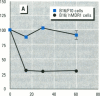
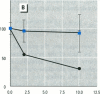
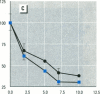
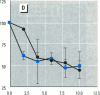
Abstract
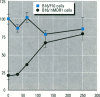
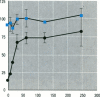
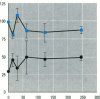
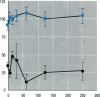
The multixenobiotic resistance phenotype is characterized by the reduced accumulation of xenobiotics by cells or organisms due to increased efflux of the compounds by P-glycoprotein (P-gp) or related transporters. An extensive xenobiotic database, consisting primarily of pesticides, was utilized in this study to identify molecular characteristics that render a xenobiotic susceptible to transport by or inhibition of P-gp. Transport substrates were differentiated by several molecular size/shape parameters, lipophilicity, and hydrogen bonding potential. Electrostatic features differentiated inhibitory ligands from compounds not catagorized as transport substrates and that did no interact with P-gp. A two-tiered system was developed using the derived structure-activity relationships to identify P-gp transport substrates and inhibitory ligands. Prediction accuracy of the approach was 82%. We then validated the system using six additional pesticides of which tow were predicted to be P-gp inhibitors and four were predicted to be noninteractors, based upon the structure-activity analyses. Experimental determinations using cells transfected with the human MDR1 gene demonstrated that five of the six pesticides were properly catagorized by the structure-activity analyses (83% accuracy). Finally, structure-activity analyses revealed that among P-gp inhibitors, relative inhibitory potency can be predicted based upon the surface area or volume of the compound. These results demonstrate that P-gp transport substrates and inhibitory ligands can be distinguished using molecular characteristics. Molecular characteristics of transport substrates suggest that P-gp may function in the elimination of hydroxylated metabolites of xenobiotics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bain L. J., LeBlanc G. A. Interaction of structurally diverse pesticides with the human MDR1 gene product P-glycoprotein. Toxicol Appl Pharmacol. 1996 Nov;141(1):288–298. doi: 10.1006/taap.1996.0286. [DOI] [PubMed] [Google Scholar]
- Christensen J. G., LeBlanc G. A. Reversal of multidrug resistance in vivo by dietary administration of the phytochemical indole-3-carbinol. Cancer Res. 1996 Feb 1;56(3):574–581. [PubMed] [Google Scholar]
- Debal V., Morjani H., Millot J. M., Angiboust J. F., Gourdier B., Manfait M. Determination of vinorelbine (Navelbine) in tumour cells by high-performance liquid chromatography. J Chromatogr. 1992 Oct 2;581(1):93–99. doi: 10.1016/0378-4347(92)80451-u. [DOI] [PubMed] [Google Scholar]
- Egorin M. J., Hildebrand R. C., Cimino E. F., Bachur N. R. Cytofluorescence localization of adriamycin and daunorubicin. Cancer Res. 1974 Sep;34(9):2243–2245. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Hofsli E., Nissen-Meyer J. Reversal of multidrug resistance by lipophilic drugs. Cancer Res. 1990 Jul 1;50(13):3997–4002. [PubMed] [Google Scholar]
- Horton J. K., Thimmaiah K. N., Houghton J. A., Horowitz M. E., Houghton P. J. Modulation by verapamil of vincristine pharmacokinetics and toxicity in mice bearing human tumor xenografts. Biochem Pharmacol. 1989 Jun 1;38(11):1727–1736. doi: 10.1016/0006-2952(89)90405-x. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Haraguchi M., Sumizawa T., Yoshimura A., Furukawa T., Hiramoto S., Sugita M., Akiyama S. Progesterone and its metabolites: the potent inhibitors of the transporting activity of P-glycoprotein in the adrenal gland. Biochim Biophys Acta. 1993 Nov 28;1158(3):201–208. doi: 10.1016/0304-4165(93)90016-2. [DOI] [PubMed] [Google Scholar]
- Kodavanti U. P., Mehendale H. M. Cationic amphiphilic drugs and phospholipid storage disorder. Pharmacol Rev. 1990 Dec;42(4):327–354. [PubMed] [Google Scholar]
- Kumar R. Simultaneous determination of some organophosphorus pesticides by high performance liquid chromatography. Biomed Chromatogr. 1989 Nov;3(6):272–273. doi: 10.1002/bmc.1130030610. [DOI] [PubMed] [Google Scholar]
- Lanning C. L., Fine R. L., Sachs C. W., Rao U. S., Corcoran J. J., Abou-Donia M. B. Chlorpyrifos oxon interacts with the mammalian multidrug resistance protein, P-glycoprotein. J Toxicol Environ Health. 1996 Mar;47(4):395–407. doi: 10.1080/009841096161726. [DOI] [PubMed] [Google Scholar]
- Negre M., Gennari M., Cignetti A. High-performance liquid chromatographic determination of Fluazifop-butyl and Fluazifop in soil and water. J Chromatogr. 1987 Jan 30;387:541–545. doi: 10.1016/s0021-9673(01)94568-6. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Jansen P. L. The role of the canalicular multispecific organic anion transporter in the disposal of endo- and xenobiotics. Pharmacol Ther. 1994 Oct;64(1):77–97. doi: 10.1016/0163-7258(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Paul S., Breuninger L. M., Kruh G. D. ATP-dependent transport of lipophilic cytotoxic drugs by membrane vesicles prepared from MRP-overexpressing HL60/ADR cells. Biochemistry. 1996 Nov 5;35(44):14003–14011. doi: 10.1021/bi9618528. [DOI] [PubMed] [Google Scholar]
- Phang J. M., Poore C. M., Lopaczynska J., Yeh G. C. Flavonol-stimulated efflux of 7,12-dimethylbenz(a)anthracene in multidrug-resistant breast cancer cells. Cancer Res. 1993 Dec 15;53(24):5977–5981. [PubMed] [Google Scholar]
- Schinkel A. H., Smit J. J., van Tellingen O., Beijnen J. H., Wagenaar E., van Deemter L., Mol C. A., van der Valk M. A., Robanus-Maandag E. C., te Riele H. P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994 May 20;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Ueda K., Okamura N., Hirai M., Tanigawara Y., Saeki T., Kioka N., Komano T., Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992 Dec 5;267(34):24248–24252. [PubMed] [Google Scholar]
- Van Lancker M. A., Bellemans L. A., De Leenheer A. P. Quantitative determination of low concentrations of adriamycin in plasma and cell cultures, using a volatile extraction buffer. J Chromatogr. 1986 Jan 24;374(2):415–420. doi: 10.1016/s0378-4347(00)83302-2. [DOI] [PubMed] [Google Scholar]
- Wolf D. C., Horwitz S. B. P-glycoprotein transports corticosterone and is photoaffinity-labeled by the steroid. Int J Cancer. 1992 Aug 19;52(1):141–146. doi: 10.1002/ijc.2910520125. [DOI] [PubMed] [Google Scholar]
- Yeh G. C., Lopaczynska J., Poore C. M., Phang J. M. A new functional role for P-glycoprotein: efflux pump for benzo(alpha)pyrene in human breast cancer MCF-7 cells. Cancer Res. 1992 Dec 1;52(23):6692–6695. [PubMed] [Google Scholar]
- Zamora J. M., Pearce H. L., Beck W. T. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells. Mol Pharmacol. 1988 Apr;33(4):454–462. [PubMed] [Google Scholar]
- van Kalken C. K., Broxterman H. J., Pinedo H. M., Feller N., Dekker H., Lankelma J., Giaccone G. Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer. 1993 Feb;67(2):284–289. doi: 10.1038/bjc.1993.54. [DOI] [PMC free article] [PubMed] [Google Scholar]