Abstract
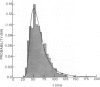
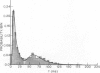
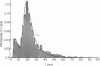
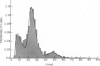
The statistics of the variability of interspike intervals of ganglion cells in the retina of goldfish are modeled by assuming the noise in an integrate-and-fire mechanism is proportional to the reciprocal of a normally distributed variable. This model meets the constraint that the coefficient of variation of the interspike. This does not change when the mean firing rate of the neuron changes. Alternative sources of variability of interspike intervals are discussed.
Full text
PDF




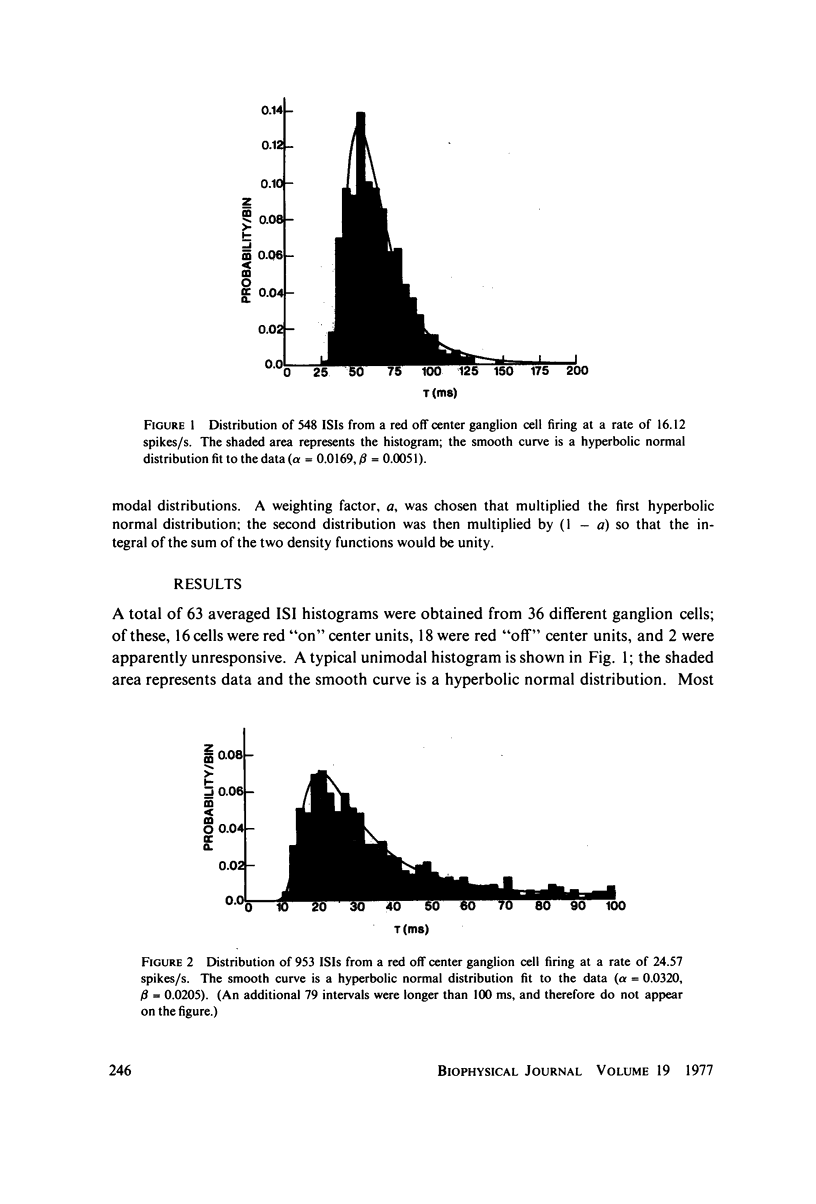
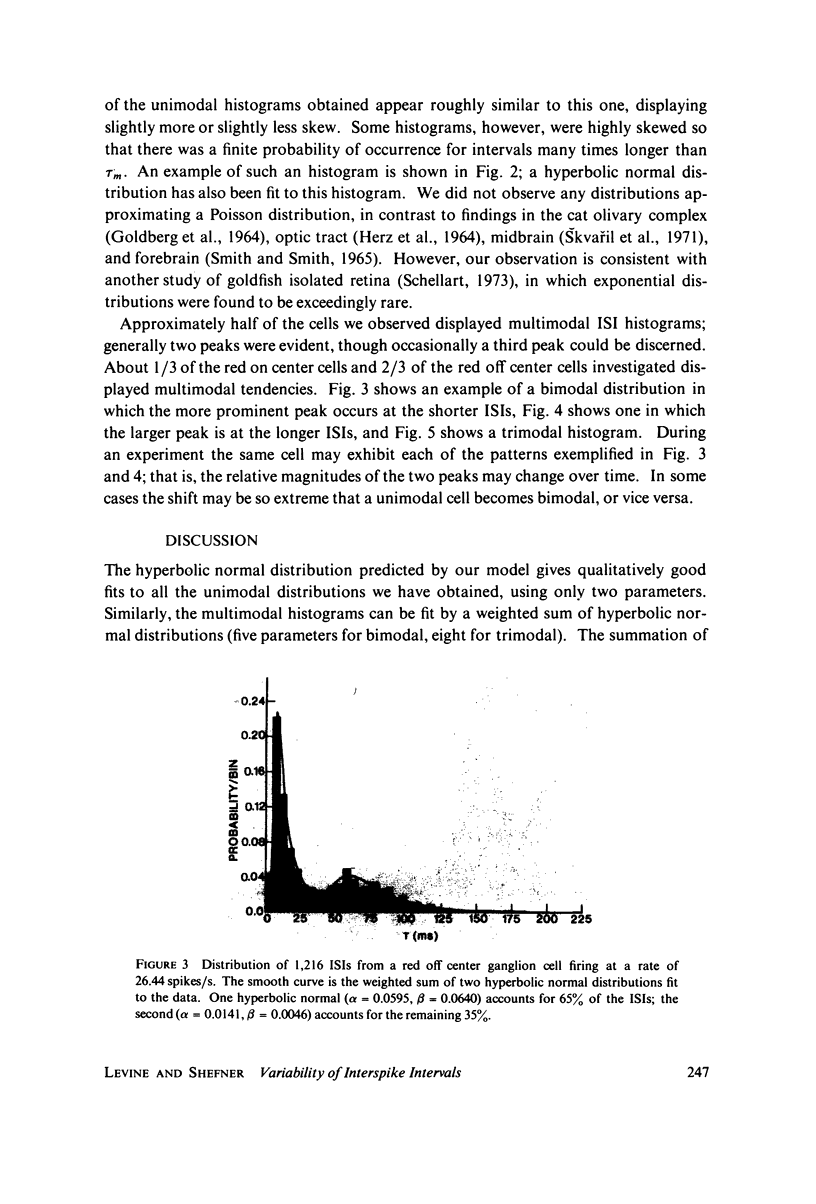
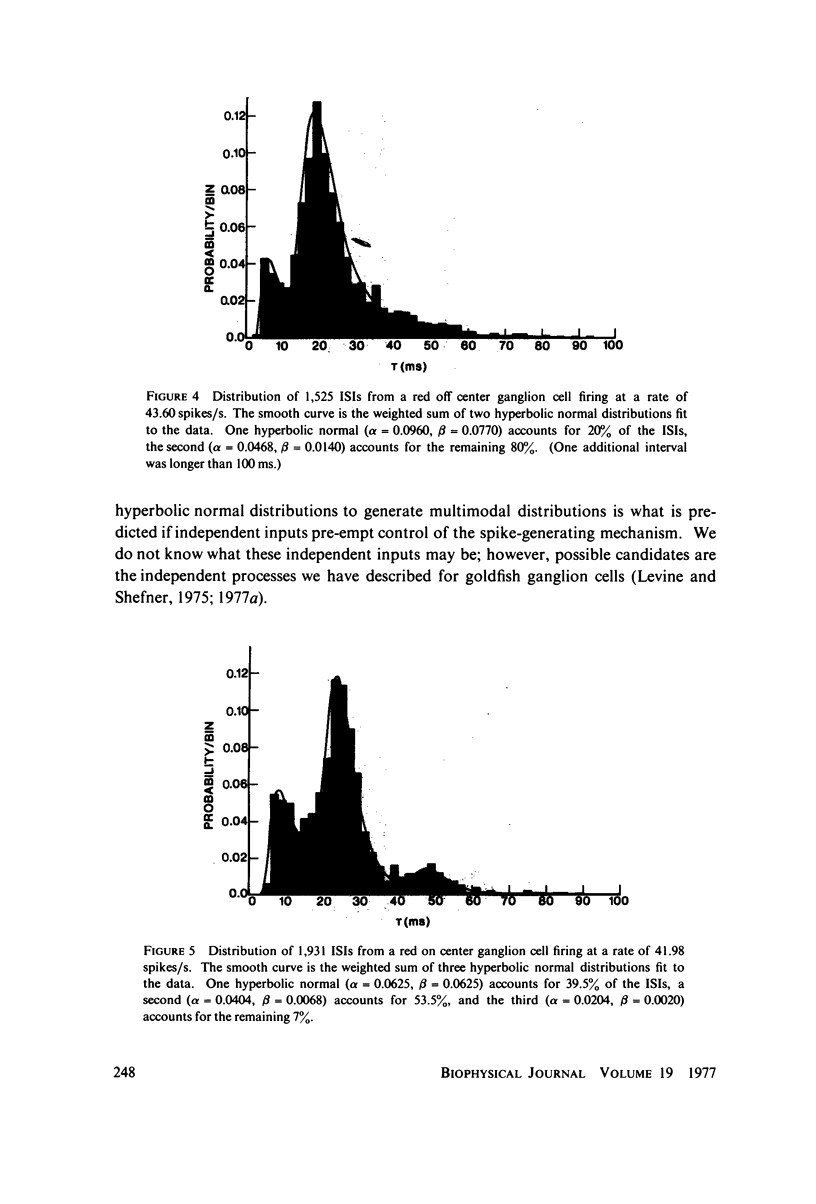




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., LEVICK W. R., WILLIAMS W. O. STATISTICAL ANALYSIS OF THE DARK DISCHARGE OF LATERAL GENICULATE NEURONES. J Physiol. 1964 Apr;170:598–612. doi: 10.1113/jphysiol.1964.sp007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall W. A., Sherebrin M. H. Spontaneous activity in amphibian second-order olfactory neurons. Brain Res. 1976 Feb 27;103(3):555–559. doi: 10.1016/0006-8993(76)90455-8. [DOI] [PubMed] [Google Scholar]
- Ekholm A., Hyvärinen J. A pseudo-Markov model for series of neuronal spike events. Biophys J. 1970 Aug;10(8):773–796. doi: 10.1016/S0006-3495(70)86335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSTEIN G. L., MANDELBROT B. RANDOM WALK MODELS FOR THE SPIKE ACTIVITY OF A SINGLE NEURON. Biophys J. 1964 Jan;4:41–68. doi: 10.1016/s0006-3495(64)86768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG J. M., ADRIAN H. O., SMITH F. D. RESPONSE OF NEURONS OF THE SUPERIOR OLIVARY COMPLEX OF THE CAT TO ACOUSTIC STIMULI OF LONG DURATION. J Neurophysiol. 1964 Jul;27:706–749. doi: 10.1152/jn.1964.27.4.706. [DOI] [PubMed] [Google Scholar]
- Geisler C. D., Goldberg J. M. A stochastic model of the repetitive activity of neurons. Biophys J. 1966 Jan;6(1):53–69. doi: 10.1016/S0006-3495(66)86639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G., Maffei L., Petracchi D. Spatial and temporal organization in retinal units. Kybernetik. 1966 Nov;3(4):196–202. doi: 10.1007/BF00290257. [DOI] [PubMed] [Google Scholar]
- Gestri G. Pulse frequency modulation in neural systems. A random model. Biophys J. 1971 Jan;11(1):98–109. doi: 10.1016/S0006-3495(71)86198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A., Creutzfeldt O., Fuster J. Statistische Eigenschaften der Neuronaktivität im ascendierenden visuellen System. Kybernetik. 1964 Jun;2(2):61–71. doi: 10.1007/BF00288559. [DOI] [PubMed] [Google Scholar]
- Hoopen M. T. Probabilistic firing of neurons considered as a first passage problem. Biophys J. 2008 Dec 31;6(4):435–451. doi: 10.1016/S0006-3495(66)86668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. W. Dynamics of encoding in a population of neurons. J Gen Physiol. 1972 Jun;59(6):734–766. doi: 10.1085/jgp.59.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W. M., Reitboeck H. J. Color-dependent distribution of spikes in single optic tract fibers of the cat. Vision Res. 1974 Jun;14(6):405–419. doi: 10.1016/0042-6989(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Abramov I. An analysis of spatial summation in the receptive fields of goldfish retinal ganglion cells. Vision Res. 1975 Jul;15(7):777–789. doi: 10.1016/0042-6989(75)90255-2. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. Independence of "on" and "off" responses of retinal ganglion cells. Science. 1975 Dec 19;190(4220):1215–1217. doi: 10.1126/science.1239079. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Masterbroek H. A., Zaagman W. H., Kuiper J. W. Intensity and structure of visually evoked neural activity: rivals in modelling a visual system. Vision Res. 1977;17(1):29–35. doi: 10.1016/0042-6989(77)90197-3. [DOI] [PubMed] [Google Scholar]
- Pernier J. Ajustement automatique des densités de probabilité d'intervalles entre potentiels d'action selon la loi de Wiener. Biometrics. 1972 Sep;28(3):737–745. [PubMed] [Google Scholar]
- Pernier J., Gerin P. Temporal patterns analysis of spontaneous unit activity in the neocortex. Biol Cybern. 1975;18(3-4):123–136. doi: 10.1007/BF00326684. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R. R., Kiang N. Y. Spike Discharge Patterns of Spontaneous and Continuously Stimulated Activity in the Cochlear Nucleus of Anesthetized Cats. Biophys J. 1965 May;5(3):301–316. doi: 10.1016/s0006-3495(65)86718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff F., Hartline H. K., Lange D. Variability of interspike intervals in optic nerve fibers of Limulus: effect of light and dark adaptation. Proc Natl Acad Sci U S A. 1968 Jun;60(2):464–469. doi: 10.1073/pnas.60.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- SMITH D. R., SMITH G. K. A STATISTICAL ANALYSIS OF THE CONTINUAL ACTIVITY OF SINGLE CORTICAL NEURONES IN THE CAT UNANAESTHETIZED ISOLATED FOREBRAIN. Biophys J. 1965 Jan;5:47–74. doi: 10.1016/s0006-3495(65)86702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN R. B. A THEORETICAL ANALYSIS OF NEURONAL VARIABILITY. Biophys J. 1965 Mar;5:173–194. doi: 10.1016/s0006-3495(65)86709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Origin of the stochastic nature of ganglion cell activity in isolated goldfish retina. Vision Res. 1973 Feb;13(2):337–345. doi: 10.1016/0042-6989(73)90111-9. [DOI] [PubMed] [Google Scholar]
- Skvaril J., Radil-Weiss T., Bohdanecký Z., Syka J. Spontaneous discharge patterns of mesencephalic neurons: interval histogram and mean interval relationship. Kybernetik. 1971 Jul;9(1):11–15. doi: 10.1007/BF00272554. [DOI] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Holden A. V. The frequency response, coherence, and information capacity of two neuronal models. Biophys J. 1972 Mar;12(3):295–322. doi: 10.1016/S0006-3495(72)86087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. THE VARIABILITY OF CENTRAL NEURAL ACTIVITY IN A SENSORY SYSTEM, AND ITS IMPLICATIONS FOR THE CENTRAL REFLECTION OF SENSORY EVENTS. J Neurophysiol. 1963 Nov;26:958–977. doi: 10.1152/jn.1963.26.6.958. [DOI] [PubMed] [Google Scholar]