Abstract
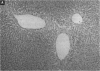
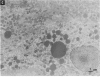
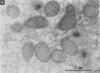
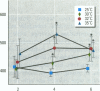
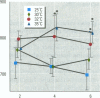
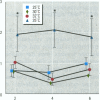
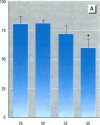
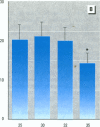
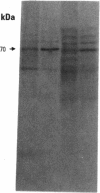
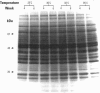
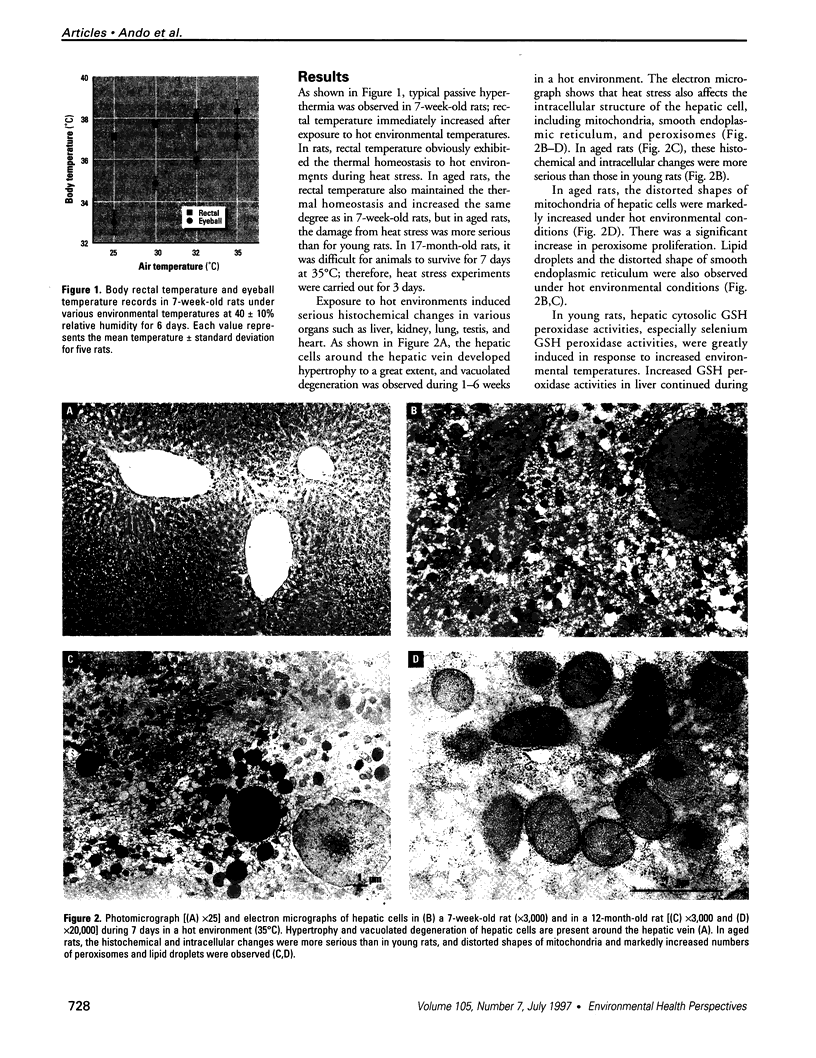
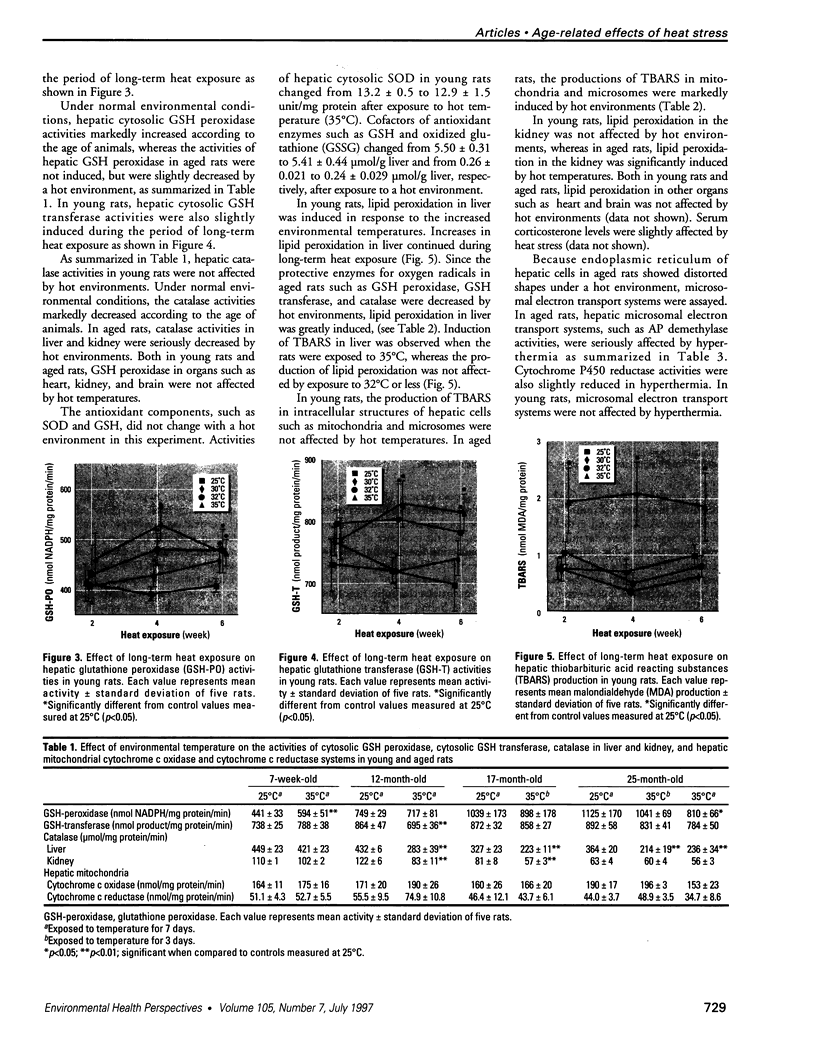
To evaluate the age-related response of essential cell functions against peroxidative damage in hyperthermia, we studied the biochemical response to heat stress in both young and aged rats. Passive hyperthermia was immediately observed in rats after exposure to hot environments. In aged rats, the rectal temperature maintained thermal homeostasis and increased to the same degree as in young rats. In these aged animals, the damage from heat stress was more serious than in young animals. In aged rats under normal environmental conditions, hepatic cytosolic glutathione peroxidase (GSH peroxidase) activities were markedly higher than those activities in younger rats. Hepatic cytosolic GSH peroxidase activities were induced by heat stress in young rats but were decreased by hot environments in aged rats. Hepatic catalase activities in young rats were not affected by hot environments, whereas in aged rats, hepatic catalase activities were seriously decreased. Catalase activities in the kidney of aged rats were also reduced by hot environments. Lipid peroxidation in the liver was markedly induced in both young and aged rats. Because the protective enzymes for oxygen radicals in aged rats were decreased by hot environments, lipid peroxidation in the liver was highly induced. In aged rats, lipid peroxidation in intracellular structures such as mitochondria and microsomes was also markedly induced by hot environments. In both young and aged rats, hyperthermia greatly increased the development of hypertrophy and vacuolated degeneration in hepatic cells. In aged rats, both mitochondria and endoplasmic reticulum of the hepatic cells showed serious distortion in shape as a result of exposures to hot environments. Microsomal electron transport systems, such as cytochrome P450 monooxygenase activities, were seriously decreased by heat stress in aged rats but not in young rats. Although the mitochondrial electron transport systems were not affected by acute heat stress in young rats, their activities were simultaneously inhibited after long-lasting heat exposure. In isolated hepatic cells and polymorphonuclear leukocytes in animals, the 70-kDa heat shock-induced proteins were markedly increased by heat stress. In conclusion, the heat stress-inducible oxygen radical damage becomes more severe according to the age of rats. Because aging and hyperthermia have a synergistic effect on lipid peroxidation, protective enzyme activities for oxygen radicals may be essential for surviving and recovering from thermal injury in aged animals and also in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Tappel A. L. Effect of dietary vitamin E on methyl ethyl ketone peroxide damage to microsomal cytochrome P-450 peroxidase. Chem Biol Interact. 1985 Nov;55(3):317–326. doi: 10.1016/s0009-2797(85)80138-1. [DOI] [PubMed] [Google Scholar]
- Ando M., Tappel A. L. Methyl ethyl ketone peroxide damage to cytochrome P-450 peroxidase activities. Toxicol Appl Pharmacol. 1985 Dec;81(3 Pt 1):517–524. doi: 10.1016/0041-008x(85)90422-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubatz J. W., Tan B. H. Age-related studies on the removal of 7-methylguanine from DNA of mouse kidney tissue following N-methyl-N-nitrosourea treatment. Mutat Res. 1993 Mar;295(2):81–91. doi: 10.1016/0921-8734(93)90004-m. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hruszkewycz A. M., Glende E. A., Jr, Recknagel R. O. Destruction of microsomal cytochrome P-450 and glucose-6-phosphatase by lipids extracted from peroxidized microsomes. Toxicol Appl Pharmacol. 1978 Dec;46(3):695–702. doi: 10.1016/0041-008x(78)90314-9. [DOI] [PubMed] [Google Scholar]
- Imai M., Shimada H., Watanabe Y., Matsushima-Hibiya Y., Makino R., Koga H., Horiuchi T., Ishimura Y. Uncoupling of the cytochrome P-450cam monooxygenase reaction by a single mutation, threonine-252 to alanine or valine: possible role of the hydroxy amino acid in oxygen activation. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7823–7827. doi: 10.1073/pnas.86.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. S., Liang A. P., Kilbourne E. M., Griffin M. R., Patriarca P. A., Wassilak S. G., Mullan R. J., Herrick R. F., Donnell H. D., Jr, Choi K. Morbidity and mortality associated with the July 1980 heat wave in St Louis and Kansas City, Mo. JAMA. 1982 Jun 25;247(24):3327–3331. [PubMed] [Google Scholar]
- Keatinge W. R., Coleshaw S. R., Easton J. C., Cotter F., Mattock M. B., Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986 Nov;81(5):795–800. doi: 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C. H., Church D. F., Winston G. W., Pryor W. A. tert-Butyl hydroperoxide-induced radical production in rat liver mitochondria. Free Radic Biol Med. 1992;12(5):381–387. doi: 10.1016/0891-5849(92)90087-w. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978 Feb;108(2):211–215. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- Loven D. P., Leeper D. B., Oberley L. W. Superoxide dismutase levels in Chinese hamster ovary cells and ovarian carcinoma cells after hyperthermia or exposure to cycloheximide. Cancer Res. 1985 Jul;45(7):3029–3033. [PubMed] [Google Scholar]
- Marmor M. Heat wave mortality in nursing homes. Environ Res. 1978 Aug;17(1):102–115. doi: 10.1016/0013-9351(78)90065-8. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Grover T. A., Nayini N., Aust S. D. Xanthine oxidase- and iron-dependent lipid peroxidation. Arch Biochem Biophys. 1993 Feb 15;301(1):1–7. doi: 10.1006/abbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J., Rahimtula A. D. A peroxidase assay for cytochrome P-450. Methods Enzymol. 1978;52:407–412. doi: 10.1016/s0076-6879(78)52045-4. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Ponti V., Dianzani M. U., Cheeseman K., Slater T. F. Studies on the reduction of nitroblue tetrazolium chloride mediated through the action of NADH and phenazine methosulphate. Chem Biol Interact. 1978 Dec;23(3):281–291. doi: 10.1016/0009-2797(78)90090-x. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Arbour N. C., Upham B., Church D. F. The inhibitory effect of extracts of cigarette tar on electron transport of mitochondria and submitochondrial particles. Free Radic Biol Med. 1992;12(5):365–372. doi: 10.1016/0891-5849(92)90085-u. [DOI] [PubMed] [Google Scholar]
- Sadis S., Raghavendra K., Hightower L. E. Secondary structure of the mammalian 70-kilodalton heat shock cognate protein analyzed by circular dichroism spectroscopy and secondary structure prediction. Biochemistry. 1990 Sep 11;29(36):8199–8206. doi: 10.1021/bi00488a001. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Takesue S., Omura T. Purification and properties of NADH-cytochrome b5 reductase solubilized by lysosomes from rat liver microsomes. J Biochem. 1970 Feb;67(2):267–276. doi: 10.1093/oxfordjournals.jbchem.a129250. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Werringloer J. Assay of formaldehyde generated during microsomal oxidation reactions. Methods Enzymol. 1978;52:297–302. doi: 10.1016/s0076-6879(78)52031-4. [DOI] [PubMed] [Google Scholar]