
RESULTS
A genetic screen for donor-choice mutants:
We devised a genetic screen for mutants deficient in the directionality of mating-type switching. Mutations randomizing donor choice are expected to increase heterologous switching in the h09 (mat2-M mat3-P) mating-type region and to decrease heterologous switching in the h90 (mat2-P mat3-M) mating-type region. Such mutations can be found using an iodine-staining procedure that reveals the efficiency of switching at the colony level in S. pombe. S. pombe spores, but not vegetative cells, are stained darkly by iodine vapors. Wild-type h90 colonies, in which mating-type switching has occurred efficiently, produce great numbers of spores uniformely distributed within colonies. Hence, h90 colonies display a dark uniform staining following sporulation. In contrast, h09 colonies are stained lightly (Thon and Klar 1993). We searched for h09 mutants displaying increased iodine staining. Colonies with increased staining obtained in the screen were examined microscopically for the presence of spores and asci. Seventy h09 mutants with elevated proportions of zygotic asci were crossed with h90 cells containing an auxotrophic marker tightly linked to the mating-type region, his2, which allowed us to genetically distinguish the two mating-type regions. Twenty-one mutations decreased iodine staining and mating efficiency in the h90 progeny of the crosses. In addition to the altered frequency of zygotic asci, low levels of haploid meiosis were observed in some of the mutants, which is indicative of a transcriptional silencing defect leading to the expression of mat2-P and/or mat3-M in these mutants. Aberrant zygotic asci were also occasionally observed, which is indicative of deficient meioses.
The trans-acting mutations defining potential directionality factors were placed into linkage groups by genetic crosses. One group was mapped to the clr1 locus (Thon and Klar 1992), one to clr4 (clr4-113 and -141 alleles), and one to rik1 (rik1-127, -128, -121, and -161 alleles); one was mapped both to swi6-mod, a locus modifying the phenotype of swi6-115 cells (Thon and Klar 1993), and to swi2 (swi2-152 allele). This latter result is surprising in the view of a recently published study proposing that swi2 and swi6-mod are unlinked genes (Jia et al. 2004b). However, we were unable to separate swi6-mod from swi2 mutant alleles in crosses; both genes displayed centromere linkage and tight linkage to lys1 as expected for swi2.
The other mutations isolated in our screen were not in previously characterized silencing or directionality factors. We chose to characterize genes acting early in the process of heterochromatin formation. Mutations in these genes would affect not only the directionality of switching, but also transcriptional silencing. As expected, mutations belonging to the clr1, clr4, or rik1 group alleviated transcriptional silencing in the mating-type region, centromeres, and telomeres. Mutations at two other loci behaved similarly, revealing that these two loci are also involved in heterochromatin formation. Because the phenotypes of the newly obtained mutations are similar to those in previously characterized clr genes, we called the two newly defined loci clr7 and clr8.
Identification of clr7 and clr8:
clr7 and clr8 were cloned by complementation of the mutant phenotypes of, respectively, h09 mat3-P (EcoRV)∷ura4+ clr7-116 and h90 mat2-P (XbaI)∷ura4+ clr8-109 cells using a library of S. pombe genomic DNA. The complementation studies identified two ORFs annotated in the S. pombe genome sequence, SPCC970.07c, complementing clr7-116, and SPCC613.12c, complementing clr8-109. The chromosomal replacement of SPCC970.07c or SPCC613.12c with kanR produced phenotypes similar to those conferred by the point mutations in clr7 or clr8. Furthermore, SPCC970.07c∷kanR displayed perfect linkage with clr7-116 and SPCC613.12c∷kanR displayed perfect linkage with clr8-109, demonstrating that the mutated genes had been cloned.
clr7 (SPCC970.07c) encodes a 636-aa protein with no clear sequence homolog in other organisms and with no recognizable domains except for a possible zinc-finger close to its carboxyl-terminal end. clr8 (SPCC613.12c) encodes a conserved 638-aa protein with four WD repeats.
Clr7 and Clr8 mediate transcriptional silencing near centromeres, telomeres, and in the mating-type region:
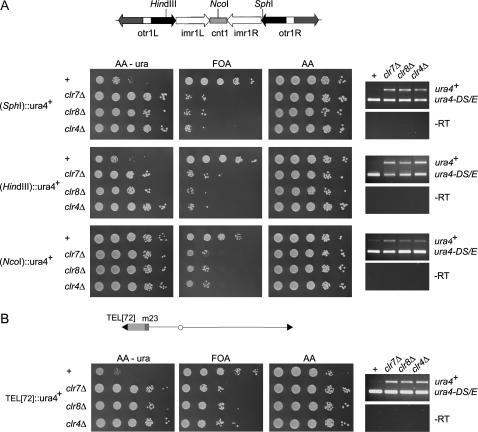
Euchromatic S. pombe genes such as ura4+ or ade6+ are repressed when placed within centromeric repeats (Allshire et al. 1994, 1995), close to the telomeric repeats of a minichromosome (Nimmo et al. 1994), or in the mating-type region (Thon and Klar 1992; Thon et al. 1994, 1999, 2002). Consequently, cells with the ura4+ or ade6+ gene at one of these locations are uracil or adenine auxotrophs. In addition, cells with a silenced ura4+ gene are able to form colonies in the presence of 5-FOA, a toxigenic substrate of the orotidine 5′-phosphate decarboxylase encoded by ura4+, while cells with a silenced ade6+ gene form red colonies on limiting concentrations of adenine. Deletion of clr7 or clr8 altered these phenotypes, derepressing the ura4+ and ade6+ reporter genes placed in heterochromatic regions (Figure 2). The derepression resulted in uracil or adenine prototrophy, FOA sensitivity in the case of ura4+, or formation of white colonies in the case of ade6+. Some FOA-resistant colonies formed by the mutant strains are most likely due to occasional loss of the ura4+ gene by recombination in centromeric regions (Figure 2A) or to loss of the minichromosome used to assay telomeric silencing (Figure 2B). ura4+ or ade6+ transcripts originating from centromeric, telomeric, or mating-type region could be easily detected in the mutant cells whereas they are in low abundance in wild-type cells (Figure 2). Furthermore, transcripts originating from the centromeric repeats per se or from genes that are naturally telomere linked were in greater abundance in the clr7 or clr8 mutant cells than in the wild-type cells, indicating that Clr7 and Clr8 have a role in the repression of these endogenous transcripts as well (see below).
Figure 2.
Effects of Clr7 and Clr8 on transcriptional silencing. Ten-fold serial dilutions of cell suspensions were spotted onto the indicated media (left) and RT-PCR was performed on the same strains to detect the ura4+ or ade6+ transcripts as indicated (right). The strains contain either a mini- ura4 gene at the ura4 locus (ura4-DS/E) or a mini-ade6 gene at the ade6 locus (ade6-DN/N) used as internal controls. clr4Δ cells are displayed for comparison. (A) Silencing in centromere 1. (Top) +, FY648; clr7Δ, SPA15; clr8Δ, PG3389; clr4Δ, PG3419. (Middle) +, FY986; clr7Δ, SPA9; clr8Δ, PG3400; clr4Δ, PG3413. (Bottom) +, FY336; clr7Δ, SPA28; clr8Δ, PG3406; clr4Δ, PG3416. (B) Telomeric silencing. +, FY520; clr7Δ, SPA14; clr8Δ, PG3408; clr4Δ, PG3420. (C) Silencing in the mating-type region. (Top) +, PG1789; clr7Δ, SPA17; clr8Δ, PG3404; clr4Δ, PG3423. (Middle) +, PG1899; clr7Δ, PG3430; clr8Δ, PG3427; clr4Δ, PG3428; (Bottom) +, Hu52; clr7Δ, SPA20; clr8Δ, PG3429; clr4Δ, PG3426.
Silencing in the mating-type region is a relatively complex phenomenon involving several overlapping pathways. A region with homology to centromeric repeats located between mat2-P and mat3-M is involved in promoting hetrochromatin formation in a manner partially redundant with the Atf1 protein for which multiple binding sites exist in the region (Grewal and Klar 1996, 1997; Thon and Friis 1997; Thon et al. 1999; Jia et al. 2004a; Kim et al. 2004). In addition, small elements adjacent to the mat2-P and mat3-M cassettes participate in silencing mat2-P and mat3-M in a manner that is not currently understood (Thon et al. 1994, 1999; Ayoub et al. 1999). These elements, REII adjacent to mat2-P and REIII adjacent to mat3-M, do not contain predicted binding sites for Atf1, nor do they display homology to centromeric repeats. Deletion of either REII or REIII has little effect on transcription, while a strong synergistic effect on the transcription of, respectively, mat2-P or mat3-M is observed when REII or REIII is deleted in a heterochromatin-deficient background, i.e., in cells with a mutated clr1, clr2, clr3, clr4, rik1, swi6, or chp2 allele (Thon et al. 1994; Thon and Verhein-Hansen 2000; G. Thon, unpublished observations). We combined the deletion of clr7 or clr8 with deletions of REII or REIII. Combination with the REII deletion caused a strong derepression of mat2-P, leading to a high frequency of haploid meiosis in unswitchable cells of the M mating type (Figure 3). Similarly, combination with the REIII deletion fully derepressed mat3-M and led to frequent haploid meioses in unswitchable P cells (Figure 3). Hence, clr7 and clr8 behave in this assay as members of the clr4 epistasis group. The alleviated transcriptional silencing suggests that heterochromatin is not formed properly in cells lacking Clr7 or Clr8.
Figure 3.
Synergistic effects of Clr7 or Clr8 with the REII and REIII silencers. (Top) All strains displayed contain an unswitchable mat1-M allele, mat1-Msmt-0. Dark iodine staining in the case of these strains is a result of haploid meiosis caused by mat2-P expression. (Bottom) Conversely, these strains contain an unswitchable mat1-P allele (mat1-PΔ17), allowing us to estimate mat3-M expression by the intensity of iodine staining. In all cases, 5 μl of cell suspensions containing ∼1000 cells/ml were spotted onto sporulation medium. Colonies were stained with iodine vapors and photographed. (Top) REII+ strains: +, PG1789; clr7Δ, SPA17; clr8Δ, PG3404; clr4Δ, PG3423. REIIΔ strains: +, PG1785; clr7Δ, SPA27; clr8Δ, PG3403; clr4Δ, PG3424. (Bottom) REIII+ strains: +, PG445; clr7Δ, SPA21; clr8Δ, PG3399; clr4Δ, PG3410. REIIIΔ strains: +, PG1403; clr7Δ, SPA25; clr8Δ, PG3397; clr4Δ, PG3409.
Defective Swi6 localization and other phenotypes suggestive of heterochromatin defects in cells lacking Clr7 or Clr8:
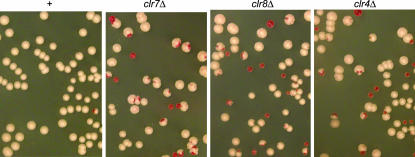
Consistent with defects in heterochromatin formation, deletion of clr7 or clr8 increased the rate of loss of a minichromosome (Figure 4; Table 2). Deletion of clr7 or clr8 also caused aberrant meioses. High proportions of zygotic asci with two or three spores were observed in these mutants and the spore viability was reduced compared with spores from a wild-type strain. Meiotic recombination occurred in the mat2-P-mat3-M interval in the mutant cells (data not shown), an event that is stringently repressed by heterochromatin in wild-type cells. In addition, the h+N configuration of the mating-type region that contains a partial duplication of the region was unstable in clr7- or clr8-deleted cells and reverted frequently to the h90 configuration, another feature characteristic of mutants lacking heterochromatin in the mating-type region.
Figure 4.
Minichromosome loss in cells lacking Clr7 or Clr8. Minichromosome loss in FY520 (+), SPA14 (clr7Δ), PG3408 (clr8Δ), or PG3420 (clr4Δ) leads to the appearance of red sectors in colonies grown on low-adenine concentration due to the loss of the ade6-M216 allele carried by the minichromosome in these strains. All strains were propagated in medium lacking adenine prior to plating to ensure that most cells would contain the minichromosome when plated.
TABLE 2.
Rates of minichromosome loss
| Genotype | No. of colonies with red sector ≥1/2 | No. of colonies | Rate of chromosome loss per cell division |
|---|---|---|---|
| + | 9 | 1667 | 0.006 |
| clr7Δ | 157 | 743 | 0.21 |
| clr8Δ | 255 | 784 | 0.33 |
| clr4Δ | 166 | 1159 | 0.14 |
The localization of the chromodomain protein Swi6 can be assayed in living cells using a GFP-tagged version of Swi6. Due to its preferential association with centromeres, telomeres, and mating-type region, GFP-Swi6 forms a few discrete bright spots in the nuclei of wild-type cells. The localization of GFP-Swi6 was disrupted in cells lacking clr7 or clr8 (Figure 5, A and B). A diffuse fluorescence was observed in these mutant cells instead of foci, demonstrating that Clr7 and Clr8 act upstream of Swi6 localization to promote heterochromatin formation.
Figure 5.
Effect of Clr7 and Clr8 on the localization of Swi6. (A) Live cells expressing EGFP-Swi6 were photographed and (B) the number of EGFP-Swi6 foci was determined in 220 cells of each strain. +, PI213; clr7Δ, SPA16; clr8Δ, PG3402.
The Pcu4 cullin interacts with Clr7 and mediates transcriptional silencing:
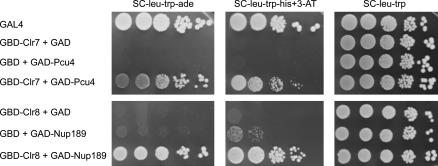
We conducted large-scale GAL4-based two-hybrid screens in S. cerevisiae using Clr7 or Clr8 as baits and an S. pombe cDNA library. We identified Pcu4 as a protein interacting with Clr7 in this system and Nup189 as interacting with Clr8 (Figure 6). Pcu4 is the S. pombe cullin 4 and Nup189 is an essential nuclear porin (Tange et al. 2002).
Figure 6.
Evidence for physical interactions involving Clr7 or Clr8. Ten-fold serial dilutions of the S. cerevisiae strain PJ69-4A expressing the indicated fusion proteins were spotted onto selective media to assay expression of the ADE2 and HIS3 reporter genes.
Cells lacking pcu4 grow poorly; however, pcu4 is not required for viability, allowing us to assay the effects of a pcu4 deletion (Liu et al. 2003) on transcriptional silencing and heterochromatin formation. Transcripts originating from centromeric repeats, mating-type region, or telomere-linked helicase genes that are normally processed into small interfering RNAs (siRNA) by the RNAi machinery (Reinhart and Bartel 2002; Volpe et al. 2002; Cam et al. 2005) were in greater abundance in cells lacking Pcu4 than in wild-type cells (Figure 7). Transcripts originating from both DNA strands were detected. These transcripts were also easily detected in cells lacking Clr7 or Clr8 (Figure 7). Hence, Clr7, Clr8, and Pcu4 either repress the transcription of centromeric and telomeric RNAs in wild-type cells or facilitate their processing into siRNA, or both.
Figure 7.
Effects of Pcu4, Clr7, and Clr8 on transcription in heterochromatic regions. Transcripts originating from noncoding regions or telomere-linked helicase genes were amplified by RT-PCR in wild-type cells and in the indicated mutants. cenHF, forward transcript originating from cenH element in the mating-type region; cenF, forward centromeric transcript as defined by Volpe et al. (2002); cenHR, reverse transcript originating from cenH; cenR, reverse centromeric transcript; tlhF, transcript originating from telomere-linked helicase genes, sense strand; tlhR, transcript originating from telomere-linked helicase genes, antisense strand; act, actin control; −RT, reverse transcription was omitted. +, FY648; clr7Δ, SPA15; clr8Δ, PG3389; clr4Δ, PG3419; pcu4Δ, PG3435. SPA15 being h− does not contain the cenH element.
Clr7, Clr8, and Pcu4 are required for histone H3 lysine 9 methylation:
The silencing defects in clr7, clr8, and pcu4 deletion strains together with the aberrant localization of Swi6 in cells lacking clr7 or clr8 suggested that histone H3 lysine 9 might not be methylated properly in these mutants. We tested this possibility by ChIP, assaying methylation levels in the mating-type region (Figure 8, A and B). We found that histone H3 lysine 9 methylation was considerably reduced in clr7Δ, clr8Δ, and pcu4Δ mutants compared with wild-type cells. The residual association of the histone H3 lysine 9 methyl mark with the mating-type region was no greater than its association with the euchromatic and actively transcribed act1 gene, revealing that Clr4 fails to operate in the three mutants. An increase in histone H3 lysine 4 methylation occurred concomitantly with the decrease in lysine 9 methylation (Figure 8C). Such an increase is commonly observed in mutants failing to methylate histone H3 lysine 9, possibly reflecting an increased accessibility of the region to the histone methyltransferase Set1. The increase in histone H3 lysine 4 methylation further demonstrates the fundamental role of Clr7, Clr8, and Pcu4 in heterochromatin formation.
Figure 8.
Effects of Clr7, Clr8, and Pcu4 on histone H3 methylation in the mating-type region and association of Clr7 with the mating-type region. (A) Representation of the K fragment amplified by PCR from the mating-type region in the experiments in B, C, and D. (B) The levels of histone H3 lysine 9 methylation in the mating-type region and at the act1 locus were estimated by ChIP in wild-type (SP837), clr7Δ (SP2121), clr8Δ (PG3393), and pcu4Δ (PG3435) cells in two experiments, one involving SP837 and SP2121 and the other involving SP837, PG3393, and PG3435. Association of the K fragment with histone H3 lysine 9 methylation was reduced ∼20-fold in clr7Δ cells relative to wild type, ∼30-fold in clr8Δ cells, and ∼35-fold in pcu4Δ cells. (C) The levels of histone H3 lysine 4 methylation were assayed by ChIP in wild-type (SP837), clr7Δ (SP2121), clr8Δ (PG3393), and pcu4Δ (PG3435) cells. Association of the K fragment with histone H3 lysine 4 methylation was increased ∼18-fold in clr7Δ cells relative to wild type, ∼22-fold in clr8Δ cells, and ∼27-fold in pcu4Δ cells. (D) The association of Clr7 with the mating-type region was assayed using a Clr7 protein tagged at its carboxyl terminus with the myc epitope and expressed from the clr7 locus under control of the clr7 promoter (SP2122).
In addition to observing aberrant histone H3 methylation patterns in clr7Δ, clr8Δ, and pcu4Δ mutant cells, we detected a physical association between Clr7 and the mating-type region in wild-type cells using a myc-tagged version of Clr7 (Figure 8D). This association supports the notion that Clr7 and Pcu4 mediate histone H3 lysine 9 methylation in a very direct manner.
DISCUSSION
We identified several factors necessary for heterochromatin formation in fission yeast, whose role had not been previously recognized: the S. pombe-specific protein Clr7, the WD repeat protein Clr8, and the Pcu4 cullin. We obtained evidence indicating that physical interactions take place between Pcu4 and Clr7, between Clr7 and the mating-type region, and between Clr8 and the nuclear-porin Nup189.
The involvement of a cullin in heterochromatin formation indicates that a ubiquitination step is essential in the process. Ubiquitination can regulate gene expression or DNA repair in several well-documented ways by affecting the stability of chromatin-associated factors or by modifying their properties as well as those of histones. For instance, in S. cerevisiae ubiquitination of histone H2B lysine 123 by Rad6 promotes methylation of histone H3 lysine 4 and lysine 79 as part of a transcriptional activation mechanism (Sun and Allis 2002; Kao et al. 2004). Ubiquitination of the proliferating cell nuclear antigen influences the choice of DNA repair pathway (Stelter and Ulrich 2003). In S. pombe, overexpression of the Rad6 homolog Rhp6 or of other putative ubiquitin-conjugating enzymes disrupts transcriptional silencing and Swi6 localization (Nielsen et al. 2002; I. S. Nielsen-Nørby and G. Thon, unpublished observations) and a specific mutant allele of rhp6, sng1, impairs the silencing of mat2-P and mat3-M following mating-type switching (Singh et al. 1998). While many examples of chromatin regulation by ubiquitination involve Rad6 or other E2 ubiquitin ligases, other observations demonstrate the role of cullin-RING complexes in similar regulatory pathways. Cullin-RING complexes constitute a large class of ubiquitin-conjugating enzymes in which cullins function as scaffolds bridging RING ubiquitin ligases with their substrates (for review, see Petroski and Deshaies 2005). The substrate specificity of cullin-RING complexes is determined by adaptor proteins making contacts with both the N-terminal part of the cullin and the substrate. Structural differences residing in the cullins N-terminal parts are believed to account for specific interactions with various adaptor proteins. Our results suggest that Clr7 functions as an adaptor for Pcu4 to mediate the effects of Pcu4 on heterochromatin formation. Furthermore, the physical association of Clr7 with heterochromatin suggests that Pcu4 does not affect chromatin structure in an indirect manner, but rather catalyzes the ubiquitination of a chromatin component. Pcu4 was found by others in a protein complex containing the Rik1 paralog Ddb1, the ribonucleotide-reductase inhibitor Spd1, and subunits of the COP9 signalosome, the purified complex being thought to regulate ribonucleotide reductase by degrading Spd1 (Liu et al. 2003; Bondar et al. 2004; Holmberg et al. 2005). Similarly, human Ddb1 associates with Cul4 (Shiyanov et al. 1999). In addition, human Ddb1 can form a dimer with the WD repeat protein Ddb2, integrating Ddb2 into the cullin-RING complex and allowing interaction of the complex with chromatin following UV irradiation (Groisman et al. 2003). Although Ddb2 and Clr8 are not closely related in sequence, they are both WD repeat proteins, suggesting that they might occupy similar positions in distinct cullin-RING complexes. These observations together with the phenotypic similarities of pcu4, clr7, clr8, and rik1 mutants suggest that Pcu4, Clr7, Clr8, and Rik1 form a complex. Ubiquitination by this complex might lead to the removal of proteins or modifed histones whose properties are incompatible with heterochromatin formation. Alternatively, it might create an epigenetic mark allowing methylation by Clr4.
Transcriptional regulation by Cullin-RING complexes is not without precedent in S. pombe. For instance, the F box protein Pof1 forms a complex with Pcu1 and Skp1 mediating the degradation of the transcription factor Zip1 (Harrison et al. 2005). Possibly more closely related to our observations, the S. pombe F box protein Pof3 complexed with Pcu1 and Skp1 is required for telomeric gene silencing (Katayama et al. 2002).
The lack of histone H3 lysine 9 methylation in cells lacking Clr7, Clr8, or Pcu4 reveals that the three proteins act early in the process of heterochromatin formation. In addition to altered histone methylation patterns, we could detect RNA molecules originating from both strands of all heterochromatic regions in the mutant strains, indicating that these RNAs are not processed by the RNAi machinery as normally occurs in wild-type cells. The possibility that Clr7, Clr8, and Pcu4 act in the RNAi pathway in a manner similar to the prototypic components Dcr1, Ago1, or Rdp1 (Volpe et al. 2002) appears unlikely, however, since the heterochromatic defects in clr7, clr8, or pcu4 deletion strains differ in several respects from the defects in RNAi mutants. In particular, clr7, clr8, or pcu4 deletions have a much greater influence on the mating-type region and telomeres than mutations in the RNAi pathway (Hall et al. 2002; Sadaie et al. 2004; this study). Mutations in the RNAi pathway do not lead to a loss of heterochromatin or transcriptional silencing in the mating-type region due to the existence of a redundant pathway of heterochromatin establishment (Jia et al. 2004a; Kim et al. 2004; Sadaie et al. 2004). Similarly, heterochromatin and transcriptional silencing persist at telomeres in RNAi mutants (Sadaie et al. 2004; K. R. Hansen and G. Thon, unpublished observations). In contrast, mutations in clr7, clr8, or pcu4 strongly affect transcriptional silencing and chromatin structure in the mating-type and telomeric regions, in addition to their effects at centromeres, which are similar to the effects of deleting rik1 or clr4 (Ekwall and Ruusala 1994; Thon et al. 1994; Nakayama et al. 2001; Noma et al. 2001; Sadaie et al. 2004; this study). Increased levels of centromeric and telomeric transcripts in the absence of Clr7, Clr8, or Pcu4 might result, on one hand, from increased transcription permitted by weakened heterochromatin, and, on the other hand, from a lack of processing caused by a mislocalization of the RNAi machinery. Histone H3 lysine 9 methylation tethers RNAi components complexed with the Chp1 chromodomain protein to heterochromatin, and hence mutations affecting histone H3 lysine 9 methylation reduce the ability of RNAi to act upon heterochromatic transcripts (Motamedi et al. 2004; Noma et al. 2004; Cam et al. 2005). More generally, all phenotypes ascribed here to the deletion of clr7, clr8, or pcu4, such as effects on the directionality of mating-type switching, localization of Swi6, transcriptional silencing, or spore viability, are likely to result from the failure in methylating histone H3 lysine 9 in these mutants. Rik1 and Clr4 associate with each other and interact with chromatin in an interdependent manner (Sadaie et al. 2004). Our results strongly suggest that they do so in conjunction with Clr7, Clr8, and Pcu4.
Correlations between gene expression levels and positioning within the nucleus have been documented in several systems. In budding yeast, tethering genes to the nuclear envelope leads to their transcriptional repression (Andrulis et al. 1998) and mutants with aberrant nuclear architecture are deficient in transcriptional silencing (Teixeira et al. 2002). The nuclear porin Nup189 that we identified by its interaction with the silencing factor Clr8 is the homolog of budding-yeast Nup145 and mammalian Nup98. Nup145 has been proposed to help localize budding-yeast telomeres to the nuclear periphery by docking Mlp2, which in turn interacts with the telomere-binding protein Yku70 (Galy et al. 2000). Similarly, Nup98 is associated with a protein network formed by the Mlp2 homolog TPR (Fontoura et al. 2001). Consistent with their being tethered to the nuclear membrane, fission yeast heterochromatic regions are close to the nuclear periphery (Pidoux et al. 2000; Kniola et al. 2001; Appelgren et al. 2003). In the case of centromeres, this localization is partly brought about by an interaction between central cores and the spindle pole body, involving the kinetochore protein Ndc80 (Kniola et al. 2001; Appelgren et al. 2003). However, centromeres do not relocalize to the central part of the nucleus even when the central core-spindle pole body interaction is genetically ablated (Appelgren et al. 2003), which is consistent with the existence of other types of tethering, possibly via Nup189. Localization of heterochromatin at the inner nuclear membrane near nuclear pore complexes would place it in close proximity to the proteasome (Wilkinson et al. 1998), conceivably facilitating the degradation of ubiquitinated substrates.
While this work was under review, two related articles appeared in the literature (Horn et al. 2005; Li et al. 2005). SPCC970.07c (clr7+) and SPCC613.12c (clr8+) were identified in a screen for factors essential to the localization of Swi6 and they were called, respectively, dos2+ and dos1+ (delocalization of Swi6; Li et al. 2005). SPCC970.07c, SPCC613.12c, and Pcu4 were identified in a complex copurifying with Rik1 by Horn et al. (2005), who named SPCC970.07c and SPCC613.12c, respectively, raf2+ and raf1+ (Rik1-interacting factor). While the results reported in the two articles are generally in excellent agreement with our own observations, Horn et al. (2005) suggested that Pcu4 might not be required for histone H3 lysine 9 methylation. This conclusion was based on the phenotype of a pcu4+ strain expressing a Pcu4 protein mutated in its neddylation site (Pcu4-K680R) from a plasmid. The differential phenotypes of the plasmid-borne pcu4-K680R gene expressed in a pcu4+ background (Horn et al. 2005) and the pcu4Δ allele (this study) might reflect the fact that pcu4-K680R is not truly dominant negative or, possibly, a weak requirement for neddylation in the heterochromatin-building function of Pcu4. Regardless of the cause of the phenotypic differences, this study establishes that Pcu4 mediates histone H3 lysine 9 methylation in fission yeast. Considering that the pathways of heterochromatin formation are tightly conserved between fission yeast and higher eukaryotes, Cullin 4-containing complexes homologous to the fission yeast complex are likely to operate in other organisms to mediate the action of histone H3 lysine 9 methyltransferases.
Acknowledgments
We thank Robin Allshire, Tony Carr, Pernilla Bjerling, and Inga Sig Nielsen-Nørby for strains and Michael Lisby for help with the microscopy. Our research was sponsored by the Danish Research Council and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement from the United States Government.
References
- Akamatsu, Y., D. Dziadkowiec, M. Ikeguchi, H. Shinagawa and H. Iwasaki, 2003. Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc. Natl. Acad. Sci. USA 100: 15770–15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire, R. C., J. P. Javerzat, N. J. Redhead and G. Cranston, 1994. Position effect variegation at fission yeast centromeres. Cell 76: 157–169. [DOI] [PubMed] [Google Scholar]
- Allshire, R. C., E. R. Nimmo, K. Ekwall, J. P. Javerzat and G. Cranston, 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9: 218–233. [DOI] [PubMed] [Google Scholar]
- Andrulis, E. D., A. M. Neiman, D. C. Zappulla and R. Sternglanz, 1998. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 394: 592–595. [DOI] [PubMed] [Google Scholar]
- Appelgren, H., B. Kniola and K. Ekwall, 2003. Distinct centromere domain structures with separate functions demonstrated in live fission yeast cells. J. Cell Sci. 116: 4035–4042. [DOI] [PubMed] [Google Scholar]
- Arcangioli, B., and G. Thon, 2004. Mating-type cassettes: structure, switching and silencing, pp. 129–147 in The Molecular Biology of Schizosaccharomyces pombe: Genetics, Genomics and Beyond, edited by R. Egel. Springer-Verlag, Berlin/Heidelberg, Germany/New York.
- Ayoub, N., I. Goldshmidt and A. Cohen, 1999. Position effect variegation at the mating-type locus of fission yeast: a cis-acting element inhibits covariegated expression of genes in the silent and expressed domains. Genetics 152: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas et al., 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromodomain. Nature 410: 120–124. [DOI] [PubMed] [Google Scholar]
- Bondar, T., A. Ponomarev and P. Raychaudhuri, 2004. Ddb1 is required for the proteolysis of the Schizosaccharomyces pombe replication inhibitor Spd1 during S phase and after DNA damage. J. Biol. Chem. 279: 9937–9943. [DOI] [PubMed] [Google Scholar]
- Cam, H., T. Sugiyama, E. S. Chen, X. Chen, P. Fitzgerald et al., 2005. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 37: 809–819. [DOI] [PubMed] [Google Scholar]
- Craven, R. A., D. J. Griffiths, K. S. Sheldrick, R. E. Randall, I. M. Hagan et al., 1998. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene 221: 59–68. [DOI] [PubMed] [Google Scholar]
- Ekwall, K., and J. F. Partridge, 1999. Fission yeast chromosome analysis: fluorescence in-situ hybridization (FISH) and chromatin immunoprecipitation (CHIP), pp. 47–57 in Chromosome Structural Analysis: A Practical Approach, edited by W. Bickmore. Oxford University Press, Oxford.
- Ekwall, K., and T. Ruusala, 1994. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics 136: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall, K., J. P. Javerzat, A. Lorentz, H. Schmidt, G. Cranston et al., 1995. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269: 1429–1431. [DOI] [PubMed] [Google Scholar]
- Ekwall, K., E. R. Nimmo, J. P. Javerzat, B. Borgstrom, R. Egel et al., 1996. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J. Cell Sci. 109: 2637–2648. [DOI] [PubMed] [Google Scholar]
- Fontoura, B. M., S. Dales, G. Blobel and H. Zhong, 2001. The nucleoporin Nup98 associates with the intranuclear filamentous protein network of TPR. Proc. Natl. Acad. Sci. USA 98: 3208–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy, V., J. C. Olivo-Marin, H. Scherthan, V. Doye, N. Rascalou et al., 2000. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature 403: 108–112. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I., and A. J. Klar, 1996. Chromosomal inheritance of epigenetic states in fission yeast during mitosis and meiosis. Cell 86: 95–101. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I., and A. J. Klar, 1997. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics 146: 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman, R., J. Polanowska, I. Kuraoka, J. Sawada, M. Saijo et al., 2003. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113: 357–367. [DOI] [PubMed] [Google Scholar]
- Hall, I. M., G. D. Shankaranarayana, K. Noma, N. Ayoub, A. Cohen et al., 2002. Establishment and maintenance of a heterochromatin domain. Science 297: 2232–2237. [DOI] [PubMed] [Google Scholar]
- Hansen, K. R., G. Burns, J. Mata, T. A. Volpe, R. A. Martienssen et al., 2005. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol. Cell. Biol. 25: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, C., S. Katayama, S. Dhut, D. Chen, N. Jones et al., 2005. SCF(Pof1)-ubiquitin and its target Zip1 transcription factor mediate cadmium response in fission yeast. EMBO J. 24: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg, C., O. Fleck, H. A. Hansen, C. Liu, R. Slaaby et al., 2005. Ddb1 controls genome stability and meiosis in fission yeast. Genes Dev. 19: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, P. J., J. N. Bastie and C. L. Peterson, 2005. A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev. 19: 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, A. V., M. J. Bonaduce, S. V. Ivanov and A. J. Klar, 1998. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat. Genet. 19: 192–195. [DOI] [PubMed] [Google Scholar]
- James, P., J. Halladay and E. A. Craig, 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144: 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, S., K. Noma and S. I. Grewal, 2004. a RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304: 1971–1976. [DOI] [PubMed] [Google Scholar]
- Jia, S., T. Yamada and S. I. Grewal, 2004. b Heterochromatin regulates cell type-specific long-range chromatin interactions essential for directed recombination. Cell 119: 469–480. [DOI] [PubMed] [Google Scholar]
- Kao, C. F., C. Hillyer, T. Tsukuda, K. Henry, S. Berger et al., 2004. Rad6 plays a role in transcriptional activation through ubiquitylation of histone H2B. Genes Dev. 18: 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, S., K. Kitamura, A. Lehmann, O. Nikaido and T. Toda, 2002. Fission yeast F-box protein Pof3 is required for genome integrity and telomere function. Mol. Biol. Cell 13: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. S., E. S. Choi, J. A. Shin, Y. K. Jang and S. D. Park, 2004. Regulation of Swi6/HP1-dependent heterochromatin assembly by cooperation of components of the mitogene-activated protein kinase pathway and a histone deacetylase Clr6. J. Biol. Chem. 279: 42850–42859. [DOI] [PubMed] [Google Scholar]
- Kniola, B., E. O'Toole, J. R. McIntosh, B. Mellone, R. Allshire et al., 2001. The domain structure of centromeres is conserved from fission yeast to humans. Mol. Biol. Cell 12: 2767–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., D. B. Goto, M. Zaratiegui, X. Tang, R. Martienssen et al., 2005. Two novel proteins, Dos1 and Dos2, interact with Rik1 to regulate heterochromatic RNA interference and histone modification. Curr. Biol. 15: 1448–1457. [DOI] [PubMed] [Google Scholar]
- Liu, C., K. A. Powell, K. Mundt, L. Wu, A. M. Carr et al., 2003. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 17: 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz, A., K. Ostermann, O. Fleck and H. Schmidt, 1994. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene 143: 139–143. [DOI] [PubMed] [Google Scholar]
- Motamedi, M. R., A. Verdel, S. U. Colmenares, S. A. Gerber, S. P. Gygi et al., 2004. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119: 789–802. [DOI] [PubMed] [Google Scholar]
- Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis and S. I. Grewal, 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292: 110–113. [DOI] [PubMed] [Google Scholar]
- Neuwald, A. F., and A. Poleksic, 2000. PSI-BLAST searches using hidden Markov models of structural repeats: prediction of an unusual sliding DNA clamp and of beta-propellers in UV-damaged DNA-binding protein. Nucleic Acids Res. 28: 3570–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, I. S., O. Nielsen, J. M. Murray and G. Thon, 2002. The fission yeast ubiquitin-conjugating enzymes UbcP3, Ubc15, and Rhp6 affect transcriptional silencing of the mating-type region. Eukaryot. Cell 1: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo, E. R., G. Cranston and R. C. Allshire, 1994. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 13: 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, K., C. D. Allis and S. I. Grewal, 2001. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293: 1150–1155. [DOI] [PubMed] [Google Scholar]
- Noma, K., T. Sugiyama, H. Cam, A. Verdel, M. Zofall et al., 2004. RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat. Genet. 36: 1174–1180. [DOI] [PubMed] [Google Scholar]
- Partridge, J. F., K. S. Scott, A. J. Bannister, T. Kouzarides and R. C. Allshire, 2002. cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr. Biol. 12: 1652–1660. [DOI] [PubMed] [Google Scholar]
- Petrie, V. J., J. D. Wuitschick, C. D. Givens, A. M. Kosinski and J. F. Partridge, 2005. RNA interference (RNAi)-dependent and RNAi-independent association of the Chp1 chromodomain protein with distinct heterochromatic loci in fission yeast. Mol. Cell. Biol. 25: 2331–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski, M. D., and R. J. Deshaies, 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6: 9–20. [DOI] [PubMed] [Google Scholar]
- Pidoux, A. L., and R. C. Allshire, 2004. Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res. 12: 521–534. [DOI] [PubMed] [Google Scholar]
- Pidoux, A. L, S. Uzawa, P. E. Perry, W. Z. Cande and R. C. Allshire, 2000. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J. Cell Sci. 113: 4177–4191. [DOI] [PubMed] [Google Scholar]
- Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun et al., 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599. [DOI] [PubMed] [Google Scholar]
- Reinhart, B. J., and D. P. Bartel, 2002. Small RNAs correspond to centromere heterochromatic repeats. Science 297: 1831. [DOI] [PubMed] [Google Scholar]
- Rose, M., F. Winston and P. Hieter, 1990. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sadaie, M., T. Iida, T. Urano and J. Nakayama, 2004. A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J. 23: 3825–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyanov, P., A. Nag and P. Raychaudhuri, 1999. Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J. Biol. Chem. 274: 35309–35312. [DOI] [PubMed] [Google Scholar]
- Singh, J., V. Goel and A. J. S. Klar, 1998. A novel function of the DNA repair gene rhp6 in mating-type silencing by chromatin remodeling in fission yeast. Mol. Cell. Biol. 18: 5511–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelter, P., and H. D. Ulrich, 2003. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425: 188–191. [DOI] [PubMed] [Google Scholar]
- Sun, Z. W., and C. D. Allis, 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418: 104–108. [DOI] [PubMed] [Google Scholar]
- Tange, Y., A. Hirata and O. Niwa, 2002. An evolutionarily conserved fission yeast protein, Ned1, implicated in normal nuclear morphology and chromosome stability, interacts with Dis3, Pim1/RCC1 and an essential nucleoporin. J. Cell Sci. 115: 4375–4385. [DOI] [PubMed] [Google Scholar]
- Teixeira, M. T., B. Dujon and E. Fabre, 2002. Genome-wide nuclear morphology screen identifies novel genes involved in nuclear architecture and gene-silencing in Saccharomyces cerevisiae. J. Mol. Biol. 321: 551–561. [DOI] [PubMed] [Google Scholar]
- Thon, G., and T. Friis, 1997. Epigenetic inheritance of transcriptional silencing and switching competence in fission yeast. Genetics 145: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., and A. J. Klar, 1992. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics 131: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., and A. J. Klar, 1993. Directionality of fission yeast mating-type interconversion is controlled by the location of the donor loci. Genetics 134: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., and J. Verhein-Hansen, 2000. Four chromo-domain proteins of Schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 155: 551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., A. Cohen and A. J. Klar, 1994. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., K. P. Bjerling and I. S. Nielsen, 1999. Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151: 945–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, G., P. Bjerling, C. M. Bunner and J. Verhein-Hansen, 2002. Expression-state boundaries in the mating-type region of fission yeast. Genetics 161: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzon, C. T., B. Borgstrøm, D. Weilguny, R. Egel, J. P. Cooper et al., 2004. The fission yeast heterochromatin protein Rik1 is required for telomere clustering during meiosis. J. Cell Biol. 165: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe, T. A., C. Kidner, I. M. Hall, G. Teng, S. I. Grewal et al., 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297: 1833–1837. [DOI] [PubMed] [Google Scholar]
- Wilkinson, C. R., M. Wallace, M. Morphew, P. Perry, R. Allshire et al., 1998. Localization of the 26S proteasome during mitosis and meiosis in fission yeast. EMBO J. 17: 6465–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, A., K. Maundrell, W. D. Heyer, D. Beach and P. Nurse, 1986. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid 15: 156–158. [DOI] [PubMed] [Google Scholar]