Abstract
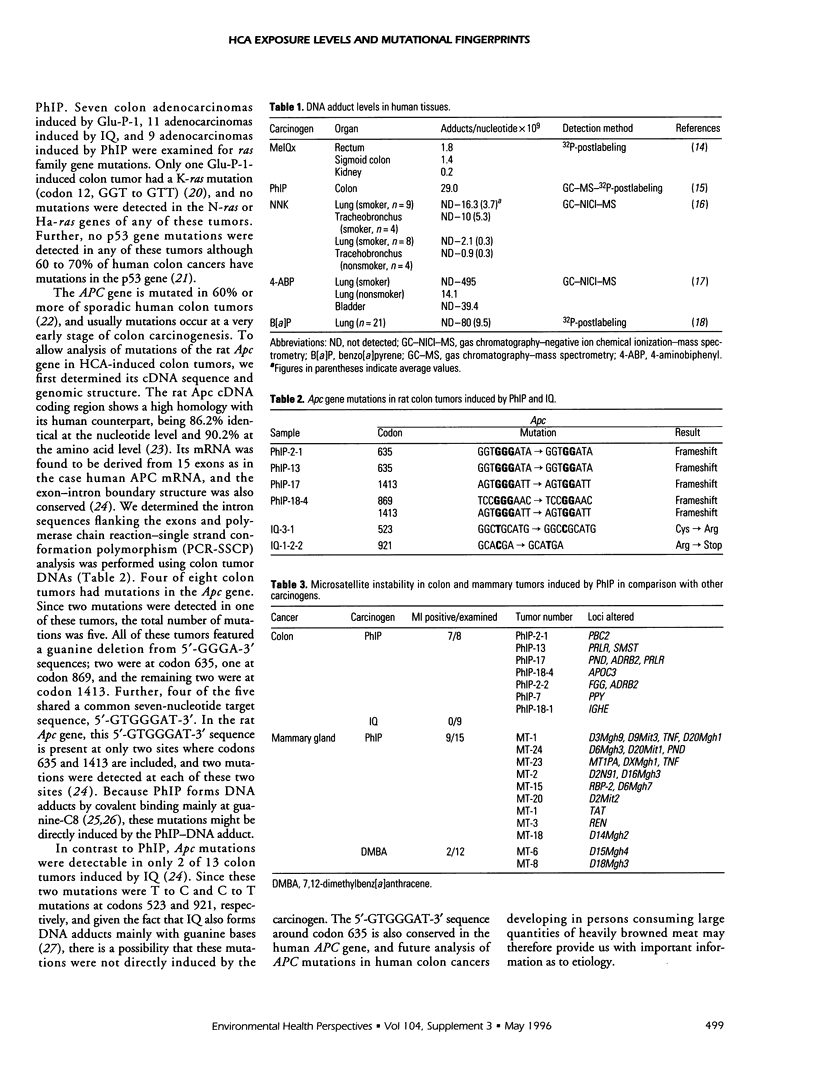
Heterocyclic amines (HCAs) are mutagens/carcinogens to which humans are exposed on almost a daily basis. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhlP) is the most abundant of the various carcinogenic HCAs (present at a level of 0.56 to 69.2 ng/g of cooked meat or fish), with 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MelQx) following it at 0.64 to 6.44 ng/g. HCAs have been found in the urine of healthy people who consume ordinary diets, while patients receiving parenteral alimentation lack, for example, PhlP and MelQx in their urine. Based on the concentrations of PhlP and MelQx in urine samples from 10 healthy volunteers, daily intake of MelQx in Japanese was calculated to be 0.3 to 3.9 micrograms/person, while that of PhlP was 0.005 to 0 micrograms. The Japanese consume more MelQx than Americans, whereas Japanese intake of PhlP was about one-third that of Americans. MelQx-DNA adducts have also detected in Japanese Kidney, colon, and rectum samples using the 32P-postlabeling method followed by identification using high-performance liquid chromatography (HPLC) analysis; the levels were 0.18, 1.8, and 1.4 per 10(9) nucleotides, respectively. In addition, we elucidated the mutational fingerprints of Phlp by analyzing Apc mutations in rat colon cancers induced by this carcinogen. Four of eight tumors had a total of five mutations in the Apc gene, four of which featured a guanine deletion from 5'-GTGGGAT-3' sequences. This specific mutation spectrum may be used as a fingerprint of PhlP in evaluating its risk potential for human colon carcinogenesis. Mutations were not found in similar 2-amino-3-methylimidazo[4,5-f]quinoline-induced colon lesions. Microsatellite instability was detected in both colon and mammary tumors induced by PhlP. The mechanisms involved in this development of microsatellite instability in PhlP. The mechanisms involved in this development of microsatellite instability in PhlP-induced cancers remain to be elucidated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canzian F., Ushijima T., Serikawa T., Wakabayashi K., Sugimura T., Nagao M. Instability of microsatellites in rat colon tumors induced by heterocyclic amines. Cancer Res. 1994 Dec 15;54(24):6315–6317. [PubMed] [Google Scholar]
- Dams E., Van de Kelft E. J., Martin J. J., Verlooy J., Willems P. J. Instability of microsatellites in human gliomas. Cancer Res. 1995 Apr 1;55(7):1547–1549. [PubMed] [Google Scholar]
- Foiles P. G., Akerkar S. A., Carmella S. G., Kagan M., Stoner G. D., Resau J. H., Hecht S. S. Mass spectrometric analysis of tobacco-specific nitrosamine-DNA adducts in smokers and nonsmokers. Chem Res Toxicol. 1991 May-Jun;4(3):364–368. doi: 10.1021/tx00021a017. [DOI] [PubMed] [Google Scholar]
- Frandsen H., Grivas S., Andersson R., Dragsted L., Larsen J. C. Reaction of the N2-acetoxy derivative of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) with 2'-deoxyguanosine and DNA. Synthesis and identification of N2-(2'-deoxyguanosin-8-yl)-PhIP. Carcinogenesis. 1992 Apr;13(4):629–635. doi: 10.1093/carcin/13.4.629. [DOI] [PubMed] [Google Scholar]
- Friesen M. D., Kaderlik K., Lin D., Garren L., Bartsch H., Lang N. P., Kadlubar F. F. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol. 1994 Nov-Dec;7(6):733–739. doi: 10.1021/tx00042a004. [DOI] [PubMed] [Google Scholar]
- Gerhardsson de Verdier M., Hagman U., Peters R. K., Steineck G., Overvik E. Meat, cooking methods and colorectal cancer: a case-referent study in Stockholm. Int J Cancer. 1991 Oct 21;49(4):520–525. doi: 10.1002/ijc.2910490408. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Ito N., Hasegawa R., Sano M., Tamano S., Esumi H., Takayama S., Sugimura T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis. 1991 Aug;12(8):1503–1506. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- Kakiuchi H., Ushijima T., Ochiai M., Imai K., Ito N., Yachi A., Sugimura T., Nagao M. Rare frequency of activation of the Ki-ras gene in rat colon tumors induced by heterocyclic amines: possible alternative mechanisms of human colon carcinogenesis. Mol Carcinog. 1993;8(1):44–48. doi: 10.1002/mc.2940080110. [DOI] [PubMed] [Google Scholar]
- Kakiuchi H., Watanabe M., Ushijima T., Toyota M., Imai K., Weisburger J. H., Sugimura T., Nagao M. Specific 5'-GGGA-3'-->5'-GGA-3' mutation of the Apc gene in rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):910–914. doi: 10.1073/pnas.92.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton D. W., Bogen K. T., Knize M. G., Hatch F. T., Johnson V. M., Felton J. S. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995 Jan;16(1):39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- Lin D., Lay J. O., Jr, Bryant M. S., Malaveille C., Friesen M., Bartsch H., Lang N. P., Kadlubar F. F. Analysis of 4-aminobiphenyl-DNA adducts in human urinary bladder and lung by alkaline hydrolysis and negative ion gas chromatography-mass spectrometry. Environ Health Perspect. 1994 Oct;102 (Suppl 6):11–16. doi: 10.1289/ehp.94102s611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Knize M. G., Boobis A. R., Gooderham N. J., Davies D. S., Murray S. Intra- and interindividual variability in systemic exposure in humans to 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline and 2-amino-1-methyl- 6-phenylimidazo[4,5-b]pyridine, carcinogens present in cooked beef. Cancer Res. 1992 Nov 15;52(22):6216–6223. [PubMed] [Google Scholar]
- Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994 Dec 23;266(5193):1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- Nagaoka H., Wakabayashi K., Kim S. B., Kim I. S., Tanaka Y., Ochiai M., Tada A., Nukaya H., Sugimura T., Nagao M. Adduct formation at C-8 of guanine on in vitro reaction of the ultimate form of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine with 2'-deoxyguanosine and its phosphate esters. Jpn J Cancer Res. 1992 Oct;83(10):1025–1029. doi: 10.1111/j.1349-7006.1992.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H., Takayama S., Sugimura T. Carcinogenicities of heterocyclic amines in cooked food. Mutat Res. 1991 Mar-Apr;259(3-4):399–410. doi: 10.1016/0165-1218(91)90130-e. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Liu B., Parsons R., Lengauer C., Palombo F., D'Arrigo A., Markowitz S., Willson J. K., Kinzler K. W. Mutations of GTBP in genetically unstable cells. Science. 1995 Jun 30;268(5219):1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Skog K., Steineck G., Augustsson K., Jägerstad M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis. 1995 Apr;16(4):861–867. doi: 10.1093/carcin/16.4.861. [DOI] [PubMed] [Google Scholar]
- Steineck G., Gerhardsson de Verdier M., Overvik E. The epidemiological evidence concerning intake of mutagenic activity from the fried surface and the risk of cancer cannot justify preventive measures. Eur J Cancer Prev. 1993 Jul;2(4):293–300. doi: 10.1097/00008469-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Tada A., Ochiai M., Wakabayashi K., Nukaya H., Sugimura T., Nagao M. Identification of N-(deoxyguanosin-8-yl)-2-amino-3,4-dimethylimidazo[4,5-f]quinoline (dG-C8-MeIQ) as a major adduct formed by MeIQ with nucleotides in vitro with DNA in vivo. Carcinogenesis. 1994 Jun;15(6):1275–1278. doi: 10.1093/carcin/15.6.1275. [DOI] [PubMed] [Google Scholar]
- Turesky R. J., Markovic J. DNA adduct formation of the food carcinogen 2-amino-3-methylimidazo[4,5- f]quinoline at the C-8 and N2 atoms of guanine. Chem Res Toxicol. 1994 Nov-Dec;7(6):752–761. doi: 10.1021/tx00042a007. [DOI] [PubMed] [Google Scholar]
- Ushiyama H., Wakabayashi K., Hirose M., Itoh H., Sugimura T., Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parenteral alimentation. Carcinogenesis. 1991 Aug;12(8):1417–1422. doi: 10.1093/carcin/12.8.1417. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Nagao M., Esumi H., Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992 Apr 1;52(7 Suppl):2092s–2098s. [PubMed] [Google Scholar]
- van Schooten F. J., Hillebrand M. J., van Leeuwen F. E., Lutgerink J. T., van Zandwijk N., Jansen H. M., Kriek E. Polycyclic aromatic hydrocarbon-DNA adducts in lung tissue from lung cancer patients. Carcinogenesis. 1990 Sep;11(9):1677–1681. doi: 10.1093/carcin/11.9.1677. [DOI] [PubMed] [Google Scholar]