Abstract
Receptors for acid hydrolases destined for the lytic compartment in yeast and mammalian cells are retrieved from intermediate, endosomal organelles with the help of a pentameric protein complex called the retromer. We cloned the Arabidopsis thaliana homologs of the three yeast proteins (Vps35, Vps29, and Vps26) constituting the larger subunit of retromer and prepared antisera against them. With these antibodies, we demonstrated the presence of a retromer-like protein complex in salt extracts prepared from Arabidopsis microsomes. This complex is associated with membranes that coequilibrate with prevacuolar compartment markers and with high-density sedimenting membranes. Immunogold negative staining identified these membranes as 90-nm-diameter coated microvesicles. Confocal laser scanning immunofluorescence studies performed on tobacco (Nicotiana tabacum) BY-2 cells revealed high degrees of colabeling between all three retromer antisera and the prevacuolar compartment (PVC) markers PEP12 and vacuolar sorting receptor VSRAt-1. The presence of plant retromer at the surface of multivesicular bodies was also demonstrated by immunogold labeling of sections obtained from high-pressure frozen/freeze-substituted specimens. Treatment of BY-2 cells with wortmannin led to swelling of the PVC and a separation of the VPS35 and VSR signals. Preliminary data suggesting that retromer interacts with the cytosolic domain of a VSR were obtained by immunoprecipitation experiments performed on detergent-solubilized microsomes with Vps35 antibodies.
INTRODUCTION
Anterograde trafficking of acid hydrolases to the lytic compartment is superficially similar in the three major classes of eukaryotic organisms. The major route involves a receptor-mediated sorting event at the trans-Golgi network (TGN), packaging into clathrin-coated vesicles with the help of an AP1-adaptor complex, and receptor-ligand dissociation in a multivesicular prevacuolar/endosomal compartment (PVC) before delivery into the lysosome/vacuole. This transport pathway is well known for mammalian (Doray et al., 2002; Ghosh et al., 2003; Gruenberg and Stenmark, 2004) and yeast (Wurmser and Emr, 1998; Costaguta et al., 2001; Deloche et al., 2001) cells and has now been established for plants (Paris and Neuhaus, 2002; Juergens, 2004; Tse et al., 2004). A second route, bypassing the PVC and involving the AP3-adaptor complex, but not clathrin, has been shown to be responsible for the transport of some enzymes, such as alkaline phosphatase, to the vacuole in yeast (e.g., Stepp et al., 1997). This second pathway also exists in mammalian cells (see review in Odorizzi et al., 1998). AP3-adaptins exist in the plant database, but a corresponding pathway remains to be established for plants.
Although the receptors responsible for the sorting of acid hydrolases at the TGN in the various eukaryotic organisms have different structures, they have in common motifs (Tyr and/or dileucine) in their cytoplasmic tails required for interaction with the μ-adaptins of the AP1-adaptor complex. This is true for the mannosyl 6-phosphate and sortilin receptors (Bonifacino and Traub, 2003; Hassan et al., 2004) in mammals, the Vps10p receptor in yeast (Dennes et al., 2002), and the BP-80–type vacuolar sorting receptors (VSRs) of plants (Happel et al., 2004). Another feature shared, at least, by the mammalian and yeast receptors is that they are recycled from the PVC to the TGN with the help of a cytosolic protein complex called the retromer (Seaman et al., 1998; Arighi et al., 2004; Seaman, 2004, 2005).
Retromer is a pentameric structure consisting of two subunits. In yeast, the proteins Vps35p, Vps29p, and Vps26p constitute the large subunit, with Vps17p and Vps5p making up the small subunit. The Vps10p receptor interacts with retromer directly via Vps35p, with Vps26p acting as a linker to the membrane of the PVC/endosomal compartment (Reddy and Seaman, 2001). Vps17p and Vps5p are members of the sorting nexin (SNX) family of proteins, which characteristically possess Phox homology (PX) domains allowing for interactions with phosphatidylinositol 3-phosphate (Yu and Lemmon, 2001). In addition, Vps17p and Vps5p have BAR (coiled-coil) domains in their C-terminal regions enabling the dimerization of these two proteins (Seaman and Williams, 2002). The smallest protein is Vps29p, which appears to function as a linker between Vps35p and the Vps17p/Vps5p dimer (Reddy and Seaman, 2001). Close homologs to four of these proteins have been identified in mammalian cells, except for Vps17p, whose function seems to be assumed by SNX2 (Haft et al., 2000). Although not yet identified, it is implicit in most studies on retromer that receptor retrieval from the PVC involves the formation of a retromer-coated vesicle. However, unlike other coated vesicles (clathrin, COPI, and COPII) the formation of retromer-coated vesicles does not seem to require a GTPase (e.g., Arf1 or Sar1) for coat recruitment (Seaman, 2005).
We have previously identified multivesicular bodies as being the PVC of suspension-cultured tobacco (Nicotiana tabacum) BY-2 cells and have characterized them as being enriched in the VSRAt-1 (Li et al., 2002; Tse et al., 2004). It seemed a logical next step to address the question as to the recycling of this receptor. We therefore embarked upon a series of experiments designed to determine whether plant cells express a retromer-like complex and where this complex is localized. To this end, we have cloned Arabidopsis thaliana homologs to Vps35p, Vps29p, and Vps26p and generated antibodies against the respective recombinant proteins. With these tools we have been able to identify biochemically a membrane binding retromer-like protein complex. Immunofluorescence confocal microscopy and immunogold electron microscopy have identified the organelle with which retromer associates as being the PVC. This was supported through an analysis of sucrose density gradients, which in addition allowed us to detect retromer-coated vesicles.
RESULTS
Identification of Plant Retromer Homologs and Cross-Reactivity of Antisera
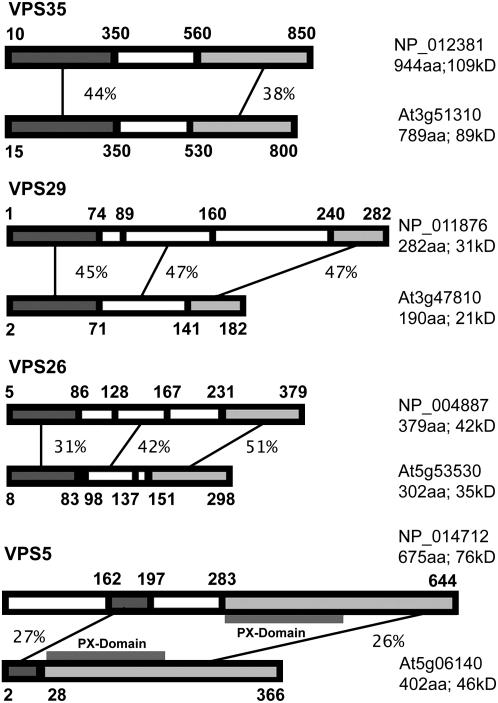
We have conducted BLAST searches in the Arabidopsis database for sequences homologous to yeast retromer proteins. Three isoforms for VPS35 were found, one for VPS29, two for VPS26, and three for VPS5 (see Methods for accession numbers). Depending on the domain, the identity ranged between 25% (VPS35) and 51% (VPS26). The PX domain, characteristic of Vps5p was also present in Arabidopsis VPS5. A sequence corresponding to Vps17p could not be found. This possibly reflects the situation in mammalian cells, where recent studies have cast doubt on the belief that SNX2 (the putative homolog to Vps17p) is a component of the retromer complex (Gullapalli et al., 2004; Seaman, 2005). On the other hand, as well as containing the Val residue (109V), which is critical to Vps35 interaction, the plant VPS29 sequence (At3g47810) also contains the site-I His residues (10H and 118H) and the water-bridging residue Asn (39N), which are present in mammalian Vps29 and are typical of divalent metal-containing phosphoesterases (Collins et al., 2005; Wang et al., 2005). Interestingly, whereas yeast Vps29p lacks the mammalian metal binding site-II His and Asp residues (86H, 62D, and 116H), the plant VPS29 sequence only lacks the Asp residue. In both yeast and plants, this is substituted by Glu.
For the purposes of recombinant expression in Escherichia coli, we selected those sequences with the highest levels of identity to the corresponding yeast partner (Figure 1). Polyclonal antibodies were generated against recombinant VPS35, VPS29, and VPS26 and tested on total membrane and cytosol fractions prepared from Arabidopsis suspension cultures and from bakers' yeast (Figure 2A). With equal amounts of loaded proteins, the signals obtained with VPS35 and VPS29 antibodies were much greater for membrane as opposed to cytosolic fractions. However, with VPS26 antibodies, the cytosolic signal was higher. The observed molecular masses for the three proteins matched exactly with the calculated masses (91 kD for VPS35, 21 kD for VPS29, and 35 kD for VPS35). These are all smaller than the yeast retromer proteins Vps35p (109 kD), Vps29p (31 kD), and Vps26p (42 kD). Only with the VPS26 antiserum did we observe a cross-reaction with the corresponding protein in yeast (Figure 2C). Yeast Vps35p antibodies did not recognize any polypeptide in plant extracts (Figure 2B).
Figure 1.
Comparisons of Yeast and Arabidopsis Retromer Proteins.
The percentage score is given for the different parts of the protein schematics showing the highest identities. Numbers in bold denote amino acid positions. Numbers of residues and estimated molecular masses are indicated. aa, amino acids.
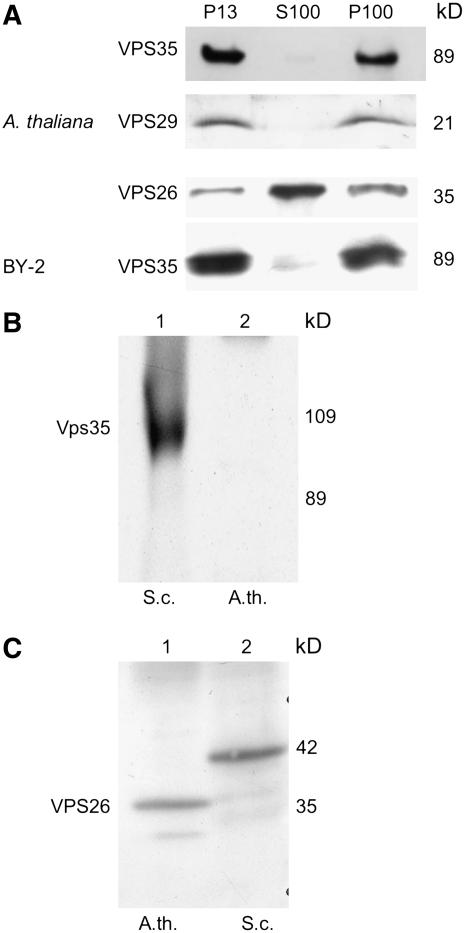
Figure 2.
Reactivity of Retromer Antibodies and Localization of Retromer Proteins in Subcellular Fractions of Arabidopsis and Tobacco BY-2 Cells.
P13 is the membrane pellet after a 13,000g centrifugation for 20 min; P100 is the pellet resulting from a subsequent 1-h centrifugation at 100,000g with the P13 supernatant; S100 is the resulting supernatant after 100,000g centrifugation. Protein gel blots were probed with VPS35 or VPS26 antibodies from Arabidopsis or yeast (Vps35p) recombinant proteins. Forty micrograms of protein was loaded in each lane.
Distribution of Retromer Proteins in Subcellular Fractions
We decided to follow the fractionation protocol of Seaman et al. (1998) and Nothwehr et al. (2000) to be able to make direct comparisons with data on the subcellular distribution of retromer in yeast. Accordingly, filtered homogenates of Arabidopsis and tobacco BY-2 cells were subjected to sequential centrifugations at 13,000g and 100,000g. The three fractions (P13 and P100 pellets and S100 supernatant) were subjected to protein gel blotting with VPS35, VPS29, and VPS26 antibodies. As seen in Figure 2, VPS35 and VPS29 are present primarily in both membrane fractions, with only small amounts detectable in the S100 cytosol fraction. This was also the case when BY-2 subcellular fractions were tested with the VPS35 antibodies (Figure 2). By contrast, considerably more VPS26 antigen was detected in the S100 than in the two membrane fractions. This closely resembles the distribution of Vps35p, Vps29p, and Vps26p in the corresponding fractions from yeast (Paravicini et al., 1992; Seaman et al., 1998). It has been proposed that Vps35p constitutively binds to the PVC acting to recruit the receptor Vps10p. Vps29p (and probably also Vps26p) are subsequently attached to the membrane and act to oligomerize the Vps35p/Vps10p complexes into budding domains (Seaman et al., 1998). This would then explain the disproportionate differences in retromer proteins in cytosolic versus membrane fractions.
Solubilized VPS35, VPS29, and VPS26 Remain Together as a Subcomplex
To ascertain the nature of the membrane association of retromer proteins, the P100 pellet of Arabidopsis was resuspended in different solutions as shown in Figure 3, rotated for 60 min, and then recentrifuged at 100,000g. The new pellet and the supernatant were screened for the presence of VPS35 proteins by protein gel blotting. A substantial proportion of VPS35 antigen dissociated from the membranes at low salt (250 mM NaCl) concentrations. Complete release and solubilization can be achieved with either 2 M urea or detergent (1% Triton X-100) treatments. This is a typical property for membrane extrinsic proteins, such as COPI/II-vesicle coat proteins (Movafeghi et al., 1999), and agrees with the solubilization data given previously for some of the proteins of yeast retromer (Horazdovsky et al., 1997; Seaman et al., 1998).
Figure 3.
Dissociation of the Large Retromer Subunit from Arabidopsis P100 Membranes.
Arabidopsis P100 membrane proteins were resuspended in homogenizing medium containing either 0.25 M NaCl, 1 M NaCl, 0.1 M Na2CO3, 2 M urea, or 1% Triton X-100 (protein end concentration: 5 mg/mL) and were incubated on a shaker for 60 min at 4°C. The resulting suspension was recentrifuged for 60 min at 100,000g, providing pellet (P) and solubilized fractions (S). For protein gel blotting, 20 μg of protein was loaded in each lane, and the corresponding blot was probed with VPS35 antibodies.
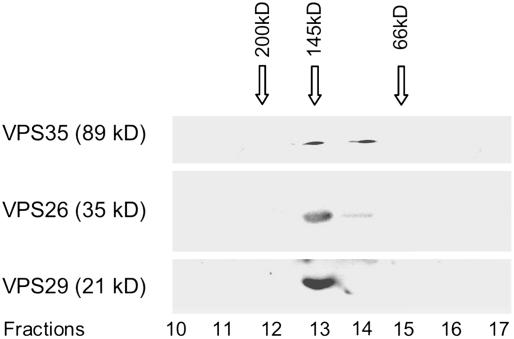
The proteins released by 250 mM NaCl were then subjected to gel filtration chromatography on Superdex 200 and the eluted fractions monitored for VPS35, VPS29, and VPS26 by protein gel blotting (Figure 4). All three retromer proteins were detected in a fraction with a size of ∼150 kD. This corresponds to the sum of these three retromer components and indicates that they remain together upon salt-induced dissociation from the membrane surface. These results are also in agreement with those of Seaman et al. (1998), who showed that Vps35p coeluted with Vps29p in a complex of ∼230 kD and that Vps5p and Vps17p eluted together in larger multimeric complexes of ∼500 kD.
Figure 4.
Superdex 200 Column Separation of a Salt-Dissociated Arabidopsis P100 Membrane Extract.
Arabidopsis P100 membranes were treated with 250 mM NaCl, and the solubilized proteins separated on a Superdex 200 column. Fractions (0.5 mL) were collected and the proteins precipitated and subjected to protein gel blotting with VPS35, VPS29, and VPS26 antisera. The gel filtration column was calibrated with molecular size markers under identical elution conditions.
Retromer Binds to PVC Membranes and Possibly to Microvesicles
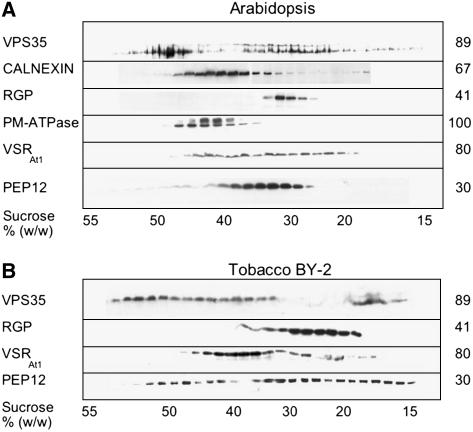
To obtain evidence on the identity of the retromer binding membranes, we performed isopycnic centrifugations of P100 membranes from Arabidopsis and BY-2 cells on linear sucrose density gradients (Figures 5A and 5B). Once again, there is a great similarity in the equilibrium distributions of VPS35 between plant (especially in the case of Arabidopsis P100 membranes) and yeast gradients (Figure 5; Seaman et al., 1998). Essentially, VPS35 collects in two regions of the gradient: a major peak around 47 to 52% sucrose and a broad band between 30 and 42% sucrose, which corresponds to the densities of VSRAt-1 and PEP12(SYP21)-bearing membranes. The VPS35 profiles for BY-2 cells are less clearly delineated into two separate peaks than for Arabidopsis but cover the same stretch of the gradient. The PEP12 and VSRAt-1 distributions are also broader but still correspond to the less dense VPS35-positive fractions.
Figure 5.
Sedimentation Characteristics of Retromer Binding Membranes in Sucrose Gradients.
(A) Arabidopsis P100 membranes were separated on an isopycnic 15 to 55% (w/w) linear sucrose gradient, and the precipitated fractions were protein gel blotted with antibodies against the ER membrane marker calnexin, the Golgi marker RGP (reverse glycosylated protein), the plasma membrane marker PM-ATPase, the prevacuolar markers VSRAt-1 and PEP12 (SYP21), and VPS35 antibodies.
(B) As for (A) but with tobacco BY-2 P100 membranes.
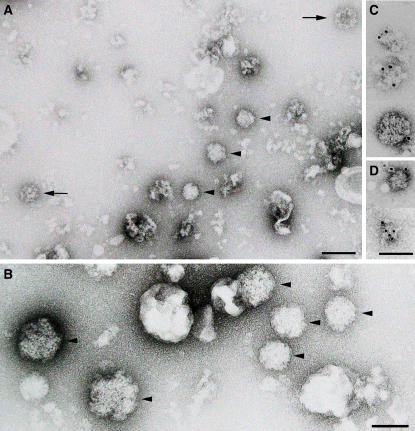
Because the majority of plant membranes, including the plasma membrane, have equilibrium densities <44% sucrose, we decided to examine the composition of the high-density VPS35-containing fractions by negative staining (Figure 6). In addition to unspecified membrane fragments, this fraction contained clearly identifiable clathrin-coated vesicles but also a population of fuzzy-coat structures, which at a diameter of ∼90 nm were slightly larger than the clathrin-coated vesicles. Since these structures reacted positively toward VPS35 and VPS26 antibodies in immunogold negative staining (Figure 6C), we tentatively claim to have identified retromer-coated vesicles. These fractions were also probed with antibodies against SEC13 and SEC21 (coat proteins on COPII and COPI vesicles, respectively; Movafeghi et al., 1999; Yang et al., 2005) but did not label the fuzzy-coat structures.
Figure 6.
Negative Contrast Immunographs of High-Density Sucrose Fractions.
Arabidopsis P100 membranes were centrifuged under isopycnic conditions in a 15 to 55% (w/w) linear sucrose gradient. High-density fractions (47 to 50%) corresponding to the strong VPS35 signals in protein gel blots at this density (Figures 5A and 5B) were negatively stained. (A) is an overview, (B) is a high-magnification detail, and (C) and (D) show putative retromer vesicles positively stained with VPS35 and VPS26 immunogold, respectively. The arrows point to clathrin-coated vesicles; the arrowheads label putative retromer-coated vesicles. Bars = 100 nm.
Retromer Localizes to Multivesicular Bodies/PVC in Situ
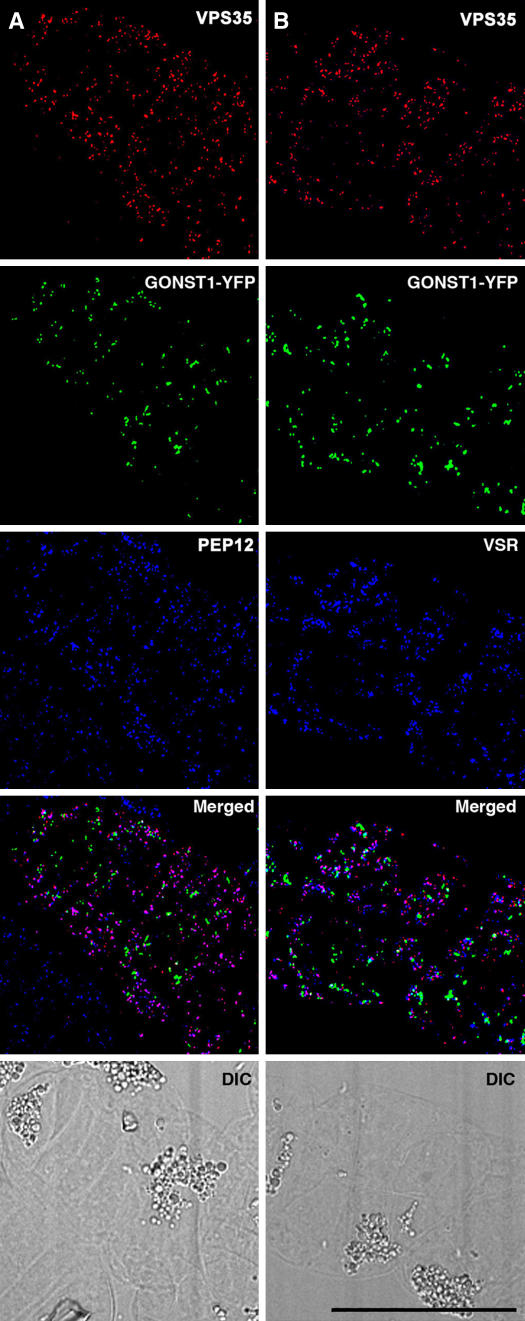
We performed immunofluorescence microscopy with protein-G purified VPS35, VPS29, and VPS26 antibodies on transgenic tobacco BY-2 cell lines stably expressing either yellow fluorescent protein Golgi (GONST1-YFP) or green fluorescent protein PVC (VSR-GFP) markers. As seen in Figure 7A, although there is hardly any colocalization between VPS35 and the Golgi marker, a substantial colocalization was registered with the PVC SNARE PEP12 (antibody staining). Exactly the same combination of labeling data was obtained when the distribution of VPS35 was compared with VSR by double antibody staining (Figure 7B). The specificity of the VPS35 antibody staining was supported by control labeling experiments with preimmune serum and by substituting the anti-rabbit secondary antibody conjugate with fluorescent anti-goat IgGs (see Supplemental Figure 1 online). A good colocalization of VPS26 with VSR (as a GFP construct) was also obtained (see Supplemental Figure 2A online). By antibody staining with Fab fragments, we were also able to demonstrate a correspondingly high degree of overlap in the images obtained with VPS26 against VPS29 (see Supplemental Figure 2B online) and VPS29 against VPS35 (see Supplemental Figure 2C online). This indicates that these three different retromer antibodies detect one and the same set of structures and that this structure was the PVC rather than the Golgi apparatus. This is convincingly underscored by a statistical analysis of the labeling results, with only ∼2% of the retromer label associated with the Golgi apparatus in comparison with >80% with the PVC markers (Table 1).
Figure 7.
Immunofluorescent Localization of Retromer in Tobacco BY-2 Cells.
(A) Double immunostaining (VPS35 and PEP12) of a cell expressing GONST1-YFP. Although the two immunostaining patterns show a high degree of overlap, they are clearly separate from the Golgi marker. DIC, differential interference contrast.
(B) As for (A) but with immunostaining for the VSRAt-1 instead of the PVC marker PEP12. Again there is a colocalization of the retromer antibody with the PVC marker but not with the Golgi marker. Bar = 10 μm.
Table 1.
Degrees of Colocalization between Retromer Antibody Staining and Golgi and PVC Markers
| Antibodies Compared | Percentage of Colocalization (mean ± sd) | n |
|---|---|---|
| VPS35, PEP12 | 82.2% ± 1.5% | 6 |
| VPS35, GONST1 | 2.3% ± 1.7% | 6 |
| VSR, VPS26 | 83.1% ± 4.0% | 7 |
| VPS26, VPS29 | 80.9% ± 4.8% | 5 |
| VPS35, VPS29 | 81.9% ± 5.9% | 6 |
| VPS35, VSR | 82.5% ± 3.8% | 5 |
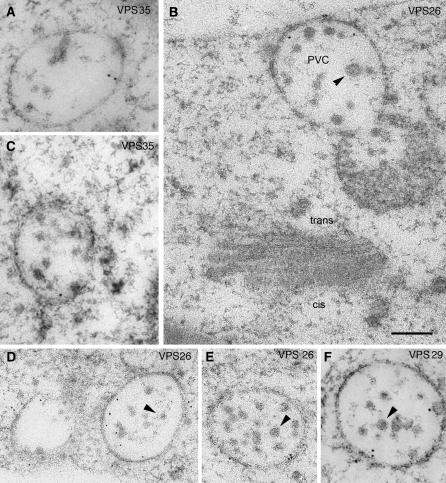
Immunogold labeling of sections prepared from high-pressure frozen/freeze-substituted BY-2 cells essentially confirmed the confocal immunofluorescence data. With labeling conditions adjusted to minimize unspecific background staining, gold particles were almost exclusively restricted to multivesicular bodies (MVBs; Figure 8), structures known to label positively for VSR and PEP12 (Tse et al., 2004). Gold label was found on or near the boundary membrane of the MVB, with little present on the interior vesicles, a situation similar to that demonstrated by us for VSRAt-1 in the MVB of BY-2 cells (Tse et al., 2004).
Figure 8.
Immunogold Localization of Retromer to MVBs in Tobacco BY-2 Cells.
Thin sections were prepared from high-pressure frozen/freeze-substituted specimens and immunostained with antibodies against the three proteins of the large retromer subunit (VPS35, VPS29, and VPS26). Labeling tends to be found at the surface of the multivesicular body. Arrowheads point to internal vesicles that are seldom labeled. Bar = 200 nm.
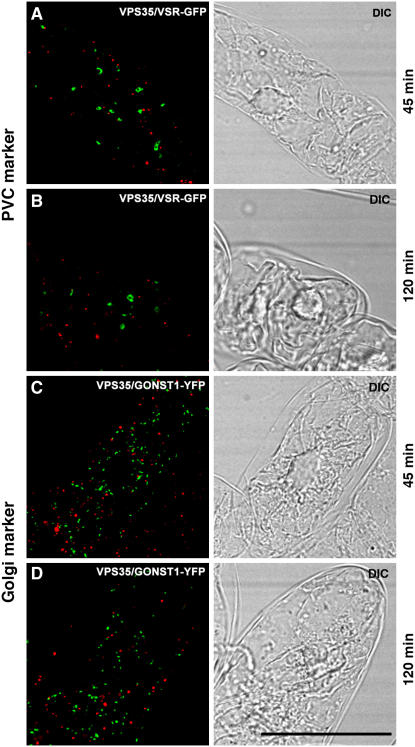
Wortmannin Treatment Separates VPS35 and VSRAt-1
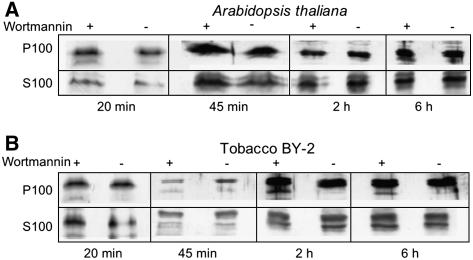
The phosphatidylinositol-3 (PI-3) kinase inhibitor wortmannin targets to multivesicular endosomes/PVC and leads to a swelling of this compartment (for references, see Tse et al., 2004; daSilva et al., 2005). To determine whether the signals for VSRAt-1 and VPS35 remained colocalized on the PVC, we therefore performed immunofluorescence microscopy on BY-2 cells treated with a concentration of wortmannin (16 μM) that was known to cause changes in PVC size and morphology (Tse et al., 2004). As previously reported, wortmannin caused the VSRAt-1 signal to change from punctate to an enlarged halo (Tse et al., 2004). This effect was not shared by the VPS35 signal, which no longer colocalized with the VSRAt-1 signal (Figure 9).
Figure 9.
Wortmannin Alters the Distribution of Retromer in Tobacco BY-2 Cells.
BY-2 cells expressing either the fluorescent PVC (VSR-GFP) or Golgi (GONST1-YFP) markers were treated with wortmannin (16 μM) for the times given, and then samples were removed and immunostained with VPS35 antibodies. Wortmannin caused a swelling of the PVC, as previously described by us (Tse et al., 2004), but also led to a separation of the PVC and retromer signals.
This observation suggested wortmannin was causing the large, VPS35-containing retromer subunit to be displaced from the surface of the PVC. We therefore isolated S100 and P100 fractions from control- and wortmannin-treated Arabidopsis and BY-2 cells and probed protein gel blots with VPS35 antibodies (Figure 10). However, at all times tested (from 20 min to 6 h), there was no significant difference in the relative signal strengths for cytosolic and membrane-bound VPS35 antigen between control and drug-treated samples (Figure 10). This indicates that wortmannin did not cause the release of VPS35 from the surface of the MVB. These protein gel blots were also performed with anti-VPS29 and anti-VPS26 with very similar results (data not shown). We surmised that the separation of the VSRAt-1 and VPS35 signals reflected their spatial redistribution to different domains in the membrane of the PVC. This notion is supported by measurements that we have made on the average separation of the two signals (Table 2). This value (1 μm) lies within the diameter that we estimate the PVC to attain after wortmannin-induced dilation, since the diameter of the multivesicular endosome/PVC in control cells is ∼0.5 μm (Tse et al., 2004). Interestingly, the average distance separating the PVC and the Golgi apparatus in such cells is considerably less (0.6 μm).
Figure 10.
Wortmannin Does Not Lead to a Release of the Large Subunit of Retromer into the Cytosol.
Arabidopsis (A) and tobacco BY-2 (B) cells were incubated with wortmannin (16 μm) for the periods indicated and then fractionated into S100 and P100 fractions as described in Methods. Protein gel blots were then made from control and treated cells with 20 μg protein loaded into each lane.
Table 2.
Determination of the Average Separation of VPS35 and VSR Signals, and Golgi and VSR Signals Induced by Treatment of Tobacco BY-2 Cells with Wortmannin (16 μM, 45 min)
| No. of Cells Measured | No. of Enlarged PVCs | No. of VPS35 Marked Organelles | Average Separate Distance (μm) |
|---|---|---|---|
| 5 | 48a | 53b | 1.0 ± 0.6c |
| 5 | 61d | 68e | 0.6 ± 0.5f |
Number of enlarged YFP-BP-80–labeled PVCs.
Number of VPS35-labeled organelles that are adjacent to the enlarged PVC.
Average distance between the enlarged YFP-BP-80–labeled PVC and the nearest VPS35-labeled organelles.
Number of enlarged PVCs labeled with VSR antibodies.
Number of GONST1-YFP–labeled Golgi stacks that are adjacent to the enlarged VSR-labeled PVC.
Average distance between the enlarged VSR-labeled PVC and the nearest GONST1-YFP–labeled Golgi stacks.
VSRAt-1 Is Immunoprecipitated with VPS35 Antibodies
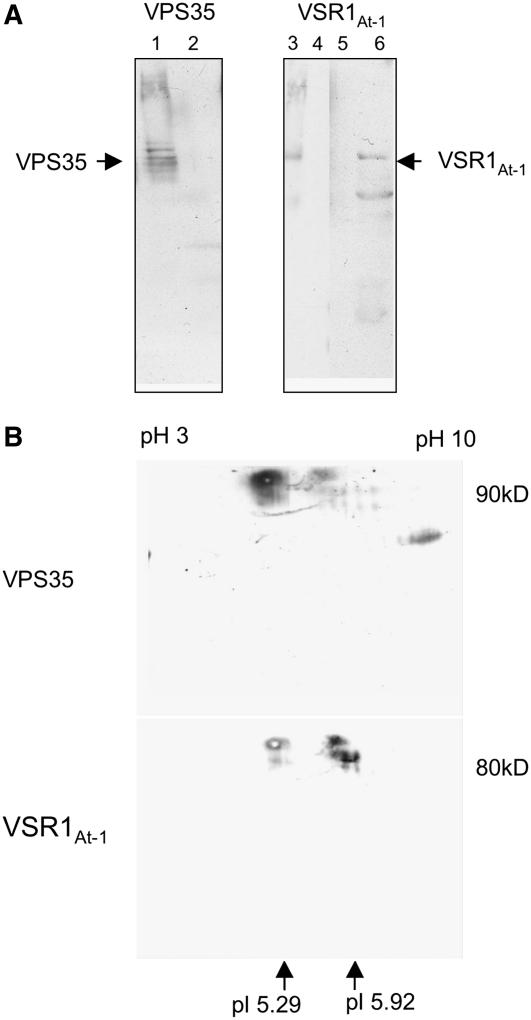
Using VPS35 antisera covalently coupled to protein-A Sepharose beads, we were able to immunoprecipitate VPS35 (Figure 11A, lane 1) and an antigen reacting with the VSRAt-1 antibodies (Figure 11A, lane 6). The protein source for these experiments was P100 membranes from the dense fractions (48 to 51% w/w) of the Arabidopsis linear sucrose gradients. These were first treated with the detergent CHAPS to solubilize the membranes, then centrifuged at 100,000g to remove a detergent-insoluble material. The supernatant was then used for the immunoprecipitation experiments. Two types of control immunoprecipitations were performed: (1) noncoupled Sepharose beads and (2) beads coupled with a different antibody (anti-γ-COP; Movafeghi et al., 1999). Neither VPS35 nor VSRAt-1 could be detected in either case (Figure 11A, lanes 2, 4, and 5). Arabidopsis dense membrane fraction served as positive control (Figure 11A, lane 3). In addition, eluates from the immunoprecipitation experiments were submitted to two-dimensional (2D) gel electrophoresis. After protein gel blot transfer of proteins in the 2D gels, membranes were probed with VPS35 antibodies and then washed and reprobed (without stripping) with VSRAt-1 antibodies (Figure 11B). In both blots, a signal was seen at the approximate expected pI and expected molecular weight for the respective proteins.
Figure 11.
VSRAt-1 Coimmunoprecipitates with Vps35 Antibodies.
(A) Fractions 5 and 6 (47 to 50% [w/w] sucrose) from an Arabidopsis P100 linear sucrose gradient were treated with CHAPS and subsequently centrifuged at 100,000g for 1 h. The supernatant was then subjected to immunoprecipitation with either VPS35 or VSRAt-1 antibodies covalently coupled to Sepharose CL-6B beads. Bound proteins were dissociated from the beads with glycine, pH 2.5, neutralized, and precipitated. Lane 1, positive control showing the precipitation of VPS35 with VPS35 antibodies; lane 2, negative control using beads coated with a nonspecific antibody (anti-γ-COP); lane 3, marker lane with detergent-solubilized membranes; lane 4, negative control as for lane 2; lane 5, negative control with uncoated beads; lane 6, eluted proteins bound to VPS35-coated beads. Protein gel blots were probed with VPS35 (lane 1) or VSRAt-1 antibodies (lanes 2 and 3). Lanes were loaded with equal amounts of protein (20 μg).
(B) VPS35 immunoprecipitated proteins from detergent-solubilized high-density P100 membranes (as in [A]) were subjected to 2D gel electrophoresis (first isoelectric focusing in a stabilized linear pH gradient followed by size separation in a 10% polyacrylamide gel) before protein gel blotting. In the 2D blot probed with VPS35 antibodies, a signal (arrow) was detected at the expected size (∼90 kD) and at the expected pI of 5.29. The blot was reprobed, without stripping, with VSRAt-1 antisera, and a signal (arrow) was seen at the expected size (∼80 kD) and at the expected pI of 5.92. Since both antisera were made in rabbits, a residual VPS35 signal was still present in (B).
DISCUSSION
Anterograde Transport to the Prelysosomal/PVC in Mammalian and Yeast Cells Is Dependent on Retromer-Mediated Receptor Recycling to the TGN
Although the mammalian mannosyl 6-phosphate receptor (MPR) and the yeast Vsp10p receptors share no sequence homology, they both rapidly recycle between the TGN and the prelysosomal/PVCs (Cooper and Stevens, 1996; Ghosh et al., 2003). This is necessary to maintain the efficient transport of soluble acid hydrolases from the TGN to the prelysosomal/PVC where low pH-mediated receptor-ligand dissociation occurs. Failure to recycle the receptors inevitably leads to a reduction in their availability at the TGN and a consequential collapse in anterograde traffic. Several lines of evidence strongly implicate retromer in receptor retrieval from the PVC in yeast and the prelysosomal compartment in mammalian cells. Vps10p retrieval from the PVC is blocked in yeast strains containing mutations in VPS35 and VPS29 genes, and anterograde traffic of the acid hydrolase carboxypeptidase Y is interrupted, with the receptor becoming localized to the vacuole (Seaman et al., 1997). Similarly, a model membrane protein A-ALP normally localized to the TGN is also redistributed to the vacuole in yeast vps35 mutant strains (Nothwehr et al., 1999).
In mammalian cells where RNA interference techniques have recently been used to reduce mammalian Vps35 and Vps26 levels, anterograde trafficking of the hydrolase cathepsin D is severely retarded (Seaman, 2004) and the cation-independent (CI)–MPR is either rapidly degraded in lysosomes or displaced to the cell surface (Arighi et al., 2004; Seaman, 2004). Moreover, a direct demonstration of the blockage of retrograde transport to the TGN caused by mVps26 knockdown has been elegantly provided by uptake studies using the T cell marker CD8 fused to CI-MPR. Normally this reporter construct reaches an mVps26-positive endosomal/prelysosomal compartment ∼8 min after being endocytosed and is subsequently transported to the TGN. However, in the mVps26 knockdown cells, the reporter remained in the prelysosomal compartment and did not reach the TGN (Seaman, 2004).
Wortmannin Perturbation of Vacuolar Protein Sorting Is an Indicator of Retromer Function
In plant cells, a large body of evidence now exists in support of the BP-80–type VSR for transport to the lytic vacuole (Paris and Neuhaus, 2002; Jiang and Rogers, 2003; Robinson et al., 2005). BP-80, a type I membrane-spanning protein, is superficially similar to Vps10p and sortilin (Marcusson et al., 1994; Petersen et al., 1997) and has a Tyr motif in its cytosolic tail that can interact with a μ-adaptin of an AP1-adaptor complex (Happel et al., 2004). Convincing evidence for the role of BP-80 and its homologs in the sorting of acid hydrolases has come from transgenic expression studies in yeast (Humair et al., 2001) and, more recently, in Arabidopsis, where the expression of a VSR-HDEL construct led to cargo retention in the endoplasmic reticulum (Watanabe et al., 2004). VSRAt-1, the Arabidopsis BP-80 isoform ATELP, localizes principally to MVB in tobacco BY-2 cells (Li et al., 2002; Tse et al., 2004), an organelle that appears to be the target for the action of the drug wortmannin both in plant (Kim et al., 2001, Pimpl et al., 2003) and mammalian (Bright et al., 2001; Sachse et al., 2002) cells.
Wortmannin has been known for sometime to differentially block vacuolar protein transport (Matsuoka et al., 1995), although the concentrations usually required to elicit a measurable response in plant systems is some 10- to 100-fold higher than those normally employed in animal cells. Recently, it has been shown that wortmannin treatment interferes with anterograde, BP-80–mediated trafficking to the lytic vacuole, resulting in the secretion of the BP-80 ligands (daSilva et al., 2005). While it does not interfere with receptor-ligand binding per se, wortmannin would seem to reduce the availability of the receptor in the Golgi apparatus, since overexpression of BP-80 partially alleviates the effect of the drug (daSilva et al., 2005). Most importantly, wortmannin perturbation of vacuolar trafficking leads to the release of BP-80 into the vacuole. Thus, in this respect, there is a certain similarity in phenotype between wortmannin action and the expression of retromer mutants in yeast and mammalian cells (see above). Is there therefore a connection between wortmannin and retromer?
Wortmannin is known to be an inhibitor of PI-3 kinases in yeast (Stack and Emr, 1994) and mammals (Vanhaesebroeck et al., 1997). It too causes the mistargeting of the MPR and its ligand in mammalian cells (Davidson, 1995; Reaves et al., 1996). PI-3 phosphate, the substrate for PI-3 kinase, localizes to multivesicular endosomes in both yeast and mammalian cells (Gillooly et al., 2000). Vps34p is a PI-3 kinase in yeast (Stack and Emr, 1994) and is also required for the efficient recycling of Vps10p to the PVC (Burda et al., 2002). It is also known that Vps34p, by binding to a complex of Vps30p/Vps38p, not only enhances PI-3 phosphate synthesis and concomitant PI-3 kinase activity but is also necessary for the binding of the retromer dimer Vps5p/Vps17p via their PX domains (Yu and Lemmon, 2001). Most importantly, it has been shown that this latter interaction is sensitive to wortmannin (Cozier et al., 2002). More conclusively, a considerable reduction in immunofluorescent label intensity for SNX1 and SNX2 (the Vps5p and Vps17p equivalents in mammals) has been observed in HeLa cells after wortmannin treatment (Arighi et al., 2004). A similar result was obtained for Snx1 in COS7 cells treated with the related PI-3 kinase inhibitor LY294002, the resulting diffuse immunofluorescent image pointing to a release of the small retromer subunit into the cytosol (Zhong et al., 2002).
Thus, there would indeed seem to be a connection between wortmannin and retromer presence/function. Nevertheless, the fate of the large retromer (mVps35, mVps29, and mVps26) subunit after wortmannin treatment in mammalian cells remains unclear. Whereas the data of Arighi et al. (2004) suggest that wortmannin causes the release of this subunit, unpublished data from other groups have not been able to substantiate this effect (M. Seaman, personal communication). Our results indicate that the large subunit of plant retromer in plant cells is not displaced into the cytosol as a result of wortmannin treatment. Lacking antibodies against VPS5, we are at the moment unable to determine whether the small retromer subunit is released after wortmannin treatment, as is known for mammalian cells. Despite this, the separation of VSRAt-1 and VPS35 signals is in full accordance with the known inhibitory effects of wortmannin on receptor-mediated vacuolar protein sorting (daSilva et al., 2005).
Retromer Localizes to the PVC and Possibly Coats Microvesicles
Despite their great elegance and compelling logic based on genetic experiments, only one of the articles dealing with the detection and function of retromer in Vps10p transport and recycling in yeast (Seaman et al., 1998) actually presents direct in situ evidence for the presence of retromer at the PVC. Even then, this was done by immunogold labeling of cryosections prepared from a class E vps mutant that has an enlarged, proliferated PVC. Because of the small size of yeast and the difficulty in recognizing the PVC, retromer localization in wild-type cells by immunofluorescence is lacking. In mammalian cells, however, both immunogold and immunofluorescent labeling have been successfully employed to localize retromer components. SNX1 and mVps26 have been localized by immunogold electron microscopy to tubular outgrowths of endosomes (Zhong et al., 2002; Arighi et al., 2004). These endosomes have also been shown to be multivesiculate (Seaman, 2004). Immunofluorescence labeling on HeLa cells has shown that mammalian Vps26 extensively colocalizes with the transferrin receptor, a marker for early endosomes, but not with the TGN marker TGN46 (Arighi et al., 2004). A partial colocalization between mVps26 and CI-MPR was also recorded in the latter study.
Our results obtained on BY-2 cells are in full agreement with the investigations on yeast and mammalian cells just described. VPS35, VPS29, and VPS26 all colocalize with standard PVC markers and with VSRAt-1 in the confocal laser scanning microscopy, and antibodies against all three retromer components label specifically MVB. Wortmannin treatment of BY-2 cells results in the dilation of the MVB/PVC and to a separation of the large retromer subunit from VSRAt-1. These data convincingly show that retromer binds to the surface of MVB in plant cells. In mammalian cells, however, retromer appears to be concentrated on tubular protrusions with only ∼25% of mVps26 immunogold label on the body of the multivesicular early endosome (Arighi et al., 2004). It has been speculated that the BAR domains of SNX1 and SNX2 (Vps5p and Vps17p) are responsible for this distribution since they are known to induce the tubulation of liposomes in vitro (Kurten et al., 2001; Peter et al., 2004) and, through their banana-like conformation, are most suited to the highly curved PI3P-enriched membranes of the early endosome (Carlton et al., 2004). At the moment, it is not possible to say whether the multivesicular PVC, which we have defined in earlier studies on BY-2 cells (Li et al., 2002; Tse et al., 2004) as being enriched in VSRAt-1 and now bearing retromer, is an early or late endosome. While it has a plaque on its surface very much like that reported as being typical for early endosomes in mammalian cells (Raposo et al., 2001; Sachse et al., 2002; Raiborg et al., 2003), there are no indications of tubular outgrowths to this structure. Whether this relates to the lack of an obvious ortholog to VPS17 in the plant database remains purely speculative.
Implicit in the articles dealing with retromer from yeast and mammalian cells is that attachment of the two retromer subunits (Vps35p,Vps29p,Vps26p) and (Vps17p,Vps5p) culminates in the formation of a retromer-coated vesicle. However, such a vesicle has neither been identified in situ nor isolated. At most the existence of retromer-coated vesicles could be inferred from protein gel blot profiles of P100 fractions separated by gel filtration chromatography on Sephacryl S1000 (Seaman et al., 1998). As mentioned previously, the distribution profiles for VPS35 on linear isopycnic sucrose density gradients prepared from plant P100 membranes are very similar to those obtained for yeast (Seaman et al., 1997, 1998). In both cases, VPS35 and Vps35p are present in a lighter set of fractions equilibrating with endosomal/PVC markers but more prominently in a denser population. Seaman et al. (1998) considered that this latter fraction could correspond to a population of coated vesicles or tubules. By negative staining we have shown that the high-density fractions in sucrose gradients from Arabidopsis P100 membranes do indeed contain 90-nm-diameter coated structures, possibly vesicles. These are distinct in morphology from clathrin-coated vesicles, and some stained positively with VPS35 and VPS26 antisera but not with COPI/COPII antisera. We cannot completely rule out the existence of COP-coated vesicles in these fractions but consider their presence unlikely for several reasons. First, COPI and COPII proteins do not extend as deeply into sucrose gradients of Arabidopsis/Brassica membranes (Movafeghi et al., 1999; Yang et al., 2005). Second, in vitro–induced COPI vesicles, both from animal (Malhotra et al., 1989) and plant (Pimpl et al., 2000) sources, have lower equilibrium densities (in the range of 40 to 45% sucrose [w/w]).
Retromer and VSR Interaction
Evidence for the interaction of Vsp35p and the yeast Vsp10p receptor Vps10p is principally genetic (Seaman et al., 1997, 1998; Nothwehr et al., 1999). In addition, through allele-specific suppression it has been possible to identify the residues that are crucial for the Vps35p–receptor interactions. In this way it has been possible to show that the Tyr-1492 residue of Vps10p and the residues 81 to 91 in the cytosolic domain of DPAP A, together with the acidic residues Asp-123 and Asp-528 of Vps35p, are crucial for receptor–retromer interactions at the PVC (Nothwehr et al., 1993, 2000). Thus, the motifs involved in receptor retrieval are different from those required for the anterograde transport of the receptor-cargo complex.
While it has been possible to immunoprecipitate a chemically cross-linked complex of Vps35p with the model recycling membrane protein A-ALP (a chimeric reporter consisting of the cytosolic domain of DPAP A fused to the transmembrane and lumenal domains of alkaline phosphatase) with Vps35p antibodies, this approach has failed to work with Vps35p and Vps10p (Nothwehr et al., 2000). It has been speculated that this may be due to an inappropriate configuration of the necessary amine groups in the interface of the Vps35-Vps10p complex. Although we were successful in immunoprecipitating VSRAt-1 bound to VPS35 with VPS35 antibodies, we have also experienced great difficulties in trying to isolate a chemically cross-linked retromer-VSRAt-1 complex. Thus, it has so far not been possible to obtain any convincing data on retromer proteins other than Vps35, or other potential receptor molecules, by this approach.
Future Prospects
The data presented in this article underscore the notion that cargo transport to the lytic compartment and receptor recycling between the TGN and the endosomal/PVCs is strongly conserved among eukaryotes. Although further experimentation on retromer–VSR interaction (e.g., through the analysis of point mutations in retromer components by transient expression or by the generation of knockouts) is needed to confirm the role of retromer in retrograde traffic from the PVC of plant cells, our results clearly place retromer at the surface of VSR-bearing MVB. The identification of retromer-coated vesicles in high-density fractions gives hope that, despite the nascent instability of coat–membrane interactions (prior to fusion, the coat proteins of all classes of coated transport vesicles must dissociate from the membrane), purified retromer-coated vesicles may soon become available for further analysis.
METHODS
Plant Material
Arabidopsis thaliana (Columbia) plants were grown in soil (TKS1; Floragard) under a 16/8-h cold light (430-W Son-T Agro bulbs; Philips) regime at 22 to 25°C and 40 to 60% relative humidity in a greenhouse. Arabidopsis cell suspension cultures (ecotype Col-0, cell line At7) were grown as previously described (Trezzini et al., 1993) using Murashige and Skoog medium (Duchefa) and subcultured every 7 d. Suspension cultures of Bright Yellow (BY-2) tobacco (Nicotiana tabacum) were maintained as described previously (Yang et al., 2005)
Cloning of Plant Retromer Components
There are three isoforms for VPS35 (with the accession numbers At1g7850, At2g17790, and At3g51310), one for VPS29 (At3g47810), two for VPS26 (At5g53530 and At4g27690), and three for VPS5 (At5g06140, At5g58440, and At5g07120). We have cloned the isoform with the highest identity to the respective yeast homolog. To specifically amplify the different Arabidopsis VPS coding regions for VPS35, VPS29, and VPS26, we prepared total first-strand cDNA from 6-week-old Arabidopsis leaves using the RNeasy plant mini kit (Qiagen) and AMV reverse transcriptase (Roche). To amplify this cDNA by PCR, we used 5′-ACATAGAATGATCGCCGACGAC-3′ (sense primer) and 5′-TTCATTCAAACCATTCCATTTTG-3′ (antisense primer) for the amplification of VPS35, 5′-CCATGGTCATGAATTATCTTCTTGGAGCTTTC-3′ (sense primer) and 5′-GGCTAGCCTCAAGATGTCTCTTCCTTGAGCCTG-3′ (antisense primer) for vps26, and 5′-TCATGACAATGGTGCTGGTATTGGCATTGG-3′ (sense primer) and 5′-GGATCCCGGCTAGCTAGCTACGGACCAGAGCTGGTAGTAG-3′ (antisense primer) for vps29. The latter two pairs had an NcoI-compatible site at 5′ of the sense and an NheI site at 5′ of the antisense primer added for insertion in frame with the glutathione S-transferase coding sequence of the vectors used. All amplified gene products were cloned into the pGEMTeasy vector (Promega) and their sequence confirmed by sequencing (MWG).
Generation of Recombinant Proteins and Preparation of Antisera
The open reading frame of Arabidopsis Vps35 (At3g51310) was cloned by standard procedures into the NotI-NotI site of the pGEX-4T-2 vector (Amersham Biosciences), and the corresponding open reading frames for Vps29 (At3g47810) and Vps26 (At5g53530) into the NcoI-NheI site of a modified pET24d vector (Novagen). All were expressed as glutathione S-transferase fusion proteins in Escherichia coli (strain BL21DE3). The fusion proteins were recovered in the insoluble pellet. The inclusion bodies were washed and solubilized as described by Sambrook et al. (1989). The recombinant proteins were purified by preparative SDS-PAGE. Polyclonal antibodies in rabbits were prepared commercially (Vps35, BioScience; Vps26 and Vps29, Eurogentec). IgGs were separated from crude serum on a protein-A Sepharose CL-4B column (No. P3391; Sigma-Aldrich) and eluted with 100 mM glycine-HCl, pH 2.9, as described by Harlow and Lane (1988).
Subcellular Fractionation
One hundred grams (fresh weight) of filtered Arabidopsis suspension culture cells were homogenized for 2 min with a hand-held mixer (ESGE-Zauberstab M150; ESGE) on ice in 100 mL of a prechilled medium (homogenization buffer) containing 0.4 M sucrose, 40 mM HEPES-KOH, pH 7.0, 10 mM KCl, 5 mM EDTA, 1 mM DTT, 2 mM o-phenanthroline, 0.5 μg/mL leupeptin, 2 μg/mL aprotinin, 1 μg/mL pepstatin, and 1 μg/mL trans-epoxysuccinyl-l-leucylamido-4-(4-guanido)-butane (E-64). The homogenate was filtered through two layers of Miracloth and centrifuged for 10 min at 3000g to remove cell debris and cell walls. The supernatant was then centrifuged for 20 min at 13,000g. The sediment (P13) was retained and the supernatant centrifuged again for 60 min at 100,000g to produce a P100 sediment. BY-2 cells were homogenized similarly except that the homogenate was passed one time through a Jeda-Press (LINCA) at 100 bar. Organelles in the P100 sediment were separated by isopycnic centrifugation on a linear 15 to 55% (w/w) sucrose density gradient (made with homogenizing medium) by centrifuging for 16 h at 100,000g. The 1.3-mL fractions were removed and the protein precipitated by addition of 10% (v/v) trichloracetic acid (TCA) (final concentration).
Solubilization of Retromer Proteins and Column Chromatography
The Arabidopsis P100 sediment was resuspended in homogenizing medium containing either 0.25 M NaCl, 1 M NaCl, 0.1 M Na2CO3, 2 M urea, or 1% Triton X-100 and incubated on a rotator for 60 min at 4°C. The resulting suspension was recentrifuged for 60 min at 100,000g, providing pellet and solubilized fractions.
For gel filtration chromatography, preparatory amounts of the 0.25 M NaCl solubilized P100 sediment were isolated and added to a Superdex-200 HR 10/30 column (Amersham Biosciences). The column was eluted with lysis buffer including 0.25 mM NaCl at a rate of 0.4 mL/min, and 0.5-mL fractions were collected and the protein of each fraction precipitated by addition of 10% TCA (final concentration). Proteins were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Pall) and immunostained with anti-Vps35, anti-Vps26, and anti-Vps29.
Immunoprecipitation with Vps35 Antibodies
Fractions corresponding to 47 to 50% (w/w) sucrose from isopycnic sucrose density gradients of P100 Arabidopsis membranes (see above) were pooled and treated with 2% (w/v) CHAPS for 1 h at 4°C. After centrifugation at 100,000g for 1 h, the supernatant was added to a suspension of anti-Vps35 covalently coupled to protein-A Sepharose CL-4B fast flow beads (Amersham Biosciences). The coupling of 1 mL of antisera (35 mg total protein/mL) to 200 μL of protein-A beads was done according to the manufacturer's instructions and was followed by cross-linking with dimethyl pimelimidate following the instructions from Pierce. Essentially, dimethyl pimelimidate was used in a 0.2 M triethanolamine buffer, pH 8.0, and quenched with glacial acetic acid. Before use, the coupled IgGs were subjected to one round of elution (see below) with 100 mM glycine, pH 2.5, to further remove nonbound IgGs. Normally, 1 mL (5 mg total protein/mL) of CHAPS-soluble extract (see above) was mixed with 100 μL of anti-Vps35 beads and batch-incubated at 4°C for 2 h while rotating, washed with 5 × 1 mL of homogenization buffer including 2% (w/v) CHAPS, and then with 5 × 1 mL of homogenization buffer without CHAPS. The beads were pelleted by low-speed centrifugation (1 min at 500g). Attached proteins were eluted from the beads with 100 mM glycine buffer, pH 2.5, directly neutralized with 100 μL of Tris-HCl, pH 8.8, per milliliter of eluate, and then precipitated with 10% (v/v) TCA (final concentration) or with methanol/chloroform (Wessel and Flugge, 1984). Eluted proteins were used for SDS-PAGE or 2D gel electrophoresis.
2D Gel Electrophoresis
Eluted proteins from immunoprecipitation experiments were precipitated with methanol/chloroform (Wessel and Flugge, 1984), and dried protein pellets were solubilized in 7 M urea, 2 M thio-urea, 2% (w/v) CHAPS, 0.5% (v/v) IPG buffer, pH 3 to 10 (Amersham Biosciences), 360 mg/mL of DTT, and a trace of bromophenol blue. After a 2-min centrifugation at 15,000g to remove insoluble aggregates, 125-μL samples containing 40 μg of protein were applied to 7-cm IPG strips with a stabilized linear gradient, pH 3 to 10, according to the manufacturer's instructions for the IPGphor system (Amersham Biosciences). Rehydration of the strips (in the protein-containing buffer) proceeded for 14 h under a tension of 30 V. Isoelectric focusing continued as follows: 500 V for 1 h, 1000 V for 1 h, and then ramping toward 8000 V (usually reaching around 5000 V) until a total of ∼30,000 Vh was achieved. Strips were equilibrated in 10 mL of 50 mM Tris-HCl, pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, and a trace of bromophenol blue for 2 × 15 min. In the first equilibration step, 10 mg/mL of DTT was added, and in the second step, 25 mg/mL of iodoacetamide was added. Equilibrated strips were assembled on top of 10% polyacylamide gels, without stacking gel, and SDS-PAGE was performed using standard conditions. Gels were stained with silver or Coomassie Brilliant Blue or subjected to protein gel blot transfer and immunostained.
Protein Determinations, Gel Electrophoresis, and Protein Gel Blotting
Protein concentrations were determined according to Bradford (1976). For one-dimensional SDS-PAGE, TCA-precipitated protein samples were separated on 10% polyacrylamide gels. Proteins were then transferred electrophoretically to nitrocellulose filters (Pall). Primary marker antibodies and their dilutions/concentrations were as follows: RGP (reversibly glycosylated polypeptide), 1:3000; calnexin, 1:5000 (Happel et al., 2004); VSRAt-1, 1 μg/mL (Tse et al., 2004); Pep12 (SYP21), 1:1000 (produced in our lab with a construct from N. Raikhel, as described in Conceição et al., 1997); PM-ATPase, 1:500 (Hinz et al., 1999); yeast Vps35p (Seaman et al., 1998), mouse Vps26 (received from M. Seaman, Cambridge, UK), and the Arabidopsis retromer antisera (Vps35, Vps26, and Vps29) were used at 1:1000 or, when purified, at 1 μg/mL. Antibody dilutions were made in 1× TBS that included 5% (w/v) fat-free milk powder or in 1× TBS that included 3% (w/v) BSA. Horseradish peroxidase–coupled secondary antibodies (Sigma-Aldrich) were diluted 1:20000 (anti-rabbit) or 1:10000 (anti-mouse) in 1× TBS including 5% (w/v) milk powder. Detection of horseradish peroxidase enhanced chemoluminescent signals essentially followed the instructions from Pierce.
Electron Microscopy
BY-2 cells were harvested from the culture medium by filtering. They were then mixed with hexadecen on the filter, excess fluid was removed, and the cells were frozen in a high-pressure freezer (HPF010; Bal-Tec). Freeze substitution was performed in a Leica freeze substitution unit (AFS) in dry acetone supplemented with 0.1% uranyl acetate at −85°C for 3 d before warming up to −35°C over 18 h. Cells were then infiltrated and embedded in Lowicryl HM20 at −35°C and polymerized for 2 d at the same temperature with UV light and for another 3 d at room temperature. Ultrathin sections were incubated with affinity-purified antisera against Vps35, Vps29, or Vps26 at primary dilutions of 1:25 to 1:100, followed by incubation with 10-nm gold-coupled secondary antibodies (Biocell GAR10) at a dilution of 1:50 in PBS supplemented with 1% of either BSA or cold water fish gelatin (Sigma-Aldrich). Sections were poststained with aqueous uranyl acetate/lead citrate and examined with a Philips CM10 transmission electron microscope operating at 80 kV. Immunogold negative staining was performed on carbon-coated mica films following the procedure previously described by Robinson et al. (1987) and Pimpl et al. (2000).
Confocal Microscopy
BY-2 cell fixation and confocal immunofluorescence were performed essentially according to Jiang and Rogers (1998) and Tse et al. (2004). Suspension-cultured BY-2 cells (2 to 3 d after subculture) or wortmannin-treated cells were first fixed in a solution containing 50 mM Na-phosphate buffer, pH 7.0, 5 mM EGTA, 0.02% Na-azide, and 4.5% paraformaldehyde for 24 h at 4°C. After washing three times with Na-phosphate-EGTA buffer, the cell walls of the fixed cells were partially digested by 1% cellulysin (Serva) in Na-phosphate buffer for 20 min, while the cell membranes were permeabilized by a 2-min treatment in 0.5% Triton X-100. For antibody single labeling, fixed cells were incubated with blocking buffer 1 (1× PBS, 1% BSA) for 30 min before adding the primary antibodies. Polyclonal primary antibodies (4 μg/mL) were then prepared in blocking buffer 2 (1× PBS, 0.25% BSA, 0.25% gelatin, 0.05% Nonidet P-40, and 0.02% Na-azide) and incubated with the fixed cells at 4°C overnight. The cells were then further washed with blocking buffer 2, followed by incubation with rhodamine-conjugated secondary antibodies at a final dilution of 1:100 for 1 h at room temperature. Finally, the labeled cells were washed twice with blocking buffer 2, mounted on slides, and used for image collection in the confocal microscope.
For double labeling using two polyclonal antibodies, first primary antibodies (4 μg/mL) were incubated at 4°C overnight followed by washing in PBST (PBS with 0.05% [v/v] Tween 20). Rhodamine-conjugated Fab fragments were then added and incubated at room temperature for 4 h prior to a second wash, followed by postfixation and washing before the addition of the second primary antibodies (4 μg/mL). The rhodamine-conjugated Fab fragment secondary antibodies were used at a dilution of 1:20, while fluorescein isothiocyanate (FITC)–conjugated/Cy-5–conjugated secondary antibodies were used at a dilution of 1:100. Controls included labeling without the use of the second primary antibodies.
All confocal fluorescence images were collected using a Bio-Rad Radiance 2100 system controlled by LaserShape2000 software with the following parameters: ×60 objective oil lens (Nikon), 1× zoom, optimal iris, and 512 × 512 box size pixel. Because primary antibodies were detected with rhodamine-conjugated secondary antibodies or rhodamine-conjugated Fab fragments while the two GFP/YFP reporters were ready for detection under the confocal microscope with the FITC setting, an FITC/rhodamine scanning method (simultaneous or sequential scanning) was thus used to collect dual images. Care was taken to ensure that the laser power and other settings (iris and gain) were set to a condition where no cross-over signals between rhodamine and FITC emissions were detected. The filter sets were used as follows: for detecting YFP, excitation wavelength 488 nm, emission filter HQ515/30; for detecting rhodamine, excitation wavelength 543 nm, emission filter HQ600/50. The YFP/GFP images were pseudocolored in green, and the rhodamine images were pseudocolored in red. The collected images were further processed using Adobe Photoshop software as described by Jiang and Rogers (1998). The percentage of colocalization between two signals was calculated as previously described (Jiang and Rogers, 1998; Li et al., 2002; Tse et al., 2004).
Accession Numbers
The Arabidopsis Genome Initiative numbers for VPS35, VPS29, and VPS26 are At3g51310, At3g47180, and At5g53530, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Control Labeling Experiments for Immunofluorescence Retromer Localizations.
Supplemental Figure 2. Colocalization of VPS35, VPS29, and VPS26.
Supplementary Material
Acknowledgments
Financial support from the State of Baden-Württemberg (Forschungsschwerpunktprogramm) is gratefully acknowledged. L.J. is supported by grants from the Research Grants Council of Hong Kong (CUHK4156/01M, CUHK4260/02M, and CUHK4580/05M) and by the University Grants Council Area of Excellence.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: David G. Robinson (david.robinson@urz.uni-heidelberg.de).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.035907.
References
- Arighi, C.N., Hartnell, L.M., Aguilar, R.C., Haft, C.R., and Bonifacino, J.S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72 395–447. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. [DOI] [PubMed] [Google Scholar]
- Bright, N.A., Lindsay, M.R., Stewart, A., and Luzio, J.P. (2001). The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic 2 631–642. [DOI] [PubMed] [Google Scholar]
- Burda, P., Padilla, S.M., Sarkar, S., and Emr, S.D. (2002). Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 115 3889–3900. [DOI] [PubMed] [Google Scholar]
- Carlton, J., Bujny, M., Peter, B.J., Oorschot, V.M.J., Rutherford, A., Mellor, H., Klumpermann, J., McMahon, H.T., and Cullen, P.J. (2004). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14 1791–1800. [DOI] [PubMed] [Google Scholar]
- Collins, B.M., Skinner, C.F., Watson, P.J., Seaman, M.J., and Owen, D.J. (2005). Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat. Struct. Mol. Biol. 12 594–602. [DOI] [PubMed] [Google Scholar]
- Conceição, A.S., Marty-Mazars, D., Bassham, D.C., Sanderfoot, A.A., Marty, F., and Raikhel, N.V. (1997). The syntaxin homolog AtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell 9 571–582. [PMC free article] [PubMed] [Google Scholar]
- Cooper, A.A., and Stevens, T.H. (1996). Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta, G., Stefan, C.J., Bensen, E.S., Emr, S.D., and Payne, G.S. (2001). Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol. Biol. Cell 12 1885–1896. Erratum. Mol. Biol. Cell 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier, G.E., Carlton, J., McGregor, A.H., Gleeson, P.A., Teasdale, R.D., Mellor, H., and Cullen, P.J. (2002). The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J. Biol. Chem. 277 48730–48736. [DOI] [PubMed] [Google Scholar]
- daSilva, L.L., Taylor, J.P., Hadlington, J.L., Hanton, S.L., Snowden, C.J., Fox, S.J., Foresti, O., Brandizzi, F., and Denecke, J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, H.W. (1995). Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol. 130 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche, O., Yeung, B.G., Payne, G.S., and Schekman, R. (2001). Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell 12 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennes, A., Madsen, P., Nielsen, M.S., Petersen, C.M., and Pohlmann, R. (2002). The yeast Vps10p cytoplasmic tail mediates lysosomal sorting in mammalian cells and interacts with human GGAs. J. Biol. Chem. 14 12288–12293. [DOI] [PubMed] [Google Scholar]
- Doray, B., Ghosh, P., Griffith, J., Geuze, H.J., and Kornfeld, S. (2002). Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297 1700–1703. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., Dahms, N.M., and Kornfeld, S. (2003). Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 4 202–212. [DOI] [PubMed] [Google Scholar]
- Gillooly, D.J., Morrow, I.C., Lindsay, M., Gould, R., Bryant, N.J., Gaullier, J.M., Parton, R.G., and Stenmark, H. (2000). Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg, J., and Stenmark, H. (2004). The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 5 317–323. [DOI] [PubMed] [Google Scholar]
- Gullapalli, A., Garrett, T.A., Paing, M.M., Griffin, C.T., Yang, Y., and Trejo, J. (2004). A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: Evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking. Mol. Biol. Cell 15 2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft, C.R., de la Luz Sierra, M., Bafford, R., Lesniak, M.A., Barr, V.A., and Taylor, S.I. (2000). Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: Assembly into multimeric complexes. Mol. Biol. Cell 11 4105–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel, N., Honing, S., Neuhaus, J.M., Paris, N., Robinson, D.G., and Holstein, S.E. (2004). Arabidopsis μA-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. 37 678–693. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Hassan, A.J., Zeng, J., Ni, X., and Morales, C.R. (2004). The trafficking of prosaposin (SGP-1) and GM2AP to the lysosomes of TM4 Sertoli cells is mediated by sortilin and monomeric adaptor proteins. Mol. Reprod. Dev. 68 476–483. [DOI] [PubMed] [Google Scholar]
- Hinz, G., Hillmer, S., Bäumer, M., and Hohl, I. (1999). Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11 1509–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky, B.F., Davies, B.A., Seaman, M.N., McLaughlin, S.A., Yoon, S., and Emr, S.D. (1997). A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell 8 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humair, D., Hernandez Felipe, D., Neuhaus, J.M., and Paris, N. (2001). Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor. Plant Cell 13 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L., and Rogers, J.C. (1998). Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J. Cell Biol. 143 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L., and Rogers, J.C. (2003). Sorting of lytic enzymes in the plant Golgi apparatus. Annu. Plant Rev. 9 114–140. [Google Scholar]
- Juergens, G. (2004). Membrane trafficking in plants. Annu. Rev. Cell Dev. Biol. 20 481–504. [DOI] [PubMed] [Google Scholar]
- Kim, D.H., Eu, Y.J., Yoo, C.M., Kim, Y.W., Pih, K.T., Jin, J.B., Kim, S.J., Stenmark, H., and Hwang, I. (2001). Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten, R.C., Eddington, A.D., Chowdhury, P., Smith, R.D., Davidson, A.D., and Shank, B.B. (2001). Self-assembly and binding of a sorting nexin to sorting endosomes. J. Cell Sci. 114 1743–1756. [DOI] [PubMed] [Google Scholar]
- Li, Y.B., Rogers, S.W., Tse, Y.C., Lo, S.W., Sun, S.S., Jauh, G.Y., and Jiang, L. (2002). BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol. 43 726–742. [DOI] [PubMed] [Google Scholar]
- Malhotra, V., Serafini, T., Orci, L., Shepherd, J.C., and Rothman, J.E. (1989). Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58 329–336. [DOI] [PubMed] [Google Scholar]
- Marcusson, E.G., Horazdovsky, B.F., Cereghino, J.L., Gharakhanian, E., and Emr, S.D. (1994). The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77 579–586. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K., Bassham, D.C., Raikhel, N.V., and Nakamura, K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movafeghi, A., Happel, N., Pimpl, P., Tai, G.H., and Robinson, D.G. (1999). Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol. 119 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S.F., Bruinsma, P., and Strawn, L.A. (1999). Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol. Biol. Cell 10 875–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S.F., Ha, S.A., and Bruinsma, P. (2000). Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S.F., Roberts, C.J., and Stevens, T.H. (1993). Membrane protein retention in the yeast Golgi apparatus: Dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J. Cell Biol. 121 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., Cowles, C.R., and Emr, S.D. (1998). The AP-3 complex: A coat of many colours. Trends Cell Biol. 8 282–288. [DOI] [PubMed] [Google Scholar]
- Paravicini, G., Horazdovsky, B.F., and Emr, S.D. (1992). Alternative pathways for the sorting of soluble vacuolar proteins in yeast: A vps35 null mutant missorts and secretes only a subset of vacuolar hydrolases. Mol. Biol. Cell 3 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, N., and Neuhaus, J.M. (2002). BP-80 as a vacuolar sorting receptor. Plant Mol. Biol. 50 903–914. [DOI] [PubMed] [Google Scholar]
- Peter, B.J., Kent, H.M., Mills, I.G., Vallis, Y., Butler, P.J., Evans, P.R., and McMahon, H.T. (2004). BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 303 495–499. [DOI] [PubMed] [Google Scholar]
- Petersen, C.M., Nielsen, M.S., Nykjaer, A., Jacobsen, L., Tommerup, N., Rasmussen, H.H., Roigaard, H., Gliemann, J., Madsen, P., and Moestrup, S.K. (1997). Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem. 272 3599–3605. [DOI] [PubMed] [Google Scholar]
- Pimpl, P., Hanton, S.L., Taylor, J.P., Pinto-DaSilva, L.L., and Denecke, J. (2003). The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., Movafeghi, A., Coughlan, S., Denecke, J., Hillmer, S., and Robinson, D.G. (2000). In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., Rusten, T.E., and Stenmark, H. (2003). Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 15 446–455. [DOI] [PubMed] [Google Scholar]
- Raposo, G., Tenza, D., Murphy, D.M., Berson, J.F., and Marks, M.S. (2001). Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves, B.J., Bright, N.A., Mullock, B.M., and Luzio, J.P. (1996). The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J. Cell Sci. 109 749–762. [DOI] [PubMed] [Google Scholar]
- Reddy, J.V., and Seaman, M.N. (2001). Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol. Biol. Cell 12 3242–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D.G., Ehlers, U., Herken, R., Hermann, B., Mayer, F., and Schürmann, F. W (1987). Methods of Preparation for Electron Microscopy: An Introduction for the Biomedical Sciences. (Berlin: Springer-Verlag).
- Robinson, D.G., Oliviusson, P., and Hinz, G. (2005). Protein sorting to the storage vacuoles of plants: A critical appraisal. Traffic 6 615–625. [DOI] [PubMed] [Google Scholar]
- Sachse, M., Urbe, S., Oorschot, V., Strous, G.J., and Klumperman, J. (2002). Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13 1313–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Seaman, M.N. (2004). Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15 68–75. [DOI] [PubMed] [Google Scholar]
- Seaman, M.N., Marcusson, E.G., Cereghino, J.L., and Emr, S.D. (1997). Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N., McCaffery, J.M., and Emr, S.D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N., and Williams, H.P. (2002). Identification of the functional domains of yeast sorting nexins Vps5p and Vps17p. Mol. Biol. Cell 13 2826–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., and Emr, S.D. (1994). Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J. Biol. Chem. 269 31552–31562. [PubMed] [Google Scholar]
- Stepp, J.D., Huang, K., and Lemmon, S.K. (1997). The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J. Cell Biol. 139 1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezzini, G.F., Horrichs, A., and Somssich, I.E. (1993). Isolation of putative defense-related genes from Arabidopsis thaliana and expression in fungal elicitor-treated cells. Plant Mol. Biol. 21 385–389. [DOI] [PubMed] [Google Scholar]
- Tse, Y.C., Mo, B., Hillmer, S., Zhao, M., Lo, S.W., Robinson, D.G., and Jiang, L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S.J., Panayotou, G., and Waterfield, M.D. (1997). Phosphoinositide 3-kinases: A conserved family of signal transducers. Trends Biochem. Sci. 22 267–272. [DOI] [PubMed] [Google Scholar]
- Wang, D., Guo, M., Fan, J., Zhu, Z., Zang, J., Zhu, Z., Li, X., Teng, M., Niu, L., Dong, Y., and Liu, P. (2005). Crystal structure of human vacuolar protein sorting protein 29 reveals a phosphodiesterase/nuclease-like fold and two protein-protein interaction sites. J. Biol. Chem. 280 22962–22967. [DOI] [PubMed] [Google Scholar]
- Watanabe, E., Shimada, T., Tamura, K., Matsushima, R., Koumoto, Y., Nishimura, M., and Hara-Nishimura, I. (2004). An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol. 45 9–17. [DOI] [PubMed] [Google Scholar]
- Wessel, D., and Flugge, U.I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138 141–143. [DOI] [PubMed] [Google Scholar]
- Wurmser, A.E., and Emr, S.D. (1998). Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J. 17 4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y.D., Elamawi, R., Bubeck, J., Pepperkok, R., Ritzenthaler, C., and Robinson, D.G. (2005). Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17 1513–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.W., and Lemmon, M.A. (2001). All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J. Biol. Chem. 276 44179–44184. [DOI] [PubMed] [Google Scholar]
- Zhong, Q., Lazar, C.S., Tronchere, H., Sato, T., Meerloo, T., Yeo, M., Songyang, Z., Emr, S.D., and Gill, G. (2002). Endosomal localization and function of sorting nexin 1. Proc. Natl. Acad. Sci. USA 99 6767–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.