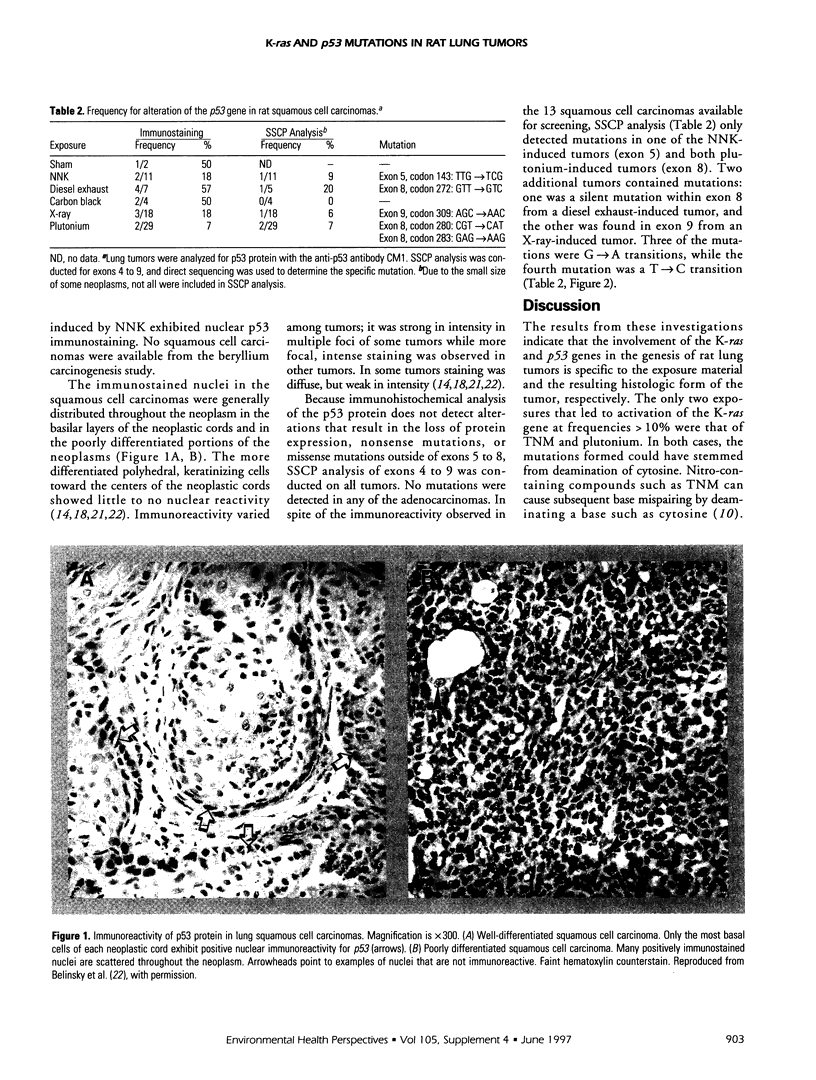
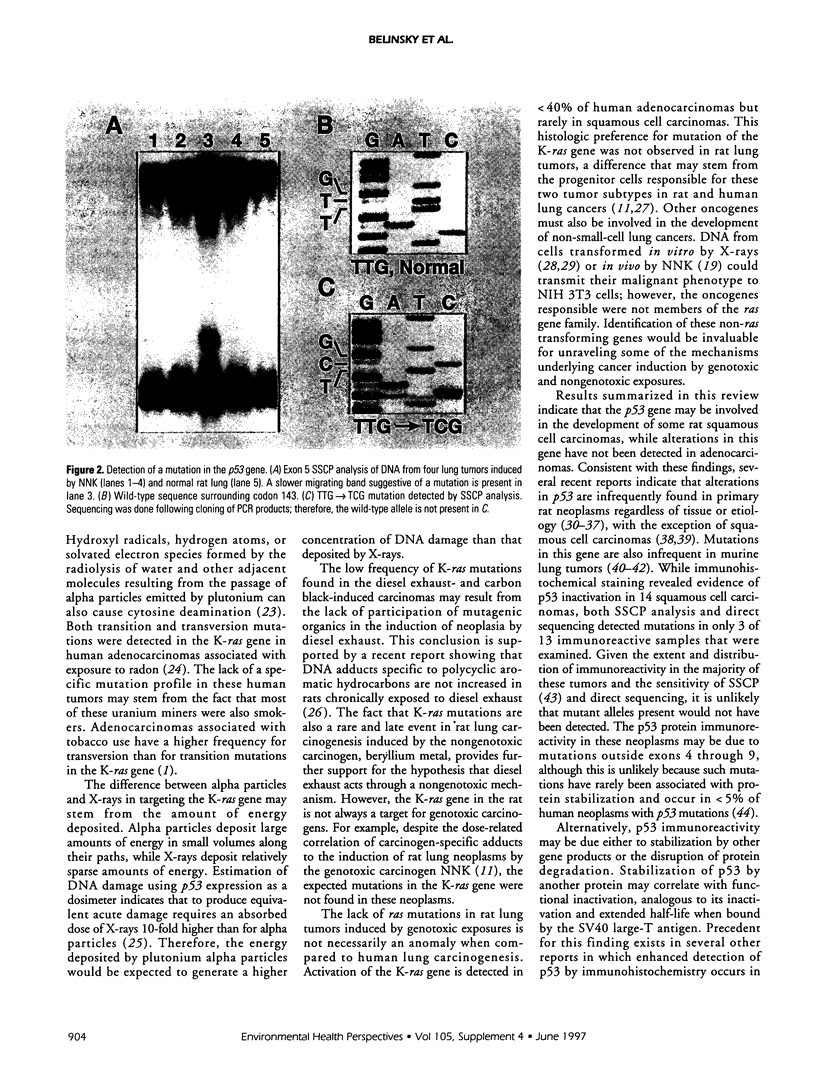
Abstract
Activation of the K-ras protooncogene and inactivation of the p53 tumor suppressor gene are events common to many types of human cancers. Molecular epidemiology studies have associated mutational profiles in these genes with specific exposures. The purpose of this paper is to review investigations that have examined the role of the K-ras and p53 genes in lung tumors induced in the F344 rat by mutagenic and nonmutagenic exposures. Mutation profiles within the K-ras and p53 genes, if present in rat lung tumors, would help to define some of the molecular mechanisms underlying cancer induction by various environmental agents. Pulmonary adenocarcinomas or squamous cell carcinomas were induced by tetranitromethane (TNM), 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK), beryllium metal, plutonium-239, X-ray, diesel exhaust, or carbon black. These agents were chosen because the tumors they produced could arise via different types of DNA damage. Mutation of the K-ras gene was determined by approaches that included DNA transfection, direct sequencing, mismatch hybridization, and restriction fragment length polymorphism analysis. The frequency for mutation of the K-ras gene was exposure dependent. Only two agents, TNM and plutonium, led to mutation frequencies of > 10%. In both cases, the transition mutations formed could have been derived from deamination of cytosine. The identification of non-ras transforming genes in rat lung tumors induced by mutagenic and nonmutagenic exposures such as NNK and beryllium would help define some of the mechanisms underlying cancer induction by different types of DNA damage. Alteration in the p53 gene was assessed by immunohistochemical analysis for p53 protein and single-strand conformation polymorphism (SSCP) analysis of exons 4 to 9. None of the 93 adenocarcinomas examined was immunoreactive toward the anti-p53 antibody CM1. In contrast, 14 to 71 squamous cell carcinomas exhibited nuclear p53 immunoreactivity with no correlation to type of exposure. However, SSCP analysis only detected mutations in 2 of 14 squamous cell tumors that were immunoreactive, suggesting that protein stabilization did not stem from mutations within the p53 gene. Thus, the p53 gene does not appear to be involved in the genesis of most rat lung tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asamoto M., Mann A. M., Cohen S. M. p53 mutation is infrequent and might not give a growth advantage in rat bladder carcinogenesis in vivo. Carcinogenesis. 1994 Mar;15(3):455–458. doi: 10.1093/carcin/15.3.455. [DOI] [PubMed] [Google Scholar]
- Asamoto M., Mann A. M., Macatee T. L., Cohen S. M. Mutations and expression of the p53 gene in rat bladder carcinomas and cell lines. Mol Carcinog. 1994 Apr;9(4):236–244. doi: 10.1002/mc.2940090408. [DOI] [PubMed] [Google Scholar]
- Belinsky S. A., Devereux T. R., White C. M., Foley J. F., Maronpot R. R., Anderson M. W. Role of Clara cells and type II cells in the development of pulmonary tumors in rats and mice following exposure to a tobacco-specific nitrosamine. Exp Lung Res. 1991 Mar-Apr;17(2):263–278. doi: 10.3109/01902149109064417. [DOI] [PubMed] [Google Scholar]
- Belinsky S. A., Foley J. F., White C. M., Anderson M. W., Maronpot R. R. Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1990 Jun 15;50(12):3772–3780. [PubMed] [Google Scholar]
- Belinsky S. A., Middleton S. K., Picksley S. M., Hahn F. F., Nikula K. J. Analysis of the K-ras and p53 pathways in X-ray-induced lung tumors in the rat. Radiat Res. 1996 Apr;145(4):449–456. [PubMed] [Google Scholar]
- Bodner S. M., Minna J. D., Jensen S. M., D'Amico D., Carbone D., Mitsudomi T., Fedorko J., Buchhagen D. L., Nau M. M., Gazdar A. F. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992 Apr;7(4):743–749. [PubMed] [Google Scholar]
- Borek C., Ong A., Mason H. Distinctive transforming genes in x-ray-transformed mammalian cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):794–798. doi: 10.1073/pnas.84.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1993. CA Cancer J Clin. 1993 Jan-Feb;43(1):7–26. doi: 10.3322/canjclin.43.1.7. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H. Ionizing radiation-induced mutagenesis. Br J Cancer. 1988 Jan;57(1):6–18. doi: 10.1038/bjc.1988.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Liu L., Castonguay A., Maronpot R. R., Anderson M. W., You M. Dose-dependent ras mutation spectra in N-nitrosodiethylamine induced mouse liver tumors and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced mouse lung tumors. Carcinogenesis. 1993 Aug;14(8):1603–1608. doi: 10.1093/carcin/14.8.1603. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Pope C. A., 3rd Lung cancer and air pollution. Environ Health Perspect. 1995 Nov;103 (Suppl 8):219–224. doi: 10.1289/ehp.95103s8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda I., Ogawa K. Detection of p53 gene mutations in rat hepatocellular carcinoma cell lines by denaturing gradient gel electrophoresis. Mol Carcinog. 1993;7(4):257–262. doi: 10.1002/mc.2940070408. [DOI] [PubMed] [Google Scholar]
- Gallagher J., Heinrich U., George M., Hendee L., Phillips D. H., Lewtas J. Formation of DNA adducts in rat lung following chronic inhalation of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis. 1994 Jul;15(7):1291–1299. doi: 10.1093/carcin/15.7.1291. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Linnoila R. I. The pathology of lung cancer--changing concepts and newer diagnostic techniques. Semin Oncol. 1988 Jun;15(3):215–225. [PubMed] [Google Scholar]
- Goodrow T. L., Storer R. D., Leander K. R., Prahalada S. R., van Zwieten M. J., Bradley M. O. Murine p53 intron sequences 5-8 and their use in polymerase chain reaction/direct sequencing analysis of p53 mutations in CD-1 mouse liver and lung tumors. Mol Carcinog. 1992;5(1):9–15. doi: 10.1002/mc.2940050105. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert R. A., Gillett N. A., Rebar A. H., Lundgren D. L., Hoover M. D., Chang I. Y., Carlton W. W., Hahn F. F. Sequential analysis of the pathogenesis of plutonium-induced pulmonary neoplasms in the rat: morphology, morphometry, and cytokinetics. Radiat Res. 1993 Apr;134(1):29–42. [PubMed] [Google Scholar]
- Hickman A. W., Jaramillo R. J., Lechner J. F., Johnson N. F. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994 Nov 15;54(22):5797–5800. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hosoe S., Shigedo Y., Ueno K., Tachibana I., Osaki T., Tanio Y., Kawase I., Yamakawa K., Nakamura Y., Kishimoto T. Detailed deletion mapping of the short arm of chromosome 3 in small cell and non-small cell carcinoma of the lung. Lung Cancer. 1994 Mar;10(5-6):297–305. doi: 10.1016/0169-5002(94)90659-9. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Jones R. F., Matuszyk J., Debiec-Rychter M., Wang C. Y. Mutation and altered expression of p53 genes in experimental rat bladder tumor cells. Mol Carcinog. 1994 Feb;9(2):95–104. doi: 10.1002/mc.2940090207. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kelly G., Stegelmeier B. L., Hahn F. F. p53 alterations in plutonium-induced F344 rat lung tumors. Radiat Res. 1995 Jun;142(3):263–269. [PubMed] [Google Scholar]
- Landers J. E., Haines D. S., Strauss J. F., 3rd, George D. L. Enhanced translation: a novel mechanism of mdm2 oncogene overexpression identified in human tumor cells. Oncogene. 1994 Sep;9(9):2745–2750. [PubMed] [Google Scholar]
- Lehman T. A., Bennett W. P., Metcalf R. A., Welsh J. A., Ecker J., Modali R. V., Ullrich S., Romano J. W., Appella E., Testa J. R. p53 mutations, ras mutations, and p53-heat shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 1991 Aug 1;51(15):4090–4096. [PubMed] [Google Scholar]
- Leuthauser S. W., Thomas J. E., Guernsey D. L. Oncogenes in X-ray-transformed C3H 10T1/2 mouse cells and in X-ray-induced mouse fibrosarcoma (RIF-1) cells. Int J Radiat Biol. 1992 Jul;62(1):45–51. doi: 10.1080/09553009214551811. [DOI] [PubMed] [Google Scholar]
- Lubin J. H., Boice J. D., Jr, Edling C., Hornung R. W., Howe G. R., Kunz E., Kusiak R. A., Morrison H. I., Radford E. P., Samet J. M. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995 Jun 7;87(11):817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- Makino H., Ishizaka Y., Tsujimoto A., Nakamura T., Onda M., Sugimura T., Nagao M. Rat p53 gene mutations in primary Zymbal gland tumors induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4850–4854. doi: 10.1073/pnas.89.11.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. W., Taylor J. A., Watson M. A., Saccomanno G., Devereux T. R. p53 and K-ras in radon-associated lung adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1995 Oct-Nov;4(7):791–793. [PubMed] [Google Scholar]
- Murphy S. E., Palomino A., Hecht S. S., Hoffmann D. Dose-response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res. 1990 Sep 1;50(17):5446–5452. [PubMed] [Google Scholar]
- Nickell-Brady C., Hahn F. F., Finch G. L., Belinsky S. A. Analysis of K-ras, p53 and c-raf-1 mutations in beryllium-induced rat lung tumors. Carcinogenesis. 1994 Feb;15(2):257–262. doi: 10.1093/carcin/15.2.257. [DOI] [PubMed] [Google Scholar]
- Nikula K. J., Snipes M. B., Barr E. B., Griffith W. C., Henderson R. F., Mauderly J. L. Comparative pulmonary toxicities and carcinogenicities of chronically inhaled diesel exhaust and carbon black in F344 rats. Fundam Appl Toxicol. 1995 Apr;25(1):80–94. doi: 10.1093/toxsci/25.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio L., Sisk S., Pluta L., Bermudez E., Gross E. A., Chen Z., Morgan K., Walker C. p53 mutations in formaldehyde-induced nasal squamous cell carcinomas in rats. Cancer Res. 1992 Nov 1;52(21):6113–6116. [PubMed] [Google Scholar]
- Smith M. L., Yeleswarapu L., Lombardi B., Shinozuka H. Lack of mutations of the p53 tumor suppressor gene in hepatocellular carcinomas induced in rats by a peroxisome proliferator. Mol Carcinog. 1993;7(2):89–93. doi: 10.1002/mc.2940070206. [DOI] [PubMed] [Google Scholar]
- Stegelmeier B. L., Gillett N. A., Rebar A. H., Kelly G. The molecular progression of plutonium-239-induced rat lung carcinogenesis: Ki-ras expression and activation. Mol Carcinog. 1991;4(1):43–51. doi: 10.1002/mc.2940040108. [DOI] [PubMed] [Google Scholar]
- Stowers S. J., Glover P. L., Reynolds S. H., Boone L. R., Maronpot R. R., Anderson M. W. Activation of the K-ras protooncogene in lung tumors from rats and mice chronically exposed to tetranitromethane. Cancer Res. 1987 Jun 15;47(12):3212–3219. [PubMed] [Google Scholar]
- Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990 Jul;5(7):1037–1043. [PubMed] [Google Scholar]
- Swafford D. S., Nikula K. J., Mitchell C. E., Belinsky S. A. Low frequency of alterations in p53, K-ras, and mdm2 in rat lung neoplasms induced by diesel exhaust or carbon black. Carcinogenesis. 1995 May;16(5):1215–1221. doi: 10.1093/carcin/16.5.1215. [DOI] [PubMed] [Google Scholar]
- Tokusashi Y., Fukuda I., Ogawa K. Absence of p53 mutations and various frequencies of Ki-ras exon 1 mutations in rat hepatic tumors induced by different carcinogens. Mol Carcinog. 1994 May;10(1):45–51. doi: 10.1002/mc.2940100108. [DOI] [PubMed] [Google Scholar]
- Ushijima T., Kakiuchi H., Makino H., Hasegawa R., Ishizaka Y., Hirai H., Yazaki Y., Ito N., Sugimura T., Nagao M. Infrequent mutation of Ha-ras and p53 in rat mammary carcinomas induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Mol Carcinog. 1994 May;10(1):38–44. doi: 10.1002/mc.2940100107. [DOI] [PubMed] [Google Scholar]
- Weghorst C. M., Dragnev K. H., Buzard G. S., Thorne K. L., Vandeborne G. F., Vincent K. A., Rice J. M. Low incidence of point mutations detected in the p53 tumor suppressor gene from chemically induced rat renal mesenchymal tumors. Cancer Res. 1994 Jan 1;54(1):215–219. [PubMed] [Google Scholar]
- You M., Candrian U., Maronpot R. R., Stoner G. D., Anderson M. W. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]