Abstract
Rpe65 knockout mice (Rpe65−/−) are unable to synthesize the visual pigment chromophore 11-cis retinal; however, if these animals are reared in complete darkness, the rod photoreceptors accumulate a small amount of 9-cis retinal and its corresponding visual pigment isorhodopsin. Suction-electrode recording of single rods from dark-reared Rpe65−/− mice showed that the rods were about 400 times less sensitive than wild-type control rods and that the maximum responses were much smaller in amplitude. Spectral sensitivity measurements indicated that Rpe65−/− rod responses were generated by isorhodopsin rather than rhodopsin. Sensitivity and pigment concentration were compared in the same mice by measuring light responses from rods of one eye and pigment concentration from the retina of the other eye. Retinas had 11–35% of the normal pigment level, but the rods were of the order of 20–30 times less sensitive than could be accounted for by the loss in quantum catch. This extra desensitization must be caused by opsin-dependent activation of the visual cascade, which leads to a state equivalent to light adaptation in the dark-adapted rod. By comparing the sensitivity of dark-reared Rpe65−/− rods to that produced in normal rods by background light, we estimate that Rpe65−/− opsin is of the order of 2.5 × 10−5 as efficient in activating transduction as photoactivated rhodopsin (Rh*) in WT mice. Dark-reared Rpe65−/− rods are less desensitized than rods from cyclic light-reared Rpe65−/− mice, have about 50% more photocurrent and degenerate at a slower rate. Retinas sectioned after 9 months in darkness show a larger number of photoreceptor nuclei in dark-reared animals than in cyclic light-reared animals, though both have fewer nuclei than in cyclic light-reared wild-type retinas. Both also have shorter outer segments and a lower free-Ca2+ concentration. These experiments provide the first quantitative measurement of opsin activation in physiologically responding mammalian rods.
RPE65 is an abundant protein in the retinal pigment epithelium and is essential for synthesis of the rhodopsin ligand 11-cis-retinal (Redmond et al. 1998). Mutations in the Rpe65 gene are thought to be responsible for several human retinal degenerations, including Lebers congenital amaurosis (Gu et al. 1997; Marlhens et al. 1997; Morimura et al. 1998; Felius et al. 2002), autosomal recessive retinitis pigmentosa (Morimura et al. 1998), and rod–cone dystrophy (Lorenz et al. 2000). In Rpe65-null (Rpe65−/−) mice, the retinas have little functional visual pigment but large amounts of the opsin apo-protein, that is opsin unliganded to chromophore (Redmond et al. 1998; Ablonczy et al. 2002; Rohrer et al. 2003), and both rods and cones degenerate (Redmond et al. 1998; Seeliger et al. 2001; Woodruff et al. 2003).
Dark-adapted rods in these animals have smaller light responses and are greatly desensitized compared with dark-adapted wild-type rods (Van Hooser et al. 2002; Woodruff et al. 2003). These effects seem to be entirely due to lack of visual chromophore, since when Rpe65−/− mice are administered 9-cis or 11-cis retinal, they completely recover normal function (Van Hooser et al. 2002; Rohrer et al. 2003). The desensitization of the rods is produced in part from the lower pigment concentration (reduced quantum catch) and in part from activation of a process equivalent to light adaptation (Woodruff et al. 2003). Responses have a smaller maximum amplitude and decay more rapidly than dark-adapted control responses, and outer segments have a lower than normal free-Ca2+ concentration (Woodruff et al. 2003). These effects are probably produced by an equivalent background light generated by the abundant opsin apo-protein, since opsin is known to activate the visual cascade and can produce an adaptation state much like that produced by real light (Cornwall & Fain, 1994; Cornwall et al. 1995). Consistent with this interpretation, the slow degeneration of Rpe65−/− rods does not occur in animals that lack both the Rpe65 protein and the G-protein transducin (Woodruff et al. 2003). This shows that activation of the transduction cascade by opsin is the probable cause of the degeneration of the rods (Lem & Fain, 2004).
It has not been possible to determine what part of the desensitization in Rpe65−/− rods is due to the decrease in pigment concentration and what part to adaptation produced by activation of the cascade by opsin, since the concentration of functional pigment in cyclic light-reared animals is too low to be measured. Recent experiments have shown, however, that when Rpe65−/− animals are kept for long periods in darkness, the rods accumulate significant quantities of 9-cis retinal and the visual pigment isorhodopsin, and they are less desensitized than Rpe65−/− animals raised in cyclic light (Fan et al. 2003). We have used these animals to make measurements of pigment concentration and rod sensitivity from the two eyes of the same animal. We show that after 15–37 weeks of dark-rearing, retinas contain isorhodopsin at a level that is 11–35% of the normal rhodopsin concentration. Dark-adapted rods nevertheless behave as if light adapted by the remaining unliganded opsin. By comparing responses of dark-adapted Rpe65−/− rods to normal rods exposed to a continuous background intensity, we show that opsin activates the cascade at an efficiency about 2.5 × 10−5 that of photoexcited rhodopsin (Rh*). This rate of activation is between 1 and 2 orders of magnitude higher than in salamander rods (Cornwall & Fain, 1994), indicating that mammalian opsin is noisier than amphibian opsin. This may have important implications for our understanding of the role of bleaching adaptation in vision.
These results were presented at the 2005 meeting of the Association for Research in Vision and Ophthalmology in Fort Lauderdale, Florida (Woodruff et al. 2005).
Methods
Suction-electrode recording and light stimulation
Techniques for recording of light responses of single mouse rods with suction electrodes have been previously described (Woodruff et al. 2002, 2003). In brief, mice kept in darkness for at least 3 h were killed in dim red illumination by cervical dislocation according to procedures approved by the Chancellor's Animal Research Committee at UCLA and in conformance with principles regarding the care and use of animals adopted by the American Physiological Society and the Society for Neuroscience. The eyes were removed and washed in 1–2 ml of Locke solution, of composition (mm): 140 NaCl, 3.6 KCl, 2.4 MgCl2, 1.2 CaCl2, 3 HEPES, 10 glucose, 5 sodium ascorbate, and 0.02 EDTA at pH 7.4. Retinas were isolated and finely chopped with a piece of razor blade under infrared illumination. The suspension of cells was transferred to the recording chamber, where it was perfused at 37°C with Dulbecco's modified Eagle's medium (D-2902, Sigma), supplemented with 15 mm NaHCO3, 2 mm sodium succinate, 0.5 mm sodium glutamate, 2 mm sodium gluconate, and 5 mm NaCl, bubbled with 5% CO2 (pH 7.4).
Suction pipettes pulled on a Flaming-Brown puller (Sutter Instruments, Novato, CA, USA) and polished on a home-made microforge were filled with Locke solution without glucose or ascorbate. Light stimuli were delivered with a conventional dual-beam optical bench, whose intensity was measured with a calibrated photodiode (Graseby Optronics, Orlando, FL, USA). The wavelength of light was varied with interference filters (Corion, Franklin, MA, USA) and the intensity, with neutral density absorption filters (Fish-Schurman Corp., New Rochelle, NY, USA). Stimulus duration was controlled with electronically driven shutters (Uniblitz, Vincent Associates, Rochester, NY, USA). Suction-pipette currents were amplified with a patch-clamp amplifier (Warner Instruments Co, Hamden, CT, USA), low-pass filtered with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA, USA), acquired with pCLAMP (Axon Instruments, Union City, CA, USA) and a PC computer, and analysed with Quattro Pro (Corel Corporation, Ottawa, Ontario, Canada) and Origin (OriginLab Corporation, Northampton, MA, USA). Data are given as means ±s.e.m. Tests of significance were done with Student's t test in Quattro Pro or Excel (Microsoft Corp., Redmond, WA, USA). Most traces shown in the figures are the averages of many individual responses, and the number of rods and/or stimulus presentations are given in the figure legends.
Extraction of pigment and spectrophotometry
A single retina from each dark-adapted Rpe65−/− mouse was isolated under infrared light. The pigment was extracted under dim red light (GBX-2 filter, Kodak) according to the following protocol. The retina was homogenized with a mini glass tissue grinder (Fisher) in 10 mm Tris buffer (pH 7.5) with 1 mm EDTA, 1 mm 4-(2-aminoethyl)-benzenesulphonyl fluoride hydrochloride (AEBSF; Roche Molecular Biochemicals, Mannheim, Germany), protease inhibitor cocktail tablet (Roche Molecular Biochemicals), and 10 μg DNase I (Sigma). The sample was centrifuged at 21 000 g for 15 min at 4°C, and the pellet was dispersed in 100 μl of 1% dodecylmaltoside (ULTROL grade; Calbiochem, La Jolla, CA, USA) in 0.1 m sodium phosphate buffer (pH 7.4) for 2 h at 4°C. The supernatant was separated by centrifugation at 25 000 g for 10 min at 4°C. Freshly neutralized hydroxylamine (20 mm) was added to the supernatant, and the sample incubated for 20 min. The absorbance of the sample was measured with a spectrophotometer (UV2101-PC, Shimadzu Scientific Instruments, Columbia, MD, USA) from 700 to 250 nm. The sample was bleached with white light for 2 min and the pigment level determined by subtracting the post-bleach from the pre-bleach spectra. The concentrations of isorhodopsin and rhodopsin were calculated using the following extinction coefficients: ɛ rhodopsin = 40 000 m−1 cm−1 (Wald & Brown, 1958; Dartnall, 1968) and ɛ isorhodopsin = 43 000 m−1 cm−1 (Yoshizawa & Wald, 1963).
Histology and counting of rod nuclei
Mice were deeply anaesthetized with pentobarbital (100 mg kg−1) and fixed by vascular perfusion for 5 min with 2.5% glutaraldehyde−2% paraformaldehyde in 0.1 m phosphate buffer (pH 7.3). Eyes were enucleated and a section of cornea removed. The eyes were again immersed in fixative for 2 h. The lens was removed and the eyes left in fixative overnight. Eyes were dehydrated through a graded series of ethanol and embedded in JB4 Plus (Ted Pella, Redding, CA, USA). The eyeball was bisected through the optic nerve from superior to inferior, approximately along the vertical plane giving temporal and nasal portions. Sagittal sections (3 μm) were mounted on glass slides and stained with toludine blue. We used the nasal half of the retina for analysis. Measurements of total retinal thickness, outer segment length and number of nuclei in the outer nuclear layer were made at four regions (2 from superior retina and 2 from inferior retina), which were spaced 250–300 μm apart. Four photographs were taken at each region, for a total of 16 photographs per eye. Placement of the areas was determined by measurement from the optic nerve head in each eye, to ensure that the areas that were compared in the different eyes were the same from one animal to the next. Three measurements were made from each photograph, for a total of 48 measurements per eye per parameter. These were averaged to give mean values for each of the eyes per condition. The means for each eye were then averaged for the three animals per condition, and it is these means and their s.e.m.'s that are given in Table 1.
Table 1.
Morphological characteristics of Rpe65−/− and WT mice
| WT cyclic light | Rpe65−/− dark | Rpe65−/− cyclic light | |
|---|---|---|---|
| Retinal thickness (μm) | 143 ± 5 | 111 ± 4 | 118 ± 7 |
| OS length (μm) | 13.9 ± 0.5 | 8.0 ± 0.2 | 7.9 ± 0.8 |
| Rows of nuclei | 9.0 ± 0.2 | 7.7 ± 0.3 | 6.5 ± 0.2 |
Errors are s.e.m.s for three animals of each condition (see Methods). Calculated average of nuclei in outer nuclear layer (ONL), outer segment length (OS), and retinal thickness for 11-month-old WT and Rpe65−/− mice. Animals were dark-reared from 2 months of age (dark) or raised in cyclic light. See Methods for description of the selection of retinal sections. The values for each animal were determined from 48 measurements per eye, 3 from each of 16 photographs (see Methods). Means ±s.e.m. are given for 3 animals per condition (i.e. 144 measurements in total).
Determination of free Ca2+ concentration
The method for measuring calcium in mouse rod outer segments has been previously described (Woodruff et al. 2002, 2003). Briefly, the Ca2+ indicator dye fluo-5F was loaded into rods as the acetomethylester and excited with an argon laser (Model 60, American Laser Corporation, Salt Lake City, UT, USA) at 488 nm. The emitted fluorescence was collected with a 505-nm dichroic and a 510-nm long-pass filter (Omega Optical, Brattleboro, VT, USA). The fluorescence was recorded from the outer segments of single mouse rods either fully isolated or protruding from small chunks of retina. A miniature thermocouple (diameter 0.05 mm, T type, Cu–CuNi; Omega Engineering) was placed within 0.5 mm of the rod to monitor the temperature at 0.1°C resolution (Model HH-25TC, Omega Engineering, Stamford, CT, USA). We noted the temperature at the time of each fluorescence measurement and used it to correct the dissociation constant of Ca2+ binding to the dye, as previously described (Woodruff et al. 2002). Tos prevent dye bleaching, the intensity of the laser was reduced to 2–5 × 1010 photons μm−2 s−1 with reflective neutral density filters (Newport Corp., Irvine, CA, USA). Fluorescence was measured with a low dark-count photomultiplier tube having a restricted photocathode (Model 9130/100A, Electron Tubes Ltd, Ruislip, UK), whose current was amplified with a low-noise, current-to-voltage converter (PDA-700, Terahertz Technology, Oriskany, NY, USA), low-pass filtered at 1 kHz with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA, USA or Kemo Ltd, UK), and digitized at 2 kHz with a PC-compatible computer.
The intracellular concentrations of Ca2+ in darkness and after illumination were determined from the initial and steady-state fluorescence after exposure to the bright light of the laser. We used the Michaelis-Menten equation together with estimates of the minimum and maximum fluorescence (Fmin and Fmax) and the dissociation constant (Kd) to convert fluorescence into [Ca2+]i (Grynkiewicz et al. 1985; Woodruff et al. 2002, 2003). The value of Fmin was determined by exposing the rod to a zero-Ca2+–ionomycin solution (140 mm NaCl, 3.6 mm KCl, 3.08 mm MgCl2, 2.0 mm EGTA, 3.0 mm HEPES with 10 μm ionomycin, pH 7.4). When the fluorescence reached a steady minimum value, we exposed the rod to a high-Ca2+–ionomycin solution (50 mm CaCl2, 3.6 mm KCl, 3.0 mm HEPES buffer, 140 mm sucrose with 10 μm ionomycin, pH 7.4) to estimate Fmax. The Kd of the dye varied from 400 nm to 543 nm over the temperature range used in these determinations (33–37°C, see Woodruff et al. 2002).
Results
Responses of dark-reared Rpe65−/− rods
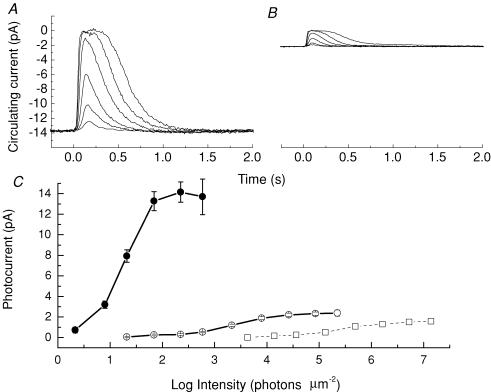
Suction-electrode recordings from rods of C57BL/6 wild-type mice raised in cyclic light (WT) and dark-reared Rpe65−/− animals are compared in Fig. 1A and B. The traces in this figure give mean responses, averaged from many rods and flash presentations, at several different flash intensities (see legend). For WT rods, we recorded a mean sensitivity of 0.38 ± 0.03 pA photon−1μm2 (n = 21). The mean flash sensitivity for the Rpe65−/− rods was 8.9 ± 1.0 × 10−4 pA photon−1μm2 (n = 37). The circulating current in darkness, which we assumed was equal to the value of the saturating light response (Baylor et al. 1979), was 14.1 ± 1.0 pA (n = 21) for WT rods and 2.3 ± 0.2 pA (n = 37) for Rpe65−/− rods. Thus dark-reared Rpe65−/− rods had a significantly smaller circulating current and sensitivity than normal rods. When, however, Rpe65−/− animals were raised in cyclic light in previous experiments (Woodruff et al. 2003), current and sensitivity were even smaller: 1.6 ± 0.3 pA and 1.2 ± 0.3 × 10−5 pA photon−1μm2 (n = 58).
Figure 1. Rods from dark-reared Rpe65−/− mice are less sensitive than wild-type mouse rods but more sensitive than rods from Rpe65−/− mice raised in cyclic light.
A, wild-type mouse rod current responses to 20 ms flashes of 500 nm light at intensities of 2.1, 7.9, 21, 69, 220 and 590 photons μm−2. Traces are global means from 10 rods, and for each rod from 10 to 12 presentations for the dimmest light, 4–10 for lights of intermediate intensity, and 2–5 for the brightest flashes. B, Rpe65−/− mouse rod currents to 20 ms flashes but at intensities of 220, 590, 2140, 7940, 26300 and 85100 photons μm−2. Responses are global means from 42 rods and 5–12 presentations per rod at each flash intensity. C, peak amplitude as function of flash intensity for rods from WT (•), dark-reared Rpe65−/− (○) and light-cycle-reared Rpe65−/− mice (□). WT data averaged from 21 rods, dark-reared Rpe65−/− from 37. The Rpe65−/− cyclic light-reared data are replotted from Woodruff et al. (2003).
Figure 1C gives the mean peak amplitude to a series of flash intensities for 21 WT rods and 37 dark-reared Rpe65−/− rods. The curve furthest to the right gives the response amplitude as a function of intensity for Rpe65−/− rods raised in cyclic light taken from Woodruff et al. (2003). Rpe65−/− animals raised in darkness had a greater circulating current and sensitivity than animals raised in cyclic light, though these values were still much smaller than for rods in WT animals.
Spectral sensitivity of rod responses
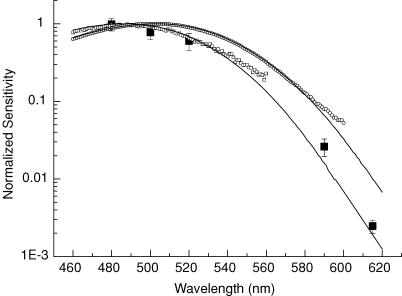
Since previous experiments have shown that raising Rpe65−/− mice in constant darkness causes the accumulation of 9-cis retinal in the retina and the production of the photopigment isorhodopsin (Fan et al. 2003), it was important to show whether the light responses we were recording were produced by isorhodopsin or by some residual 11-cis retinal and rhodopsin. Figure 2 plots the spectral absorption curves of the photopigments in WT and Rpe65−/− retinas from the same animals used to measure sensitivity (see Fig. 3). The small open squares give the mean relative absorption of the pigment from one retina each of nine Rpe65−/− mice and have been fitted with the nomogram of Lamb (1995) for a λmax of 487 nm, the wavelength of peak absorption for mouse isorhodopsin (Fan et al. 2003). The open circles give the mean relative absorption from one WT retina and have been fitted with a nomogram for a λmax of 503 nm. For both rhodopsin and isorhodopsin, the absorbance values are greater than predicted by the nomograms at long wavelengths, probably as the result of contamination and the small pigment concentration.
Figure 2. Spectral sensitivity of dark-reared Rpe65−/− rods follows isorhodopsin absorption spectrum.
Mean relative absorption of pigment from one retina each of 9 Rpe65−/− mice (□) was fitted with isorhodopsin nomogram calculated as in Lamb (1995) for a λmax of 487 nm. Mean relative absorption from one retina of a WT mice (○) was fitted with a rhodopsin nomogram for a λmax of 503 nm. Mean dark-adapted sensitivities of 7 Rpe65−/− rods each measured at five wavelengths (▪) were normalized by dividing by the sensitivity at 480 nm, and the result was then multiplied by the value of relative sensitivity at 480 nm predicted from the nomogram for isorhodopsin (0.986).
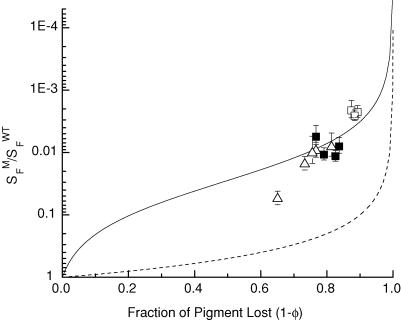
Figure 3. Dependence of sensitivity of rods in Rpe65−/− mice on pigment concentration.
Mean dark-adapted sensitivity (SFM) from 3 to 7 Rpe65−/− photoreceptors for each of 12 animals (total of 63 rods from all animals). Eight animals were placed in darkness for a period of 15 weeks (□) or 37 weeks (▴) beginning 3 weeks after birth, and 4 were kept in darkness from birth for 20 weeks (▪). Sensitivities were corrected for the difference in quantum efficiency between isorhodopsin and rhodopsin (0.22 versus 0.67) and normalized to mean dark-adapted sensitivity of WT animals (SFWT). From each Rpe65−/− retina, pigment concentration was measured and normalized to pigment concentration in WT after adjusting for the loss of photoreceptors in Rpe65−/− retinas and the smaller length of the rod outer segments compared with wild-type. This fraction (Φ) was then subtracted from unity to give the fraction of pigment lost. Dashed curve is loss in sensitivity expected from loss in quantum catch alone. Continuous curve is fit to eqn (5). See text.
The large filled squares give the relative dark-adapted sensitivity of Rpe65−/− rods at five wavelengths, calculated by dividing the measured sensitivity at each wavelength by the measured sensitivity at 480 nm and then multiplying the result by the value of relative sensitivity at 480 nm predicted from the nomogram for isorhodopsin (0.986). The satisfactory agreement of the measured spectral sensitivity with the nomogram for isorhodopsin, even at very long wavelengths, indicates that the response of the rod is produced by the 9-cis chromophore and not measurably by 11-cis retinal.
Dependence of sensitivity on pigment concentration
Previous experiments have shown that even when Rpe65−/−mice are reared for many months in darkness, they accumulate only a fraction of the normal concentration of pigment (Fan et al. 2003). Though rods in Rpe65−/− mice are less sensitive than rods in normal mice (Fig. 1), part of this decrease in sensitivity must be attributed to the lower pigment concentration and consequent decrease in quantum catch. Other experiments, however, indicate that rods in Rpe65−/− mice behave as if light adapted by activation of the visual cascade by opsin (Woodruff et al. 2003). In order to distinguish the decrease in sensitivity produced by loss of quantum catch from that produced by an equivalent background light produced by opsin, we measured sensitivity and pigment concentration in the same animals.
For 12 Rpe65−/− animals reared in darkness for periods of 15–37 weeks, we removed one eye to measure pigment concentration (see Methods) and the other to record rod light responses and measure sensitivity. Pigment concentrations varied from 21.4 to 69.8 pmol per retina, consistent with previous measurements (Fan et al. 2003). There was a clear trend for the concentration to be larger in animals left in darkness for longer times, as also previously observed (Fan et al. 2003). Extractions from three WT retinas prepared under identical conditions gave a mean pigment concentration of 408 pmol per retina. After correcting for the smaller outer segment length and loss of rods in Rpe65−/− animals (Table 1), we calculate that the isorhodopsin concentration was from 11 to 35% that of WT retinas, with a mean value of about 20%.
If all of the sensitivity loss in Rpe65−/− rods were due to loss in quantum catch, these measurements indicate that sensitivity should be of the order of a factor of 5 smaller than in WT rods. The response–intensity curves in Fig. 1C show, however, that the decrease in sensitivity is much greater than this. In order to investigate this relationship explicitly, we measured the mean dark-adapted sensitivity from three to seven photoreceptors for each of the 12 animals from which the pigment was extracted and its concentration measured. We call this SFM, the dark-adapted flash sensitivity of the mutant rods. These mean values (with standard errors) were adjusted for the smaller length of the outer segment (and thus smaller collecting area– see Table 1) and for the lower quantum efficiency of isorhodopsin than of rhodopsin (0.22 versus 0.67, see Hurley et al. 1977). These values were then divided by the mean dark-adapted flash sensitivity of WT rods (SFWT) to give the relative sensitivity, SFM/SFWT. We then calculated the fraction of pigment present as isorhodopsin in the rod, which we call Φ. To do this, we have divided the pigment concentration in each Rpe65−/− animal by the mean pigment concentration in the WT animals. We adjusted for the smaller outer segment length of the Rpe65−/− rods by assuming that the total number of molecules of photopigment in the outer segment is approximately proportional to the length (i.e. that pigment concentration per unit outer segment volume is nearly constant). Spectrophotometric measurements of total opsin from Rpe65−/− retinas by pigment regeneration confirmed this supposition. Finally, we adjusted Φ for the loss of rods from the retina, since we have measured the retinal pigment concentration but require the pigment concentration per rod. The data in Table 1 indicate that Rpe65−/− retinas after 9 months of dark rearing have on average 86% of the number of rods of a wild-type retina, and we have used this value even though some of the animals used for single-cell recording were much younger than this and probably had fewer rods lost. The values for Φ were then subtracted from one to give 1 −Φ, or the fraction of pigment lost from the rod.
In Fig. 3 we have plotted SFM/SFWTversus 1 −Φ. Each symbol gives a measurement from a single retina. The open symbols are for animals placed in darkness 3 weeks after birth for a period of 15 or 37 weeks (see figure legend), whereas the filled squares are for animals born in darkness and kept there for 20 weeks without any exposure to light. The errors are the s.e.m.s of the sensitivity measurements. No error is given for pigment concentration, since only one measurement was made from each animal. The dashed curve gives the loss in sensitivity expected from the decrease in quantum catch. The sensitivity decrease is of the order of 20–30 times greater than expected from quantum catch alone.
For the continous line in Fig. 3, we assumed that the decrease in sensitivity in the Rpe65−/− rods consisted of two components, one due to the loss in quantum catch and an extra component due to activation of the cascade by the remaining opsin (see Jones et al. 1996). Since the relative loss in sensitivity due to the loss in quantum catch is equal simply to Φ, the fraction of pigment remaining, we calculated the component due to cascade activation, ΔSFM/SFWT, by removing the component due to loss in quantum catch from the total loss in sensitivity by dividing by Φ, that is:
| (1) |
We then assumed that the intensity of the equivalent light produced by opsin is proportional to the opsin concentration (Cornwall & Fain, 1994), that is to 1 −Φ, the fraction of pigment lost (strictly speaking, the fraction as un-liganded opsin). In background light, sensitivity decreases according to the Weber-Fechner relation, that is:
| (2) |
where SF is the flash sensitivity in the presence of a background light of intensity IB, and SFD is the flash sensitivity in darkness. I0 is a constant equal to the background light intensity required to reduce the sensitivity by a factor of 2. This may be re-arranged to give (Baylor & Hodgkin, 1974):
| (3) |
This equation says that the inverse of fractional sensitivity minus one is directly proportional to the intensity of the background light, that is to the number of stimulated rhodopsin molecules. Since activation of transduction is also directly proportional to opsin concentration though with a much smaller gain (Cornwall & Fain, 1994), we reasoned by analogy that:
| (4) |
with k a constant. For the 12 retinas we calculated a mean value of k of 34 ± 5 (s.e.m.), about twice as large as the value of 16.2 previously obtained from a similar analysis of opsin activation after bleaching in salamander rods (Jones et al. 1996).
Equation (1) for the loss in sensitivity due to the decrease in quantum catch can be combined with eqn (4) to give the total loss of sensitivity (Jones et al. 1996):
| (5) |
This is the continuous line in Fig. 3.
Adaptation of rods by real light
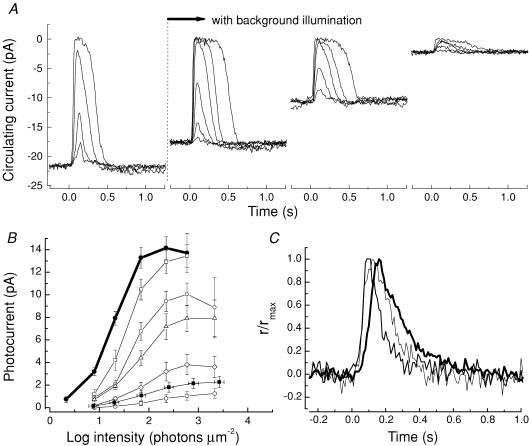
To compare the desensitization of the rod produced by opsin activation in Fig. 3 to that produced by real light, we exposed wild-type mouse rods to steady background lights and measured the decrease in sensitivity. Figure 4A shows results from a typical experiment. Recordings were made from a single rod to a series of increasing flash intensities first in the absence of a background (traces to left). A steady background light was then turned on and after 60–90 s, responses to flashes were recorded. The background intensity was then increased, and after another 60–90 s, responses to flashes were again recorded, and so on for all of the backgrounds to which the rod was exposed. The responses in the presence of three representative background levels are shown to the right in Fig. 4A. Steady background light decreased the circulating current and altered the sensitivity of the response, as has been previously observed for rods in mouse and other species (for example Fain, 1976; Baylor et al. 1979; Tamura et al. 1989, 1991; Matthews, 1991; Mendez et al. 2001; Makino et al. 2004). In Fig. 4B, we show collected results of peak response amplitude versus intensity from 21 rods. The dark-adapted data are the same as those given in Fig. 1C for WT rods, and the other curves are for these same cells but in the presence of background light.
Figure 4. Comparison of adaptation by background light in WT rods with desensitization by opsin activation in Rpe65−/− rods.
A, suction-electrode responses to 20 ms, 500 nm flashes from single WT rod, in darkness (left-most traces) and in presence of background light of 500 nm at intensities of 311, 939 and 2900 photons μm−2 s−1. Each trace is the mean of 5–12 presentations. B, mean peak response amplitude plotted as function of flash intensity for 21 dark-adapted WT rods (•), taken from Fig. 1C and light-adapted WT rods (open symbols) at background intensities (increasing for curves from left to right) of 27 (□), 101 (○), 311 (▴), 939 (⋄), and 2900 (⊥) photons μm−2 s−1. Also shown are responses of 37 dark-adapted Rpe65−/− rods replotted from Fig. 1C (▪) after adjusting light intensities for loss in quantum catch (see text). Error bars give s.e.m.s. C, mean small-amplitude responses normalized to peak amplitude from 10 dark-adapted WT rods (thick line), 36 dark-adapted, dark-reared Rpe65−/− rods (medium line), and 19 light-adapted WT rods (thin line). Actual peak amplitudes and flash intensities for three responses (dark-adapted WT, dark-adapted Rpe65−/−, light-adapted WT): 3.6 pA, 15 photons μm−2 0.48 pA, 1150 photons μm−2; 0.71 pA, 135 photons μm−2.
The filled squares in Fig. 4B show the data for the 37 rods from dark-reared Rpe65−/− animals, which have been replotted from Fig. 1C after adjusting the flash intensities (IF) for each rod by the loss of quantum catch. That is, for each rod at each of the intensities IF used in our experiments, we calculated new intensities, I′F, equal to IF/Φ. This compensates for the loss in quantum catch in Rpe65−/− rods and makes it possible to compare adaptation produced by opsin with adaptation produced by background light. The light intensities were further corrected for the difference in quantum efficiency between rhodopsin and isorhodopsin (0.67 versus 0.22, see Hurley et al. 1977). The vertical error bars give the s.e.m. for the amplitude of the response, and the horizontal error bars give the s.e.m. for the calculated values of I′F due to variability in the pigment concentration in the different Rpe65−/− retinas from which the flash responses were recorded. These errors were small by comparison with the overall difference in sensitivity between Rpe65−/− rods and WT rods.
Comparison of the filled squares with the response–intensity curves from WT rods exposed to different backgrounds indicates that the opsin in the Rpe65−/− animals adapted the rod light response more than the second brightest background (940 photons μm−2 s−1) but less than the brightest background used in our experiments (2900 photons μm−2 s−1). When we compared the waveform of the responses for dark-adapted Rpe65−/− rods with those for dark-adapted WT rods (Fig. 4C), we found that the response of Rpe65−/− rods decayed more rapidly than the WT response, as previously observed for Rpe65−/− rods raised in cyclic light (Woodruff et al. 2003). Figure 4C also shows that the dark-reared Rpe65−/− responses decayed more rapidly than WT rods exposed to the brightest background.
Comparison of activation of opsin and real light
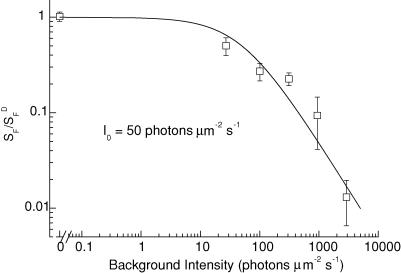
In Fig. 5, we summarize measurements of the change in sensitivity of WT rods in the presence of background light. The continuous line fitted to the data is the Weber-Fechner relation, eqn (2), with a value for the constant I0 of 50 photons μm−2 s−1. For a collecting area of 0.5 μm2 (see for example Field & Rieke, 2002), this intensity is equivalent to 25 Rh* s−1.
Figure 5. Weber-Fechner curve for WT mouse rods.
Mean sensitivity of from 4 to 12 WT rods in presence of background light normalized to dark-adapted WT sensitivity and plotted as function of background intensity (intensity values as in legend to Fig. 4B). Curve is eqn (2) with I0 set to 50 photons μm−2 s−1.
For the 12 dark-reared Rpe65−/− retinas from which we measured both sensitivity and pigment concentration, the mean value of ΔSFM/SFWT, that is the relative flash sensitivity of Rpe65−/− rods to WT, was 4.9 ± 1.1 × 10−2, after correction for loss of quantum catch and the difference in quantum efficiency between rhodopsin and isorhodopsin. From the value of I0 and eqn (2), the background light intensity (IB) that would reduce WT rods to 4.9 × 10−2 of the dark-adapted sensitivity has a mean value of the order of 1000 photons μm−2 s−1 or 500 Rh* s−1. For the Rpe65−/− retinas, a mean of 80% of the pigment was present as opsin. Rods in dark-reared Rpe65−/− retinas have a mean outer segment length of about 8 μm, shorter than in WT retinas (see Table 1). Since the outer segment diameter is about 1.5 μm, the outer segments have a volume of about 1.4 × 10−14 l. If photopigment is expressed in Rpe65−/− retinas at the same concentration as in normal retinas (about 3 mm), the outer segment contains about 2.5 × 107 pigment molecules, of which on average 0.5 × 107 are present as isorhodopsin and 2.0 × 107 as apo-opsin. Thus we can calculate that apo-opsin in the dark-reared Rpe65−/− mice is of the order of 2.5 × 10−5 as effective in stimulating the cascade as 1 Rh* s−1 in a wild-type mouse.
Degeneration in dark-reared Rpe65−/− retinas
The photoreceptors of Rpe65−/− mice raised in cyclic light degenerate by a mechanism that depends upon the activation of the transduction cascade by opsin (Woodruff et al. 2003). Since in dark-reared Rpe65−/− animals, opsin is present at a somewhat lower concentration because of the slow formation of isorhodopsin in the outer segments, we reasoned that the rate of degeneration might also be somewhat slower. To test this hypothesis, we raised Rpe65−/− mice in both cyclic light and in constant darkness for a period of 9 months. Three animals for each condition and three WT animals of the same age raised in cyclic light were then killed, and measurements were made of retinal thickness, outer segment length, and number of rows of nuclei (see Methods).
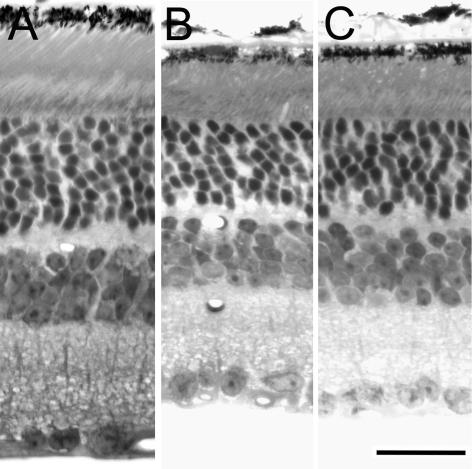
These measurements are summarized in Table 1, and representative photographs are given in Fig. 6. Retinal thickness and outer segment length were smaller for Rpe65−/− animals than for cyclic light-reared WT animals but were not significantly different for Rpe65−/− animals raised in constant darkness and cyclic light. The number of rows of nuclei was also smaller for Rpe65−/− retinas than for WT retinas, and this difference was significant both for Rpe65−/− raised in cyclic light (P < 0.0003, Student's t test) and for Rpe65−/− raised in constant darkness (P < 0.015). In addition, the number of rows of nuclei was smaller for Rpe65−/− raised in cyclic light than for Rpe65−/− raised in darkness, and this difference was also significant (P < 0.02).
Figure 6. Retinal histology of WT and Rpe65−/− mice.
Light micrographs from age-matched 11-month-old cyclic light-reared WT (A), cyclic light-reared Rpe65−/− mice (B), and Rpe65−/− mice reared in dark for 9 months (C). Micrographs were taken from the same region in each eye 250 μm superior to the optic nerve head. Scale bar, 25 μm.
Measurement of free-calcium concentration
Some evidence suggests that the apoptosis of rods can be triggered by a very low free-Ca2+ concentration (Lem & Fain, 2004). Since the circulating current of Rpe65−/− rods raised in darkness is larger than that of Rpe65−/− rods raised in cyclic light (see Fig. 1), and since dark-reared rods die at a somewhat slower rate (Table 1), it seemed possible that the free Ca2+ concentration in the outer segments of dark-reared rods might not be as low as that in the outer segments of cyclic light-reared photoreceptors. To investigate this possibility, we measured the calcium concentration in rod outer segments of Rpe65−/− mice raised in darkness, with methods previously used for wild-type (Woodruff et al. 2002) and cyclic light-reared Rpe65−/− rods (Woodruff et al. 2003). For nine rods, the free Ca2+ concentration in the outer segments of dark-reared Rpe65−/− rods was 141 ± 26 nm (mean ±s.e.m.) in darkness and 41 ± 13 nm in bright light. These were not significantly different from the concentrations measured from cyclic light-reared Rpe65−/− rods (138 ± 14 nm in darkness, 40 ± 5 nm in light, see Woodruff et al. 2003).
Discussion
These experiments confirm previous observations (Fan et al. 2003) that mice lacking the Rpe65 protein raised in constant darkness accumulate 9-cis retinal and the photopigment isorhodopsin. The isorhodopsin is functional and responsible for the light responses and sensitivity of the rods in these animals, since the spectral sensitivity of dark-reared Rpe65−/− rods matches that of isorhodopsin and not of rhodopsin (Fig. 2). Even after many months in darkness, however, only a modest fraction of the opsin contains the 9-cis retinal chromophore, and most of the photopigment is present as apo-opsin (Fan et al. 2003).
Rods in Rpe65−/− animals, whether raised in cyclic light (Woodruff et al. 2003) or in constant darkness, are significantly desensitized by comparison with wild-type rods (Fig. 1). When rod sensitivity and pigment concentration are measured from two eyes of the same dark-reared Rpe65−/− animals so that the decrease in pigment concentration can be measured directly, the desensitization is of the order of 20–30 times larger than can be accounted for by the decrease in pigment, that is by the loss of quantum catch (Fig. 3). Since apo-opsin has been shown to activate the visual cascade (Cornwall & Fain, 1994; Cornwall et al. 1995; Melia et al. 1997), the activation of transduction by the apo-opsin must produce an equivalent background light that desensitizes the rods (Woodruff et al. 2003).
This adaptation by apo-opsin resembles adaptation by real light (Fig. 4). Both produce a decrease in circulating current and sensitivity (Figs 1C and 4B), and both cause an acceleration in the falling phase of the light response (Fig. 4C). Adaptation to real light in a mouse rod produces a decrease in sensitivity consistent with the Weber-Fechner relation (Fig. 5), as others have previously shown (Mendez et al. 2001; Makino et al. 2004). From a comparison of the decrease in sensitivity from the equivalent background light produced by apo-opsin and from real background light, we estimate that apo-opsin in Rpe65−/− rods is about 2.5 × 10−5 as efficient as 1 Rh* s−1 in activating the transduction cascade.
This number is subject to a number of uncertainties. Measurement of the fraction of pigment in Rpe65−/− rods obtained from spectra from single eyes, especially for the small pigment amounts in the mutant retinas, is likely to be imprecise. Although this error cannot be estimated systematically since only a single absorbance measurement can be made per animal, the data in Fig. 3 from several animals all kept in darkness for the same time suggest that these measurements are probably accurate to within at least a factor of two. A fraction of as much as 20% of the apo-opsin is phosphorylated (Ablonczy et al. 2002), and this is true even in dark-reared animals. Although the effect of phosphorylation on apo-opsin activity is unknown, were it completely to inhibit the activation of transducin, the mean effective apo-opsin concentration used in our calculations (1 −Φ) would decrease from 0.80 to 0.64. This would produce a corresponding decrease in our estimate of the number of active apo-opsin molecules and an increase in the calculated value of the efficiency of apo-opsin.
In a similar manner, the efficiency of opsin would be greater if Rpe65−/− rods contained less opsin than we have estimated. We have assumed that the opsin amount scales with the length of the outer segment, but if there is less opsin as the result of damage to the outer segment from incipient degeneration, the remaining apo-opsin would be more active than we have calculated. Finally, there may also be error in our determination of I0 in Fig. 5. Other experiments have given values as much as 2–3 times larger than the one we obtained (see Mendez et al. 2001; Makino et al. 2004), and this would also have the effect of increasing the estimate of the efficiency of apo-opsin. These considerations suggest that our estimate of 2.5 × 10−5 is likely to be a lower limit, and that the opsin efficiency may very well be a factor of 2–3 greater than this.
The number we have obtained for the relative activation of transduction by opsin is of some interest, since the most reliable previous measurement of the activity of apo-opsin from mammalian retina (bovine, Melia et al. 1997) gave a value for the opsin efficiency of 10−6 that of Rh*, over an order of magnitude lower. These experiments were performed on isolated rod outer segment membranes. Although the activity of both opsin and Rh* were measured and compared from the same preparations, it is possible that the activity of opsin was for some reason lower in the isolated membranes than in intact photoreceptors or that the Rh* activity was higher. It is certainly conceivable that Rh* lifetime in isolated membranes is longer as a result of slower phosphorylation and arrestin binding, or that Ca2+-dependent turn-off of the cascade is slower. Finally, opsin activity may be greater in Rpe65−/− mouse rods than in wild-type mouse rods. This later possibility seems to us unlikely, since when Rpe65−/− mice are fed 9-cis retinal the rods completely recover sensitivity, maximum amplitude of response, and waveform (van Hooser et al. 2002). It is therefore unlikely that the physiology of the rods is significantly different in Rpe65−/− and WT mice apart from the lack of chromophore.
Our value for opsin efficiency in mouse is between one and two orders of magnitude greater than previously determined for intact salamander rods (Cornwall & Fain, 1994). Some of this discrepancy may be attributed to the difference in temperature during the experiments (salamander 20–22°C, mouse 37°C), but it is unlikely that this would account for all of the difference. Another possibility is that the difference in the ratio of efficiencies of opsin and Rh* is due not to a difference in opsin activation but rather a difference in the lifetime of Rh* or the efficacy of Ca2+ feedback mechanisms in the two species. We think this is also unlikely, since the size of the single quantum response is nearly the same in the two (salamander, Jones, 1998; mouse, see for example Xu et al. 1997), and approximately the same number of Rh* molecules delivered as a steady background light are required to reduce sensitivity by a factor of two in mouse and salamander (25 in mouse, this study; 32 in salamander, see Matthews et al. 1988). Our experiments seem therefore to indicate that mouse opsin, at least as measured in Rpe65−/− rods, is intrinsically noisier than amphibian opsin.
Why should this be so? One consideration is anatomy: the volume of a salamander rod (Lamb et al. 1986) is 50–100 times larger than the volume of a mouse rod. Since the concentration of rhodopsin appears to be nearly uniform among species (see for example Liebman, 1972), this means that a mouse rod contains a total of about 50–100 times fewer photopigment molecules. Since approximately the same number of Rh* molecules are required to reduce sensitivity by a factor of two, the bleaching of a similar percentage of pigment in the rods of the two animals would produce a similar concentration of apo-opsin and therefore a similar opsin-dependent desensitization. In a salamander, for example, a value for I0 of 32 Rh* s−1 and an efficiency ratio for opsin per Rh* s−1 of 5 × 10−7 (midway between the limits given by Cornwall & Fain, 1994) means that about 6.4 × 107 opsin molecules would be required to reduce sensitivity by a factor of two. This is equivalent to about a 2% bleach. The comparable value in mouse, with an I0 of 25 Rh* s−1 and an opsin per Rh* s−1 efficiency of 2.5 × 10−5, is also of the order of 2% of the total pigment concentration. We have used for this calculation an outer segment length in a wild-type mouse rod of 13.9 μm (see Table 1). This comparison is meaningful only if Rpe65−/− opsin and WT mouse opsin activate the cascade to the same extent. This is presently unknown.
Dark adaptation in rods is complex (see Fain et al. 2001); small bleaches are likely to desensitize by activation of transduction by photointermediates and by stimulation of opsin by all-trans retinal (Jager et al. 1996). For larger bleaches, steady-state desensitization is produced largely by activation of the cascade by opsin after the decay of photointermediates and hydrolysis of all-trans retinal to retinol (Kennedy et al. 2001). Our measurements suggest that the equivalent background produced by large bleaches may be roughly the same for mammalians and amphibians, and that both species would recover sensitivity after bright light exposure with approximately the same time course, as is known to be true at least within a factor of 2–3 (for amphibians, see for example Donner & Reuter 1965). This suggests that the prolonged desensitization of the rods by large bleaches serves some useful purpose, perhaps keeping the channels closed to reduce energy consumption.
The knocking out of the Rpe65 gene has previously been shown to produce a shortening of outer segment length and degeneration (Redmond et al. 1998; Seeliger et al. 2001). Although dark-rearing of Rpe65−/− mice has not previously been thought to provide protection from apoptosis (Woodruff et al. 2003), our experiments show a small but significant slowing of the rate of loss of photoreceptor nuclei in dark-reared as compared with light-reared Rpe65−/− mice, though we could detect no effect on outer segment length (see Table 1). The decrease in outer segment length may in part be related to the eventual apoptosis of the cells (see Redmond et al. 1998), but it is also possible that the outer segments are reduced as a consequence of photostasis (Penn & Williams, 1986). Outer segment length has been shown to decrease when the ambient light intensity is increased provided the high light exposure is maintained for many days. If this can occur for real light, it may also happen during a constant, equivalent light in both dark-reared and light-cycle-reared Rpe65−/− mice.
Both the change in outer segment length and the loss of photoreceptor nuclei in Rpe65−/− mice can be prevented by inactivation of the transducin gene and elimination of transduction (Woodruff et al. 2003). Since Rpe65−/− mice raised in cyclic light have a much smaller circulating current than WT rods (Woodruff et al. 2003), one possibility is that apoptosis is triggered by a prolonged lowering of outer segment Ca2+ concentration (Lem & Fain, 2004). Rpe65−/− rods raised in darkness have a somewhat larger circulating current than Rpe65−/− rods raised in cyclic light (Fig. 1), and it might therefore be expected that they would degenerate somewhat more slowly than cyclic light-reared animals. This expectation is confirmed by the data in Table 1. When we measured the Ca2+ concentration, however, we could not distinguish any difference between animals raised in cyclic light and in darkness. It remains possible that the Ca2+ concentration is sufficiently different under the two conditions to account for the difference in rate of degeneration, but not different enough for us to distinguish reliably, given the relatively large error of our determinations.
Acknowledgments
This work was supported by grants from the NIH (EY01844 to G.L.F. and EY04939 to R.K.C.) and an unrestricted grant from Research to Prevent Blindness, Inc., to the Medical University of South Carolina. R.K.C. is an RPB Senior Scientific Investigator. We thank Y. Koutalos and M.C. Cornwall for reading an earlier draft of the manuscript.
References
- Ablonczy Z, Crouch RK, Goletz PW, Redmond TM, Knapp DR, Ma JX, Rohrer B. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277:40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Hodgkin AL. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974;242:729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. The membrane current of single rod outer segments. J Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994;480:261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Matthews HR, Crouch RK, Fain GL. Bleached pigment activates transduction in salamander cones. J General Physiol. 1995;106:543–557. doi: 10.1085/jgp.106.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall HJA. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968;8:339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Donner KO, Reuter T. The dark-adaptation of single units in the frog's retina and its relation to the regeneration of rhodopsin. Vision Res. 1965;5:615–632. doi: 10.1016/0042-6989(65)90035-0. [DOI] [PubMed] [Google Scholar]
- Donner KO, Reuter T. Dark-adaptation processes in the rhodopsin rods of the frog's retina. Vision Res. 1967;7:17–41. doi: 10.1016/0042-6989(67)90023-5. [DOI] [PubMed] [Google Scholar]
- Fain GL. Sensitivity of toad rods: dependence on wave-length and background illumination. J Physiol. 1976;261:71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci U S A. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felius J, Thompson DA, Khan NW, Bingham EL, Jamison JA, Kemp JA, Sieving PA. Clinical course and visual function in a family with mutations in the RPE65 gene. Arch Ophthalmol. 2002;120:55–61. doi: 10.1001/archopht.120.1.55. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nature Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Hurley JB, Ebrey TG, Honig B, Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977;270:540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Jager S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- Jones GJ. Membrane current noise in dark-adapted and light-adapted isolated retinal rods of the larval tiger salamander. J Physiol. 1998;511:903–913. doi: 10.1111/j.1469-7793.1998.903bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Cornwall MC, Fain GL. Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander. J General Physiol. 1996;108:333–340. doi: 10.1085/jgp.108.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Lee KA, Niemi GA, Craven KB, Garwin GG, Saari JC, Hurley JB. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 2001;31:87–101. doi: 10.1016/s0896-6273(01)00340-3. [DOI] [PubMed] [Google Scholar]
- Lamb TD. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Res. 1995;35:3083–3091. doi: 10.1016/0042-6989(95)00114-f. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Matthews HR, Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Fain GL. Constitutive opsin signaling: night blindness or retinal degeneration? Trends Mol Med. 2004;10:150–157. doi: 10.1016/j.molmed.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Liebman PA. Microspectrophotometry of photoreceptors. In: Dartnall HJA, editor. Handbook of Sensory Physiology VII/I, Photochemistry of Vision. Berlin: Springer; 1972. pp. 481–528. [Google Scholar]
- Lorenz B, Gyurus P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J General Physiol. 2004;123:729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber's congenital amaurosis. Nature Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- Matthews HR. Incorporation of chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. J Physiol. 1991;436:93–105. doi: 10.1113/jphysiol.1991.sp018541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Murphy RL, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98:9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Williams TP. Photostasis: regulation of daily photon-catchy by rat retinas in response to various cyclic illuminances. Exp Eye Res. 1986;43:915–928. doi: 10.1016/0014-4835(86)90070-9. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nature Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Goletz P, Znoiko S, Ablonczy Z, Ma JX, Redmond TM, Crouch RK. Correlation of regenerable opsin with rod ERG signal in Rpe65-/- mice during development and aging. Invest Ophthalmol Vis Sci. 2003;44:310–315. doi: 10.1167/iovs.02-0567. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Reme CE, Humphries P, Hofmann F, Biel M, Fariss RN, Redmond TM, Wenzel A. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nature Genet. 2001;29:70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nakatani K, Yau KW. Light adaptation in cat retinal rods. Science. 1989;245:755–758. doi: 10.1126/science.2772634. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nakatani K, Yau KW. Calcium feedback and sensitivity regulation in primate rods. J General Physiol. 1991;98:95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser JP, Liang Y, Maeda T, Kuksa V, Jang GF, He YG, Rieke F, Fong HK, Detwiler PB, Palczewski K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G, Brown PK. Human rhodopsin. Science. 1958;127:222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Fan J, Cilluffo MC, Crouch RK, Fain GL. Opsin-dependent activation of transduction in mouse rods. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 4630. [Google Scholar]
- Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nature Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Wald G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]