Abstract
Cxs (connexins), the protein subunits forming gap junction intercellular communication channels, are transported to the plasma membrane after oligomerizing into hexameric assemblies called connexin hemichannels (CxHcs) or connexons, which dock head-to-head with partner hexameric channels positioned on neighbouring cells. The double membrane channel or gap junction generated directly couples the cytoplasms of interacting cells and underpins the integration and co-ordination of cellular metabolism, signalling and functions, such as secretion or contraction in cell assemblies. In contrast, CxHcs prior to forming gap junctions provide a pathway for the release from cells of ATP, glutamate, NAD+ and prostaglandin E2, which act as paracrine messengers. ATP activates purinergic receptors on neighbouring cells and forms the basis of intercellular Ca2+ signal propagation, complementing that occuring more directly via gap junctions. CxHcs open in response to various types of external changes, including mechanical, shear, ionic and ischaemic stress. In addition, CxHcs are influenced by intracellular signals, such as membrane potential, phosphorylation and redox status, which translate external stresses to CxHc responses. Also, recent studies demonstrate that cytoplasmic Ca2+ changes in the physiological range act to trigger CxHc opening, indicating their involvement under normal non-pathological conditions. CxHcs not only respond to cytoplasmic Ca2+, but also determine cytoplasmic Ca2+, as they are large conductance channels, suggesting a prominent role in cellular Ca2+ homoeostasis and signalling. The functions of gap-junction channels and CxHcs have been difficult to separate, but synthetic peptides that mimic short sequences in the Cx subunit are emerging as promising tools to determine the role of CxHcs in physiology and pathology.
Keywords: apoptosis, ATP release, calcium channels, cell communication, Gap 26 and 27, mimetic peptides, rotigaptide
Abbreviations: CMTX, Charcot-Marie-Tooth X-linked disease; Cx, connexin; Hcs, hemichannels; PKC, protein kinase C
INTRODUCTION
Cxs (connexins) are ubiquitous channel-forming proteins found in vertebrates [1] and some invertebrates [2]. They have molecular masses between 25 and 62 kDa, and this size difference is currently used to name the proteins. They are found mainly in the plasma membrane as paired multisubunit channels that facilitate intercellular communication. Cxs inserted into the endoplasmic-reticulum membrane assemble into oligomeric structures constructed of six protein subunits arranged concentrically around a central channel; these CxHcs (connexin hemichannels), often referred to as connexons [3,4], are delivered to the plasma membrane, where they diffuse laterally into cell-contact regions to dock head-to-head with partner Cxs present on neighbouring cells. This interaction produces a gap junction channel that is defined as a protein-lined conduit providing an intercellular communication pathway directly connecting adjacent cell cytoplasms. The number of gap-junction channels compressed into plaques, often characterized by regular geometric symmetry, varies, and they form by accretion as channels attach to the edges of the plaques, a process balanced by concurrent removal of channels from the central areas of the plaques [5,6]. Gap-junction plaques, observed by freeze–fracture electron microscopy, vary enormously from a few channels to as many as 200 000, as seen in cardiac intercalated discs [7] and the organ of Corti in the inner ear [8]. However, in most tissues, and in confluent cell cultures, the number of units comprising a plaque is generally much smaller.
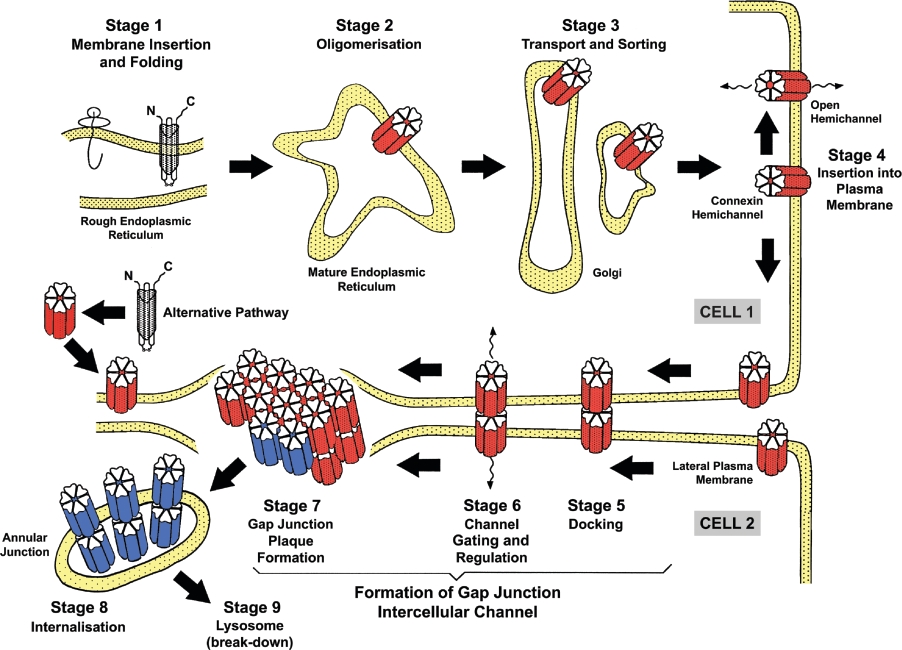
Cxs are short-lived proteins. In the mammalian heart, a high producer of Cx43 and the most widespread Cx, a half-life of 2–4 h has been calculated [9,10], but there is likely to be variation, with Cx49, for example, expressed in ovine lens tissue, having a half-life of 10 h [11]. Gap junction proteins are degraded after transfer to lysosomes via multivesicular endosomes [12,13]. The overall trafficking and assembly of CxHc into gap junctions and their degradation (Figure 1) is rapid and highly regulated [14,15]. Formation of CxHcs begins in the endoplasmic reticulum–Golgi environs of the secretory pathway [15,16] (Figure 1). Cx26 differs from most other Cxs, since it is also assembled into gap junctions by a non-classical mechanism [17], with the formation of Cx26 gap junctions continuing after treatment of cells with the Golgi-disrupting drugs brefeldin A and ilimaquinone [18–20]. After the docking of CxHcs, two previously segregated intracellular environments are introduced to each other, and solutes of molecular mass up to about 1–1.5 kDa diffuse across pathways insulated from external fluctuations in the cell's environment.
Figure 1. Assembly and breakdown of gap junctions emphasizing the genesis and trafficking of CxHcs.
Formation of CxHcs occurs early in the secretory pathway (Stage 1) and they are transported along the secretory pathway (Stages 2 and 3), implicating regions of the endoplasmic reticulum and the Golgi apparatus. A second assembly mechanism that is poorly characterized also operates with Cx26 and possibly other connexins has been reported which does not directly feature the Golgi apparatus. CxHcs, after insertion into the plasma membrane (Stage 4), dock with partners (Stage 5) located on an attached cell, and gate to an open configuration (Stage 6), a process that may occur concurrently with the aggregation of the gap junction channels into large adhesive plaques (Stage 7). Stages 7 and 8 involve internalization of gap junctions into one of the attached cells and break down by hydrolysis in lysosomes (Stage 9).
The significance in physiology and pathology of unapposed CxHcs when in transit in the plasma membrane prior to incorporation into gap junction plaques has recently attracted interest [21,22]. As well as fulfilling signalling functions, their exposed position in non-contacting cells makes CxHcs especially vulnerable to external fluctuations. On the other hand, the exposure of CxHcs to the cell's external environment has allowed studies of their roles in intercellular signalling, especially that involving Ca2+, and it is becoming evident that they are implicated in cellular responses to various metabolic and external stresses.
ASSEMBLY OF Cxs INTO CHANNELS
Over 20 different Cx proteins characterized by regions of high and low amino-acid-sequence conservation are found in chordates [1], raising fundamental questions about the need for multiple Cx subtypes. Cxs are characterized by four transmembrane segments with α-helical conformation and N- and C-terminal tails projecting into the cytoplasm. Two extracellular loops (EL1 and EL2), containing about 31 and 34 amino acids respectively are highly conserved and covalently connected by three invariant disulphide bonds. The C-terminal tail varies in length and is a major determinant of the Cx molecular mass; for example, Cx43 has a C-terminal tail of 156 amino acids which interacts with proteins present in other cell junctions and with cytoskeletal components [23], as amplified below. The tail also contains multiple phosphorylation sites for PKC (protein kinase C), MAPK (mitogen-activated protein kinase) and Src kinase [24,25]. In contrast, the C-terminal tail of Cx26 has 16 amino acids and is not phosphorylated. The N-terminal sequence of approx. 20 amino acids is highly conserved in all Cxs and contributes to the transjunctional voltage sensor [26]. Multiple amino-acid-sequence determinants dictating oligomerization and intracellular targeting have been identified in many Cx domains [4,15]. The bulk of the cytoplasmic tail is not essential for CxHc trafficking to the plasma membrane and the formation of gap junctions [27]. Domains governing channel conductance and permeability have been extensively explored in gap junctions, although many operational aspects remain uncharted at the molecular level, with similar gaps remaining in unravelling how CxHcs function [28–32,75]. Of especial interest in studying CxHcs are the two environmentally exposed loops that crucially dictate the geometry of the interacting CxHcs during formation of gap junctions. In Cx46Hc, the first extracellular loop is an important determinant of channel charge selectivity [33] and in Cx32Hc of gating polarity [34]. The overall structure of the extracellular loops has been studied predominantly in gap junctions, where they adopt an antiparallel β-barrel conformation [35–38]. The importance of the invariant intramolecular disulphide linkages covalently linking the loops has been demonstrated in cells expressing Cx43 in which the relevant cysteine residues have been replaced by other amino acids; these mutated Cx43Hcs were permanently closed, and no gap junctions were formed [39]. The topography of specific Cx regions may be different in CxHcs compared with gap junctions (Figure 2), as illustrated by the high susceptibilty of Cx43Hc compared with gap junctions to cellular redox potentials, which implicates intramolecular disulphide bonds [40].
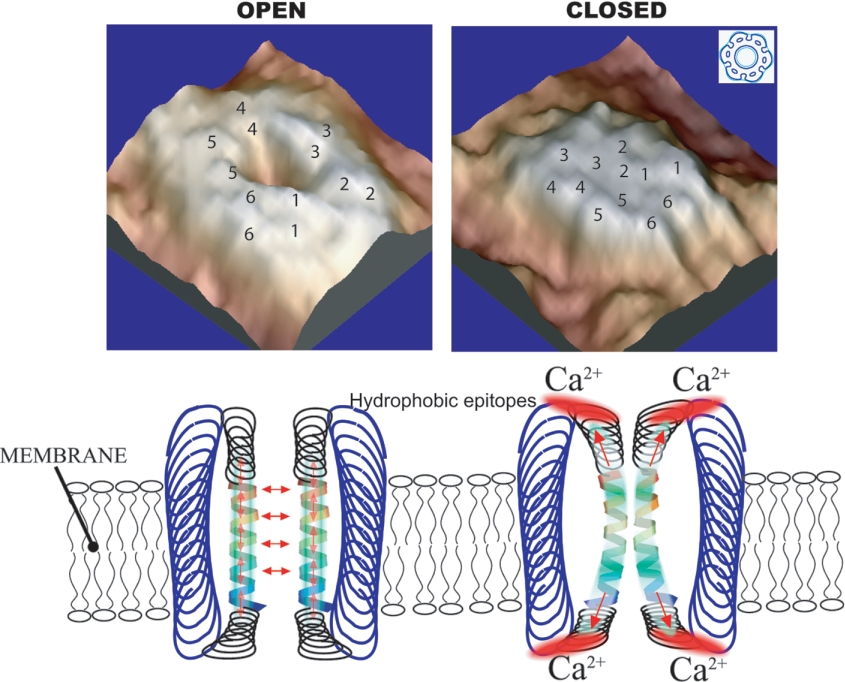
Figure 2. Model depicting extracellular Ca2+-sensitive open and closed conformations of CxHcs deduced from samples examined by atomic-force microscopy.
This Figure is modified from Figures 3 and 5 of [77] and is reproduced with the permission of the authors and the publishers. © 2005 The American Society for Biochemistry and Molecular Biology (http://www.jbc.org).
The permeability of gap junctions to a range of small molecules has been extensively investigated [41,42]. The Cx make-up of homomeric and heteromeric channels is likely to be a major determinant governing channel selectivity and the underpinning of fine tuning of signalling molecules passing across gap junctions [43,44]. In contrast, the permeability of CxHcs has not yet been explored in such detail. An important question in analysing the functions of CxHcs concerns where they become associated with accessory proteins during transit to the plasma membrane. Proteins interacting with the cytoplasmic C-terminal tail of Cx43 include the tight-junction proteins ZO1, occludin and claudins, tubulin and Src kinase [23]. Other proteins associating with the Cx43 are continually being identified, and now include drebrin [45], p120 catenin and CIP150 [46]. Integrin α5 associates specifically with Cx43Hc and influences channel gating during shear stress (J. X. Jiang, personal communication; [9]). Interaction of Cxs with the Ca2+-binding protein calmodulin is important [51], and two Ca2+-dependent calmodulin-binding sites on the N- and C-terminal tails have been detected in Cx32 [47]. Surface-plasmon-resonance approaches showed that calmodulin binds in a Ca2+-dependent manner to the C-terminal tails of perch Cx35 and Cx34.7, which are related to Cx36 in mouse brain [48]. Calmodulin is integrated into gap junctions early in the biogenetic pathway [49–50], suggesting interaction with intracellular CxHcs. Other studies show that calmodulin is important in the oligomerization of CxHcs [51] and is a candidate channel gating regulator prior to their insertion into the plasma membrane.
A central issue arising is how CxHcs are kept mainly in a closed configuration, especially during transit inside the cell and after insertion into the plasma membrane. CxHcs, like other ion channels in the cell's endomembranes, segregate the luminal and cytoplasmic intracellular milieux, for they are positioned to act as gates to large and energy-dependent Ca2+ gradients, especially in excitable cells. One possibility is that disparate localized Ca2+ levels separated by these endomembrane systems, and maintained by an array of receptors and ion pumps underpinning signalling [52], ensure that CxHcs are maintained in a closed configuration. Intracellular CxHcs are most likely maintained constitutively in a closed configuration [12], a view that led to the precept that all CxHcs are closed, whereas gap junctions operate in an open-channel configuration [40]. This precept extended to CxHcs after their insertion into the plasma membrane, where a further range of ion channels and pumps operate [52].
PROPERTIES OF CxHcs
The concept of CxHcs functioning exclusively as mere structural precursors of gap junctions began to be questioned after they had been detected on the horizontal cell dendrites of vertebrate retina that regulate transmitter binding to cone receptors [53]. At the same time, studies on lens Cx46 expressed in Xenopus oocytes showed that the Hcs had a high (300 pS) unitary channel conductance and a relatively large permeability, especially to cations. The channel was closed by elevated levels of H+ and Ca2+ ions on the cytoplasmic side, but was opened by low extracellular Ca2+ [28,55]. It was argued that the exceptional gating behaviour of Cx46Hc observed in oocytes might have a functional basis in lens tissue, for the avascular nature of this organ utilizes Cx channels, among others (e.g. aquaporins), to provide an extensive intercellular communication network of open channels to deliver nutrients across the lens tissue. These model systems led to further characterization of CxHc gating properties. Several Cxs, including many of the commonly studied vertebrate Cxs, as well as ovine and the endogenous Xenopus oocyte Cx38, chicken Cx56 and Cx45.6 and zebrafish (Danio rerio) Cx35 were induced to form open CxHcs when bathed in low-Ca2+/Mg2+ solutions [54–61]. A further early demonstration that open CxHcs could be detected in the plasma membrane emerged from studies showing that small fluorescent dyes entered cultured cells via this route [64]. CxHcs have since emerged as useful models for exploring their gating properties and the details of the pore region, in view of easier external accessibility of single channels [62,63].
Importantly, genetic mutations appear to modify CxHc functioning. For example, mutations in the C-terminus of Cx32 are linked to dysfunction in the peripheral neuropathy CMTX (Charcot-Marie-Tooth X-linked disease), producing CxHcs that are leaky [65,66]. Mutations in Cx30Hc cause abnormal channel activity in hidrotic ectodermal dysplasia [67], and mutations in Cx26Hc are detected in the supporting cells of the cochlea. Macrophage recruitment in atherosclerosis is regulated by Cx37Hc in the endothelium [69]. Six mutations in Cx43Hc are linked to oculodentodigital dysplasia – a development disorder characterized by craniofacial and limb disorders – and result in changes in dye traffic through the channels [9,203].
PREPARATION AND STRUCTURE OF CxHcs
The preparation and biochemical characterization of gap junctions [71] has allowed procedures for their dissociation into constituent CxHcs. The solubility of CxHcs in detergents such as Triton X-100 and N-lauryl sarcosinate, in contrast with the insolubility of gap-junction plaques in these mild detergents, has been a key aspect of the isolation procedures. Further elaborations in CxHc preparation, deploying either reducing conditions with high salt in conjunction with non-ionic detergents followed by chromatography [72,73] or immunoaffinity chromatography [74], have also been described. These approaches have provided material for determining the physical characteristics of aggregated CxHcs and allowed their reconstitution into lipid bilayers. In general, these studies show that CxHcs possess permeability characteristics similar to those of gap junctions (Table 1) [75,76]. However, atomic-force microscopy shows that, despite overall similarity of CxHcs and gap junctions, for example, their hexagonal geometry, the extracellular domains of free Hcs are structurally different [77]. Also, CxHcs appear less ordered in the lipid bilayer and, in contrast with gap junctions, partition into lipid rafts [78,79], which are membrane subdomains enriched in cholesterol and sphingomyelin and implicated in cell signalling [80]. In physiological buffers, CxHcs are closed, but they change to an open conformation as the Ca2+ concentration is lowered, with the pore diameter increasing from 1.8 to 2.5 nm. These results suggest that hydrophobic extracellular domains are key areas in regulating the widely observed Ca2+-dependent conformational changes [82] depicted in models (Figure 2). A qualification to many of these studies is that CxHcs were produced by peeling apart gap junctions, and the possibility remains that unassembled CxHcs in transit and prior to docking may be subtly different in structure and operation. As described above, access to the extracellular and intracellular aspects of the CxHcs has allowed the nature of the amino acids lining the channel to be studied by techniques such as SCAM (substitute cysteine accessibilty mutagenesis) [81]. Although there is likely to be some structural variation between individual Cx isoforms, a current view is that the following areas contribute to the Hc pore: EL1 at the extracellular end; TM1 within the transmembrane space; NT (N-terminus) and/or CL (cytoplasmic loop) at the pore's cytoplasmic aspect. A Ca2+-binding site has been detected on the outer aspect of CxHcs [82]. Atomic resolution of gap junctions and CxHc obtained at greater than 6 Å (1 Å=0.1 μm) has progressed, although current models obtained with 5.7 Å in plane and 19.8 Å vertical resolution identify the positions and tilt angles for the 24 α-helices within each Hc [36–38,83]. Progress in determining the three-dimensional structure also continues by alternative methods that introduce specific Cx mutations, mainly of genetic origin, to map channel characteristics governing operation [84,85].
Table 1. Cx mimetic peptides – structures and properties.
| Name | Cx numbers/sequence | Protein domain | Properties | Reference(s) |
|---|---|---|---|---|
| Gap peptides | ||||
| Gap 26 | 43, 32, 26 | 64–76 EL1 | Inhibits CxHc within minutes and gap junctions later | [131,136] |
| VCYDKSFPISHVR | ||||
| Gap 27/43 | 43, 37, 32, 26 | 201–211 EL2 | Inhibits CxHc within minutes and gap junctions later | [131–139] |
| (43Gap 27) | SRPTEKTIFII | |||
| Gap 27/40 | 40 | 201–221 EL2 | Inhibits Cx40 gap junctions (effects on CxHc not investigated) | [115,131] |
| (40Gap 27) | SRPTEKNVFIV | |||
| Gap 24 | 32 | 110–122 IL | Inhibits Cx32Hc and not gap junctions | [146] |
| GHGDPLHLEEVKC | ||||
| L2 peptide | 43 | 119–144 IL | Influences gap junction channel gating | [148] |
| Other peptides | ||||
| No name | 43 | 180–195 EL2 | Inhibits Cx43 gap junctions | [205] |
| SLSAVYTCKRDPCPHE | ||||
| No name | 40 | 177–192 EL2 | Inhibits Cx40 gap junctions | [205] |
| FLDTLHVCRRSPCPHP | ||||
| No name | 32 | 52–63 EL1 | Inhibits Cx32 gap junctions in hepatocytes | [156] |
| ICNTLQPGCNSV | ||||
| No name | 32 | 41–42 EL1 | One of several peptides inhibiting gap junctions in oocytes | [204] |
| ESVWGDEKSSFI |
REGULATION OF CxHcs
CxHcs in the plasma membrane are closed (i.e. have a low open probability) under resting conditions, but can be activated to open under the influence of stimuli such as low extracellular Ca2+, membrane depolarization, mechanical membrane stress and metabolic inhibition. Current evidence indicates that Hcs composed of Cx26, Cx30, Cx32, Cx35, Cx37, Cx38, Cx43, Cx44, Cx46, Cx50 and Cx56 open when exposed to one of these conditions [40,75]. In addition, CxHcs are influenced by numerous other factors, such as extra- and intra-cellular pH [75], phosphorylation status [25,86–88] and redox status [89]. Various methods have been applied to investigate CxHc responses to these triggers or modulators, including electrophysiology, uptake or release of reporter dyes and release of messenger substances. A major advance was the demonstration by several groups that ATP release from cells occurs via CxHcs [100,101,110]. Cellular ATP release by cells is an important and widespread physiological response [90,91], and the implication of CxHc had to be demonstrated against a historical background that other ATP-release mechanisms preponderate, including release from exocytotic vesicles [92,93], transport by ABC (ATP-binding cassette) transporters [94] and diffusion via P2X7 receptor channels [94,95] or other less-characterized channels, such as the maitoxin activated pore [96]. Subsequent work showed that other messengers – provided they fulfil size and shape criteria – may also pass via CxHcs, such as glutamate released by astrocytes [96,206], NAD+ released by fibroblasts [97] and prostaglandins released by mechanosensitive osteoclasts [98].
The dependency of CxHcs on extracellular Ca2+ has emerged as an universal observation, and exposing cells to low or zero Ca2+ environments is a general strategy to trigger their opening [99,102,103]. In addition, other extracellular ions can further modulate CxHc opening, such as Mg2+, non-physiological bivalent ions like Ba2+ or Sr2+ [103], univalent anions such as Cl− [107] and cations like Na+ [108]. Depolarization is another patent and thoroughly investigated stimulus that activates Hcs composed of a large family of Cx subtypes [54,82,103]. Mechanical membrane stress is still another well-appreciated stimulus of CxHc opening. For example, CxHcs open when cells are subjected to shear stress [98], point mechanical stimulation of a single cell [104,105], negative pressure applied via a patch pipette [106], changes in osmolarity [22,99] and even sound waves in the inner ear [68]. It is conceivable that a mechanical component of stimulation is somehow involved in all CxHc stimulation paradigms that involve a medium change (e.g. switch to low Ca2+ conditions; R. G. Johnson, personal communication). Metabolic inhibition with drugs interfering with the mitochondrial electron-transfer chain [89,107,108] or by applying ischaemic conditions (T. Clarke, O. Williams, P. M. Martin and W. H. Evans, unpublished work) are known to trigger CxHc opening. The exact signalling cascade involved in CxHc opening is not known under these conditions, but dephosphorylation, probably due to calcineurin activation, changes in the redox state by free radicals and products of the arachidonic acid metabolism, have been put forward as possible explanations. CxHcs have been reported to be inhibited by certain kinases such as PKC and v-Src [87,109], and dephosphorylation is thus expected to result in increased opening. It is, however, known that kinases may have opposing effects on gap-junction channels, depending on the cell type, and further work is required to further understand phosphorylation effects at the various known phosphorylation consensus sites on the Cx subunit [25,87]. Recent work suggests that growth factors, such as basic fibroblast growth factor, and inflammation-inducing substances, such as lipopolysaccharide, amplify CxHc opening (E. DeVuyst, E. Decrock, M. DeBock, H. Yamasaki, C. C. Naus, W. H. Evans and L. Leybaert, unpublished work).
As the build-up of evidence for open CxHcs on a range of cells continues, the possibility that they are a mere laboratory artefact and may not play a significant role in normal metabolism recedes. CxHcs have been studied mainly in isolated, experimentally manipulated cells using environmental conditions rarely encountered in normal circumstances, and the release by stressed cells of ATP across CxHcs cannot be sustained for long periods [110]. However, evidence has accrued that cells appear to utilize CxHcs adaptively to injury and inclemental environments, and evidence discussed below shows that that CxHc gating is closely interlinked with intracellular Ca2+ signalling responses. Open CxHcs are detected in cultured cells, and there are increasing reports that they are also found in tissues, for example heart [108,111], microglia [113] and cochlear organ cultures [68,112]. The demonstration that ATP is released in a Cx-dependent process in retina and parenchymal microglia of brain provides further evidence for their operation in intact tissues and suggests new roles for CxHcs in the cellular injury responses discussed below [114].
EXPERIMENTAL MANIPULATION OF Cx-MEDIATED INTERCELLULAR COMMUNICATION
A diverse range of compounds inhibit intercellular communication across gap junctions. However, most of these display limited specificity towards Cx channels over other membrane channels, for they act either by modifying general membrane ionic permeability or by inducing metabolic inhibition [115,116]. For example, the aliphatic alcohols octanol and heptanol and the anaesthetic halothane provoke local disorder at lipid/protein interfaces in membranes, leading to compression of CxHcs, gap junctions and other membrane channels. Oleic acid [117] and arachidonic acid [118] influence membrane channels after embedding themselves in membranes and changing membrane fluidity; with gap junctions they modify gating induced by voltage [119]. Other widely used inhibitors, such as the glycyrrhizic metabolites 18α- and 18β-glycyrrhetinic acid and carbenoxolone enter cells and elicit metabolic changes often reflected in dephosphorylation of Cx43 [120]. Phosphorylation of several sites on the C-terminal tail of Cxs modifies channel gating, but the effects are complex and vary from cell to cell [86–88]. Fenamates, such as niflumic and flufenamic acids, advocated as reversible gap-junction blockers [121], are anti-inflammatory drugs acting on lipoxygenase and cyclo-oxygenase pathways [122]. Tamoxifen, a synthetic lipophilic non-steroidal triphenylethylene derivative, inhibits gap junctions reversibly, but also affects other channels such as L- and T-type Ca2+ channels [123]. Polyamines inhibit Cx43 gap junctions [124], and quinine inhibits gap junctions constructed of Cx38 and Cx50, but not Cx26, Cx32, Cx40 and Cx43 [125], possibly influencing channel pH gating. Spermine action on Cx40 has been studied in molecular detail and appears to involve action at basic amino acids in the first membrane-spanning region of Cxs [126]. These data are mentioned because these inhibitors also probably act on CxHc, as demonstrated by blocking of CxHc with halothane [107].
Cx MIMETIC PEPTIDES AS Cx CHANNEL BLOCKERS
Cx mimetic peptides, i.e. peptides that are identical with a short amino acid sequence on the Cx subunit, were initially designed to produce Cx-selective blockage of gap junctions [127,128], but have recently emerged as tools to block CxHcs with little or no immediate effects on gap junctions (Table 2). Cx mimetic peptides were selected following examination in a chick myocyte bioassay of a range of peptides spanning most of the two extracellular-loop regions of Cx [128]. The bioassay probed gap-junctional coupling in myocytes on the basis of the achievement of synchronous contraction of cell aggregates; intracellular peptides of similar length as the active versions were used as controls in these experiments. The most widely used peptides, Gap 26 and Gap 27, correspond to amino acid sequences on the first and second extracellular loop of Cx43 respectively (Table 1) [129]. The selected sequences comprise conserved domains (VCYD in Gap 26 and SRPTEK in Gap 27) and interface with the membrane. Other peptides have been developed that incorporate small changes in the non-conserved peptide moieties, resulting in a variety of peptides directed at the ‘Gap 26 region’ or the ‘Gap 27 region’ [130,131]. Gap 26 and Gap 27 peptides appear to act in a Cx-specific manner and have now been widely applied to block gap junctions composed of Cx37, Cx40 and Cx43 [133,136,137]. These peptides inhibit gap junctional transfer of fluorescent dyes [131], electrical coupling [132] and synchronized Ca2+ oscillations in smooth-muscle cells [137]. Gap 26 and 27 peptides also inhibit the intercellular propagation of Ca2+ waves in monolayer cell cultures [134,135], but inhibition of Ca2+ wave propagation may also result from CxHc inhibition, as detailed further below. In addition to electrical or dye coupling, the peptides also inhibit several cellular integrative processes mediated by Cxs and/or gap junctions, such as synchronized rhythmic activity of arteries [138], endothelial smooth-muscle interactions governing relaxation of blood vessels [131,132], co-operation of T and B lymphocytes [139], dendritic cells [140] and the production of immunoglobulins and cytokines [141].
Table 2. Comparison of the properties of CxHcs and gap junctions.
| Property | Gap junctions | CxHcs | Selected references |
|---|---|---|---|
| Structure | Dodecameric channels composed of two CxHcs arranged end-to-end, straddling two membranes and creating confluence of two internal environments | Hexameric Cx channels, straddling a single membrane | [36–38,77,83] |
| Function | Operate between cells in contact | Operate in absence of cell contact | See the text |
| Selectivity | Up to 1.5 kDa, depending on the Cx subtype | [41–44] | |
| Gating | Each junction constructed of two or more subtypes having characteristic conductances. Regulated by voltage, pH, phosphorylation and calmodulin | Connexin subtypes have characteristic conductance regulated by voltage, pH and phosphorylation | [40,50,75] |
| Ca2+ effects | Moderate levels generally close, acting mainly from the cytoplasmic aspect. Store-operated Ca2+-entry | Increase in Ca2+ (≈500 μM) opens Cx32 channels from inside. Low Ca2+ (below ≈30 μM) opens most channels from outside | [50,114,146] |
| Inhibition, regulation | Channel is insulated from external changes and difficult to block specifically. Cx mimetics inhibit after 2 h exposure | Channel external aspect regulation by ionic and other external changes. Amenable to reversible and rapid inhibition by Cx mimetics and activation by rotigaptides | [115,116,151] |
| Stress response | Response to stress situations probably via indirect mechanisms; not mechanosensitive | Sensitive to osmotic, mechanical, shear, hypoxic and other external stresses, and to metabolic inhibition. Directly mechanosensitive | See the text |
CxHcs, THE PRIMARY TARGET OF Cx MIMETIC PEPTIDES
Braet et al. [142,143] were the first to report that Cx mimetic peptides also inhibit CxHcs. This observation was not unexpected, given the fact that the extracellular-loop sequences, with which the peptides are likely to interact, are freely available in the CxHc form [144]. Both Gap 26 and Gap 27 block uptake of the reporter dye propidium iodide and ATP release in Cx43-expressing cells, and the effect became apparent after short incubations, typically in the order of 10–30 min [142,143]. For comparison, the effects of Cx mimetic peptides on gap junctions typically require longer incubations of the order of about 1 h or more. Further work by other groups has confirmed the strong inhibition by Gap 26 of CxHc-related ATP release and dye uptake in Cx43-expressing corneal endothelium [104] and retinal pigment epithelium [114]. Some of the mimetic peptides have been demonstrated to selectively inhibit CxHc composed of a specific Cx isoform, e.g. Cx43-directed Gap 27 (also described as 43Gap 27 [138,145]) selectively inhibited Cx43Hc without influencing Cx32Hc [142]. The field was advanced a step further when it was shown that the Gap 26 peptide also blocked ATP-mediated mitogenic effects on neural retina cells involving the inhibition of CxHc [114]. A further peptide, Gap 24 (Table 1), corresponding to an intracellular-loop (IL) sequence of Cx32, specifically blocked ATP release and dye uptake in Cx32-expressing cells, but not in Cx43-expressing cells; significantly, Gap 24 had no effect on gap-junctional intercellular coupling [146]. An open question is how the Gap 24 peptide makes its way into the cell. Several possibilities exist, including endocytosis, entry through open CxHcs or the presence of a cell-penetrating peptide sequence, but none of these has been positively confirmed – Gap 24 does not contain any currently known cell-penetrating peptide sequence [147]. Peptides similar to Gap 24 have been used to investigate the ball-and-chain gating mechanism in gap junctions composed of Cx43 and Cx40 [148].
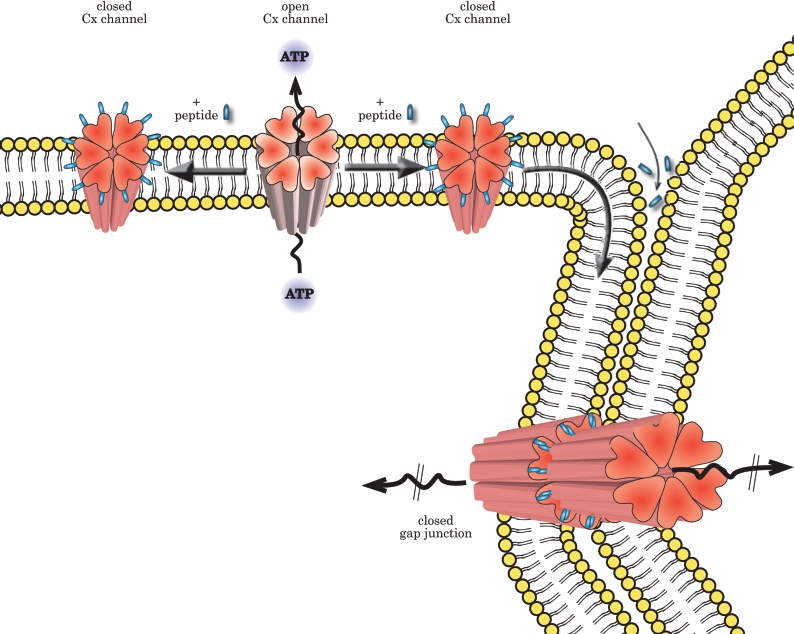
How can one understand the selective effects of Gap 26 or 27 peptides on CxHcs given their widely documented effects on gap junctional coupling? The answer lies mainly in the duration of exposure to the mimetic peptides, as pointed out above. Patch-clamp studies have shown that the bound Cx mimetic peptides reduce within minutes the voltage-induced opening of Cx43 channels expressed in HeLa cells, with inhibition of cell coupling following about 30 min later (T. Desplantez, V. Verma, W. H. Evans and R. Weingart, unpublished work). Inhibition of ATP release or dye uptake via CxHcs follows similar kinetics, with effects becoming apparent after 15 min exposure, whereas dye coupling via gap junctions was not affected until 2 h and was fully inhibited following overnight incubation of various Cx43-expressing cells (E. DeVuyst and L. Leybaert, unpublished work). To understand the action of the peptides, it is important, however, to consider also their effects on gap junctions. The exact mechanism of gap-junctional block is currently not known, but several possibilities have been put forward, including the reduced docking of two preformed CxHcs with peptides on their extracellular loops, the breaking apart of existing gap-junction channels and interactions of the peptides with an accessible Cx target followed by conformational changes that lead to gap-junction channel closure (Figure 3) [129]. The first possibility, i.e. decreased assembly of CxHc into gap junctions upon their attachment to the edges of a plaque [149], is less likely, because Cx mimetic peptides had little discernable effect on the size of gap-junction plaques in HeLa cells expressing Cx43–GFP (Cx43–green fluorescent protein) [150]. Further possibilities include the diffusion of the peptides in the extracellular space surrounding the gap-junction channels and into the intercellular clefts, a process that may take longer, resulting in a separation of independent effects on gap-junctional coupling (at a time scale of hours) from those on CxHcs (inhibited within minutes).
Figure 3. Proposed mechanism of action by which Cx mimetic peptides inhibit cell signalling.
Within minutes of application, the peptide binds to CxHcs, lowering channel conductance and restricting, for example, ATP release. Later, as CxHcs become incorporated by accretion to the edges of gap juctions, intercellular coupling is inhibited. Cx mimetic peptides may also prevent assembly of CxHcs into newly formed functional gap junction channels, break existing gap junction channels apart or diffuse into the intercellular cleft and induce direct blockage of gap junctions. See the text for discussion.
Cx mimetic peptides acting on cell communication offer opportunities for application as therapeutic tools. Their inhibitory actions on gap junctions and CxHcs is opposite to that caused by rotigaptides such as ZP123 [151], a class of anti-arrhythmia peptide drugs that selectively open gap junctions constructed of Cx43, resulting in an increase in dye coupling. Rotigaptides also enhanced the pulsatile release of ATP across CxHcs in cardiac cells subject to ischaemic stress; in contrast, Gap26 inhibits this process (T. Clarke, O. Williams, P. M. Martin and W. H. Evans, unpublished work).
CxHcs AND Ca2+ SIGNALLING
CxHc and gap junctions play a prominent role in cellular Ca2+ signalling, more specifically at the level of communication between cells, i.e. intercellular Ca2+ signalling. Cxs entered this field upon the demonstration that InsP3 permeated through gap-junction channels, thereby communicating a Ca2+ message by triggering Ca2+ release from endoplasmic-reticulum stores in the target cell [152,153]. In homogenous confluent cell layers, intercellular Ca2+ signals take the form of intercellular Ca2+ waves (Figure 4). Further work demonstrated that intercellular Ca2+ signals/waves were also sustained by the paracrine messenger ATP, which is released by cells and diffuses into the extracellular space and acts on G-protein-coupled receptors on nearby cells [154,155]. Phospholipase C activation then triggers InsP3 formation, with subsequent release of Ca2+ from stores in the target cell(s). Cxs were revisited in the intercellular-Ca2+-signalling field following the demonstration that CxHcs were a likely pathway for ATP release [143,144]. The triggers for CxHc opening initially came from pathology, such as mechanical stimulation [106], lowered extracellular Ca2+ [56], metabolic inhibition [89,114], as pointed out above. Depolarization of the cell also triggers CxHc opening [157], and an increase in InsP3 in cells was also demonstrated to trigger CxHc opening [142,143,158], indicating that physiological manipulations acting on the membrane potential or cytoplasmic Ca2+ (via InsP3) control CxHc opening. Further support favouring involvement of CxHcs in the control of cytoplasmic Ca2+ levels came from the observation that buffering cytoplasmic Ca2+ with BAPTA [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid] inhibited CxHc-related ATP release [143]. Specific block of CxHcs using Cx mimetic peptides proved crucial in distinguishing their involvement from other Ca2+-dependent release mechanisms, such as exocytosis [146]. Further work on native retinal pigment epithelium [114] suggested a role for cytoplasmic Ca2+ as a component regulator of CxHc opening, based on the observation that ‘trigger cells’ only initiated an intercellular Ca2+ wave when Ca2+ transients in these cells were associated with uptake of the reporter dye propidium iodide. The concept of trigger cells was demonstrated by luminometric extracellular ATP imaging, which showed that ATP was released as a point-source burst originating from a single cell that was subsequently identified by its uptake of propidium iodide entering the cell via CxHcs [159]. This led to the suggestion that there was an association between Ca2+ changes and CxHc opening in trigger cells. Subsequent work showed that an increase in cytoplasmic Ca2+, triggered by photoactivation of caged Ca2+ or Ca2+ entry stimulated by ionophores, was sufficient to open CxHc in Cx32-expressing cells [146]. The ATP-release or dye-uptake responses to cytoplasmic Ca2+ changes were characteristic, since concentrations above 200 nM and below 1000 nM elicited CxHc opening, with lower and higher concentrations being inactive (bell-shaped dose–response curve). Cx43-expressing cells display a similar, but narrower, bell-shaped response peak at 500 nm at which propidium iodide entered and ATP left the cell via CxHcs (E. De Vuyst and L. Leybaert, unpublished work). In recent work, Hcs composed of pannexins may also form a pathway for ATP release triggered by cytoplasmic Ca2+ changes [160].
Figure 4. CxHcs and Ca2+ signalling.
(A) CxHcs open in response to cytoplasmic Ca2+ changes and thereby form a conduit for the release of messengers such as ATP and others; CxHcs only open in so-called ‘trigger cells’. (B) ATP diffuses into the extracellular space and activates G-protein-coupled serpentine receptors on neighbouring cells. This results in the activation of phospholipase C, the formation of InsP3 and the release of Ca2+ from the endoplamic reticulum. This pathway underlies paracrine cell–cell communication of Ca2+ signals. (C) Ca2+ signals can also be communicated by the diffusion of InsP3 or Ca2+ via gap junctions connecting cells. (D) The extracellular ATP concentration gradually decreases and the communication of Ca2+ signals stops unless another trigger cell is encountered that regenerates the ATP signal. (E) Ca2+-triggered ATP release via CxHcs may also be involved in Ca2+ oscillations in the cell via an autocrine signalling path (see the text). (F) Cytoplasmic Ca2+ changes can trigger CxHc opening, and conversely, open CxHcs may magnify Ca2+ changes by Ca2+ entry from the extracellular space.
CxHcs may also be involved in the development of Ca2+ oscillations, which are repetitive cytoplasmic Ca2+ changes in individual cells occurring in the time domain only. Ca2+ oscillations necessitate a regenerative signalling loop, and Ca2+-triggered ATP release via CxHc followed by autocrine action of ATP on cell-surface receptors and subsequent regeneration of the cytoplasmic Ca2+ signal constitutes a putative signalling loop leading to Ca2+ oscillations (Figure 4). In line with this hypothesis, Cx mimetic peptides applied briefly to block CxHcs indeed inhibit spontaneous Ca2+ oscillations in HeLa cells expressing Cx26 or Cx43 and in dispersed non-confluent cardiac myocytes (V. Verma, D. Burke, M. H. Hallett, P. E. Martin and W. H. Evans, unpublished work). Another regenerative loop may be formed by Ca2+-triggered NAD+ release via CxHcs, followed by its conversion into cADP-ribose by the ectoenzyme CD38, re-uptake of cyclic ADP ribose into the cell [161] and activation of ryanodine receptors with subsequent Ca2+ release from the stores. Due to the different kinetics of Ca2+ changes triggered in this manner [162], this regenerative loop is likely to be slower. It remains to be determined whether signal regeneration according to this scheme contributes to the development of Ca2+ oscillations in certain cell types.
A question emerging from the cytoplasmic Ca2+-sensitivity of CxHc is whether there is an endogenous Ca2+ sensor located on Cx subunits or ancillary proteins are involved. Direct interaction of Ca2+ with cytoplasmic Cx domains is unlikely [50], but Ca2+–calmodulin interactions may be involved. It was demonstrated that Ca2+-triggered CxHc opening was largely suppressed by calmodulin inhibition using the inhibitor W7, the extent of inhibition being similar in extent to that obtained with Cx mimetic peptides. In addition, the Ca2+ sensitivity of Cx32Hc opening (200–1000 nM) [146] is in the Kd range for Ca2+–calmodulin interactions (500 nM–5 μM) [163], pointing to the involvement of calmodulin in the signalling cascade. Cx32 has two calmodulin interaction sites – one in the N-terminus and one close to the C-terminal tail [47] – whereas Cx43 has one on its C-terminus [48]. CxHc composed of both Cx32 [146] and Cx43 (L. Leybaert, unpublished work) subtypes open in response to Ca2+ changes, results compatible with direct Ca2+–calmodulin interactions with Cx. However, an indirect cascade via interposed calmodulin-dependent kinases is equally possible, and further work is needed to distinguish these two possibilities.
Elevated cytoplasmic Ca2+ concentrations have been known for a long time to inhibit gap junctions, raising a pertinent question regarding the mechanism of Ca2+ -triggered CxHc opening: how can one reconcile Ca2+-triggered closure of gap junctions with the opening of CxHcs? At the functional level, gap-junction closure prevents the spread of pathological signals to healthy neighbours, whereas open CxHcs cause loss of ATP and dissipation of ion gradients. To resolve these apparent contradictions, one needs to understand the differential effects of Ca2+ changes on hexameric versus dodecameric proteins composed of the same subunits. Definitive answers are currently unavailable, but the following observations may shed some light. First, there may be a difference in the range of Ca2+ concentrations acting on gap-junction channels and CxHcs, the latter being opened by 200–1000 nM Ca2+ [146], whereas the former are closed by somewhat higher levels in the range of 500–2000 nM [50]. Secondly, microdomain Ca2+ concentrations in junctional areas, i.e. just below gap junction plaques, may be substantially different from those in non-junctional sites where CxHcs reside [164,165]. As a consequence, considerations on the effects of global cytoplasmic Ca2+ concentrations on gap junction channels and CxHcs will remain incomplete as long as microdomain Ca2+ information is lacking. Thirdly, different Cx in homo- or hetero-meric assemblies may show differing responses to ambient Ca2+ levels. Fourthly, not only the magnitude of the Ca2+ changes per se, but also the source of the Ca2+ increase, may be decisive. Indeed, it was elegantly demonstrated recently that only capacitative Ca2+ entry via store-operated channels is effective in blocking gap junctions, whereas Ca2+ entry triggered by Ca2+ ionophores was without effect [166].
CxHc: TWO-WAY TRAFFIC?
Gap junctions, in contrast with synaptic junctions, rarely exhibit rectification [167], and when it is observed, as in neural tissues, where Cx36 is a major player [168], the possibility cannot be discounted that coupling or the existence of electrical synapses may reflect the operation of intercellular communication channels constructed of pannexins, channel-forming proteins that are Cx orthologues [169,170,171]. Current evidence indicates that CxHcs can allow two-way traffic. Thus small fluorescent molecules can enter or exit cells across CxHcs [146], and cell-signalling molecules released via these channels move down their concentration gradients. Size/shape discrimination may also operate, since ATP (molecular mass 501 Da), but not lactate (molecular mass 89 Da), was released by ischaemic neonatal cardiac myocytes (T. Clarke, O. Willams, P. E. Martin and W. H. Evans, unpublished work). Simultaneous measurements of the cytoplasmic Ca2+ concentration and propidium iodide dye uptake in Cx32-expressing cells showed that resting cells with open CxHc, exemplified by dye uptake, had a cytoplasmic Ca2+ concentration that was only marginally above the resting level in cells with closed CxHc (≈95 nM versus ≈60 nM respectively) [146]. This suggests that opening of high-conductance CxHc conduits (single-channel conductance of ∼90 pS for Cx32 [75]) did not result in an immediate collapse of the transmembrane ion gradients, presumably because of a low open probability of these channels or because the pumps have sufficient capacity to counteract this leakage pathway. Much larger changes related to CxHc opening have been described for ventricular myocytes that express Cx43 under conditions of metabolic inhibition [108].
Open CxHcs may provide pathways for foreign and possibly infectious agents to enter cells. Although the pore dimensions of CxHc would be expected to discriminate against entry of viruses and micro-organisms, Shigella infection was shown to induce indirectly the opening of CxHc in an actin- and phospholipase-C-dependent manner and culminating in ATP release into media that accounted for the intercellular propagation of Ca2+ transients [172]. Viral infections also influence Cx expression [173] and thus CxHc function. Since small interfering RNA [174] and peptides [175] are transferred between cells via gap junctions, the possibility arises that they may also enter cells via CxHcs.
CxHc, INJURY CONTROL AND CELL DEATH
Cxs play a role in cell death/cell survival, via gap-junction channels that may communicate pro-apoptotic or cell-protective messages to neighbouring cells, but also via Cx-intrinsic effects that are not related to gap-junction channels [176,177]. CxHcs may add to this death/survival repertoire. CxHcs in open configuration are detrimental to the efficient operation of cell metabolism and ultimately lead to cell death for maintenance of ionic balances between the cell interior and exterior cannot be sustained over long time periods. In other words, open CxHcs can behave as pathogenic pores. As such, they can be decisive in the balance between cell death via necrosis or apoptosis, as they may substantially influence the intracellular ATP concentration [178] which is an important outcome factor in the progression towards necrosis or apoptosis [179]. Cellular ATP depletion may in its turn activate CxHcs [180], thereby instigating further pathogenic effects of CxHc pores. Apoptotic stimuli, for example staurosporin, open Cx43Hcs, and staurosporin-triggered apoptosis was inhibited in CxHc composed of C-terminal truncated Cx43Hc (forming non-functional CxHcs) [181]. Other studies have reported closure of CxHc during the development of apoptosis [182]. Alendronate, a biphosphonate drug used in the treatment of osteoporosis, inhibited apoptosis of osteocytes in a CxHc-dependent manner [183,184], indicating that CxHc opening may also transduce anti-apoptotic effects. A further link between CxHcs and apoptosis comes from the cellular-Ca2+-homoeostasis field, showing that it is also an important player in the apoptotic cascade [185]. Apoptosis is associated with cytoplasmic Ca2+ elevation, which may trigger CxHc opening and, conversely, open CxHc, generating high-conductance Ca2+ entry channels with possible impact on cytoplasmic Ca2+ regulation. Similarly, membrane depolarization, one of the consequences of oxidative stress, opens CxHcs and allows reactive oxygen to penetrate cells, resulting in damage that leads to apoptosis. Changes to CxHc gating ultimately disrupt internal ionic homoeostasis and may activate Ca2+-dependent kinases, leading to a plethora of injurious consequences such as mitochondrial poisoning (S. Ramachandran, S. Subramanian and R. Lal, personal communication). The importance of CxHcs in the apoptosis field is currently not fully established, but specific inhibition of CxHcs using Cx mimetic peptides will allow detailed studies on their involvement and may provide new insights into the signalling cascades leading to apoptosis in Cx-expressing cells.
In heart, where release of ATP across CxHcs is a stress response that vasodilates blood vessels and facilitates increased delivery of oxygen and energy to the organ [186], continued loss of ATP cannot be sustained [187] and ultimately leads to cell death. Cardioprotection by ischaemic preconditioning features phosphorylation of Cx43 and is generally believed to implicate gap-junctional communication, and a possible role for Cx43Hc located at non-junctional areas in cardiac myocytes has been advocated [188,189]. ATP release through CxHcs elicits a cellular response to injury [113,190], and stroke/hypoxia models [191,192] suggest that similar molecular mechanisms that implicate open CxHcs contribute to the death of neurons and interacting astrocytes. Indeed, in hypoxia, astrocytes release ATP and glutamate via CxHcs [193]. The development of pharmaceutical tools for cardio- and neuro-protection in ischaemic injury is a major goal and has already focused on traditional non-specific gap-junction inhibitors such as halothane and octanol, which reduce the extent of brain injuries in animal models [192]. The characterization of CxHc and identification of their roles in ATP release and ATP-dependent intercellular Ca2+ signalling, combined with the identification of CxHc as the primary targets of Cx mimetic peptides, indicate that the peptide mimetics stand as promising tools with which to counteract necrotic and apoptotic cell death ensuing in heart attack and stroke.
FUTURE DIRECTIONS
Just as human beings have learnt to adapt to stressful conditions, cells also adjust to potentially harmful environmental conditions. CxHcs appear as key structures in stress response, since their exposed position makes them vulnerable as membrane conduits across which cells release small molecules and ions. The release of ATP across CxHcs and its role in paracrine intercellular signalling is now well documented, although that of glutamate by astroglia is hotly debated [193]. It is possible that further molecules, many with signalling potential, utilize these channels, thereby adding to the range of small biochemical messengers underpinning near-neighbour junctional cellular cross-talk. The increasing attribution of Cx mutations to Hc signalling misfunction, shown in CMTX [3,4] and dysplasias [203] may open up a Pandora's Box of connexin channelopathies.
Cells respond to their adhering partners by formation of junctions. In addition, they are increasingly perceived as being able to sense or monitor their external microenvironment, as illustrated by the presence of cell-surface Ca2+ sensors [194]. The opening of CxHcs by intracellular Ca2+ levels that differ from those that close gap junctions can be regarded as testimony to the spatial control of the operation of these channels. This is possibly an evolutionary development to allow cells to cope with environmental changes, as illustrated in hypoxia, where a number of genes are rapidly activated. The regulation of CxHcs by graded Ca2+ levels at the inner and outer aspect of the channel makes it difficult to distinguish between direct and indirect effects, since the Ca2+ microenviroment may vary beneath the various regions of the cell surface, especially in polarized cells. On a broader signalling front, Cx43Hcs are linked to intracellular signalling pathways that, in turn, are key regulators of apoptosis [183–185].
Cx channels are implicated in embryonic development and patterning [195], and early important observations on their roles as gap junctions, allowing intercellular coupling in Xenopus and mouse embryo development [196], are ripe for re-investigation in a broader signalling context, since proteins other than Cxs are also likely to feature; for example, Eph receptors and ephrin, among others, are advocated as components of the cell-to-cell communication apparatus [197]. In a similar vein, Cxs have also entered the stem-cell arena, with Cx43Hcs advocated as negative markers in human limbal epithelial stem cells [198]. The identification of innexins in protostomes [199,200] and pannexins in vertebrate brain [169–171], with both families of proteins forming cell-to-cell communication channels, despite possessing different protein structures, prompts fundamental questions regarding the evolution of mechanisms effecting cellular cross-talk [201]. Indeed, pannexin channels also release ATP under mechanical stress [202]. It is thus possible that pannexins also operate as Hcs in the plasma membrane, underwriting paracrine signalling and implicating purinergic receptors and intracellular Ca2+ [160].
References
- 1.Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Guldenagel M., Deutsch U., Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 2.White T. W., Wang H., Mui R., Litteral J., Brink P. R. Cloning and functional expression of invertebrate connexins from Halocynthia pyriformis. FEBS Lett. 2004;577:42–48. doi: 10.1016/j.febslet.2004.09.071. [DOI] [PubMed] [Google Scholar]
- 3.Evans W. H., Martin P. E. Gap junctions: structure and function. Mol. Membr. Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 4.Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 5.Lauf U., Giepmans B. N. G., Lopez P., Braconnot S., Chen S. C., Falk M. M. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryerse J. S., Nagel B. A., Hammel I. The role of connexon aggregate fusion in gap junction growth. J. Submicrosc. Cytol. Pathol. 1984;16:649–657. [PubMed] [Google Scholar]
- 7.Severs N. J. The cardiac muscle cell. Bioessays. 2000;22:188–199. doi: 10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Forge A., Becker D., Casalotti S., Edwards J., Evans W. H., Lench N., Souter M. Gap junctions and connexin expression in the inner ear. Novartis Found. Symp. 1999;219:134–156. doi: 10.1002/9780470515587.ch9. [DOI] [PubMed] [Google Scholar]
- 9.Laird D. W. 2005 International Gap Junction Conference overview. Cell Commun. Adhes. 2005;12:73–74. [Google Scholar]
- 10.Laird D. W. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breidert S., Jacob R., Ngezahayo A., Kolb H. A., Naim H. Y. Trafficking pathways of Cx49–GFP in living mammalian cells. Biol. Chem. 2005;386:155–160. doi: 10.1515/BC.2005.019. [DOI] [PubMed] [Google Scholar]
- 12.Rahman S., Carlile G., Evans W. H. Assembly of hepatic gap-junctions – topography and distribution of connexin-32 in intracellular and plasma-membranes determined using sequence-specific antibodies. J. Biol. Chem. 1993;268:1260–1265. [PubMed] [Google Scholar]
- 13.Leithe E., Brech A., Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem. J. 2006;393:59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthoud V. M., Minogue P. J., Laing J. G., Beyer E. C. Pathways for degradation of connexins and gap junctions. Cardiovasc. Res. 2004;62:256–267. doi: 10.1016/j.cardiores.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Das Sarma J., Meyer R. A., Wang F. S., Abraham V., Lo C. W., Koval M. Multimeric connexin interactions prior to the trans-Golgi network. J. Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 16.Diez J. A., Ahmad S., Evans W. H. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur. J. Biochem. 1999;262:142–148. doi: 10.1046/j.1432-1327.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim J., Scott S. V., Klionsky D. J. Alternative protein sorting pathways. Int. Rev. Cytol. 2000;198:153–201. doi: 10.1016/s0074-7696(00)98005-7. [DOI] [PubMed] [Google Scholar]
- 18.Martin P. E. M., Blundell G., Ahmad S., Errington R. J., Evans W. H. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- 19.Cruciani W., Mikalsen S. O. Ilimaquinone inhibits gap junctional communication in a connexin isotype-specific manner. Exp. Cell Res. 2005;304:136–148. doi: 10.1016/j.yexcr.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad S., Evans W. H. Post-translational integration and oligomerization of connexin 26 in plasma membranes and evidence of formation of membrane pores: implications for the assembly of gap junctions. Biochem. J. 2002;365:693–699. doi: 10.1042/BJ20011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stout C., Goodenough D. A., Paul D. L. Connexins: functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.John S., Cesario D., Weiss J. N. Gap junctional hemichannels in the heart. Acta Physiol. Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 23.Herve J. C., Bourmeyster N., Sarrouilhe D. Diversity in protein–protein interactions of connexins: emerging roles. Biochim. Biophys. Acta. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Warn-Cramer B. J., Lau A. F. Regulation of gap junctions by tyrosine protein kinases. Biochim. Biophys. Acta. 2004;1662:81–95. doi: 10.1016/j.bbamem.2003.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solan J. L., Lampe P. D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Purnick P. E. M., Benjamin D. C., Verselis V. K., Bargiello T. A., Dowd T. L. Structure of the amino terminus of a gap junction protein. Arch. Biochem. Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 27.George C. H., Kendall J. M., Evans W. H. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J. Biol. Chem. 1999;274:8678–8685. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- 28.Ebihara L., Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebihara L., Berthoud V. M., Beyer E. C. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys. J. 1995;68:1796–1803. doi: 10.1016/S0006-3495(95)80356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebihara L. New roles for connexons. News Physiol. Sci. 2003;18:100–103. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- 31.Ebihara L. Physiology and biophysics of hemi-gap-junctional channels expressed in Xenopus oocytes. Acta Physiol. Scand. 2003;179:5–8. doi: 10.1046/j.1365-201X.2003.01195.x. [DOI] [PubMed] [Google Scholar]
- 32.Harris A. L., Bevans C. G. Exploring hemichannel permeability in vitro. Methods Mol. Biol. 2001;154:357–377. doi: 10.1385/1-59259-043-8:357. [DOI] [PubMed] [Google Scholar]
- 33.Trexler E. B., Bukauskas F. F., Kronengold J., Bargiello T. A., Verselis V. K. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys. J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh S., Rivkin S., Tang Q. X., Verselis V. K., Bargiello T. A. Determinants of gating polarity of a connexin 32 hemichannel. Biophys. J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foote C. I., Zhou L., Zhu X., Nicholson B. J. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J. Cell Biol. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unger V. M., Kumar N. M., Gilula N. B., Yeager M. Expression, two-dimensional crystallization, and electron cryo-crystallography of recombinant gap junction membrane channels. J. Struct. Biol. 1999;128:98–105. doi: 10.1006/jsbi.1999.4184. [DOI] [PubMed] [Google Scholar]
- 37.Unger V. M., Kumar N. M., Gilula N. B., Yeager M. Electron cryo-crystallography of a recombinant cardiac gap junction channel. Novartis Found. Symp. 1999;219:22–30. doi: 10.1002/9780470515587.ch3. [DOI] [PubMed] [Google Scholar]
- 38.Unger V. M., Kumar N. M., Gilula N. B., Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 39.Bao X., Chen Y., Reuss L., Altenberg G. A. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J. Biol. Chem. 2004;279:9689–9692. doi: 10.1074/jbc.M311438200. [DOI] [PubMed] [Google Scholar]
- 40.Saez J. C., Retamal M. A., Basilio D., Bukauskas F. F., Bennett M. V. Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elfgang C., Eckert R., Lichtenbergfrate H., Butterweck A., Traub O., Klein R. A., Hulser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa-cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ek-Vitorin J. F., Burt J. M. Quantification of gap junction selectivity. Am. J. Physiol. Cell Physiol. 2005;289:C1535–C1546. doi: 10.1152/ajpcell.00182.2005. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg G. S., Moreno A. P., Lampe P. D. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J. Biol. Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- 44.Weber P. A., Chang H. C., Spaeth K. E., Nitsche J. M., Nicholson B. J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butkevich E., Hulsmann S., Wenzel D., Shirao T., Duden R., Majoul I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 2004;14:650–658. doi: 10.1016/j.cub.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama M., Ishida N., Ogawa T., Yogo K., Takeya T. Molecular cloning and functional analysis of a novel Cx43 partner protein CIP150. Biochem. Biophys. Res. Commun. 2005;335:1264–1271. doi: 10.1016/j.bbrc.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Torok K., Stauffer K., Evans W. H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997;326:479–483. doi: 10.1042/bj3260479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burr G. S., Mitchell C. K., Keflemariam Y. J., Heidelberger R., O'Brien J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem. Biophys. Res. Commun. 2005;335:1191–1198. doi: 10.1016/j.bbrc.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peracchia C., Sotkis A., Wang X. G., Peracchia L. L., Persechini A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 2000;275:26220–26224. doi: 10.1074/jbc.M004007200. [DOI] [PubMed] [Google Scholar]
- 50.Peracchia C. Chemical gating of gap junction channels Roles of calcium, pH and, calmodulin. Biochim. Biophys. Acta. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad S., Martin P. E. M., Evans W. H. Assembly of gap junction channels – mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001;268:4544–4552. doi: 10.1046/j.1432-1327.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- 52.Parekh A. B., Putney J. W. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 53.Malchow R. P., Qian H., Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J. Neurosci. Res. 1993;35:237–245. doi: 10.1002/jnr.490350303. [DOI] [PubMed] [Google Scholar]
- 54.Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma-membrane of Xenopus oocytes. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebihara L., Xu X., Oberti C., Beyer E. C., Berthoud V. M. Co-expression of lens fiber connexins modifies hemi-gap-junctional channel behavior. Biophys. J. 1999;76:198–206. doi: 10.1016/S0006-3495(99)77189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebihara L., Liu X. Q., Pal J. D. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys. J. 2003;84:277–286. doi: 10.1016/S0006-3495(03)74848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ripps H., Qian H. H., Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell. Mol. Neurobiol. 2004;24:647–665. doi: 10.1023/B:CEMN.0000036403.43484.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivas M., Kronengold J., Bukauskas F. F., Bargiello T. A., Verselis V. K. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys. J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valiunas V., Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Archiv. Eur. J. Physiol. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 60.Puljung M. C., Berthoud V. M., Beyer E. C., Hanck D. A. Polyvalent cations constitute the voltage gating particle in human connexin37 hemichannels. J. Gen. Physiol. 2004;124:587–603. doi: 10.1085/jgp.200409023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valiunas V., Mui R., McLachlan E., Valdimarsson G., Brink P. R., White T. W. Biophysical characterization of zebrafish connexin35 hemichannels. Am. J. Physiol. Cell Physiol. 2004;287:C1596–C1604. doi: 10.1152/ajpcell.00225.2004. [DOI] [PubMed] [Google Scholar]
- 62.Pfahnl A., Dahl G. Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys. J. 1998;75:2323–2331. doi: 10.1016/S0006-3495(98)77676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bader P., Weingart R. Conductive and kinetic properties of connexin45 hemichannels expressed in transfected HeLa cells. J. Membr. Biol. 2004;199:143–154. doi: 10.1007/s00232-004-0682-y. [DOI] [PubMed] [Google Scholar]
- 64.Li H. Y., Liu T. F., Lazrak A., Peracchia C., Goldberg G. S., Lampe P. D., Johnson R. G. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro C., Gomez-Hernandez J. M., Silander K., Barrio L. C. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J. Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang G. S. L., de Miguel M., Gomez-Hernandez J. M., Glass J. D., Scherer S. S., Mintz M., Barrio L. C., Fischbeck K. H. Severe neuropathy with leaky connexin32 hemichannels. Ann. Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- 67.Essenfelder G. M., Bruzzone R., Lamartine J., Charollais A., Blanchet-Bardon C., Barbe M. T., Meda P., Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 68.Zhao H. B., Yu N., Fleming C. R. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong C. W., Christen T., Foglia B. F., Roth I., Chanson M., Goodenough D. A., Kwak B. R. Connexin37 participates in atherosclerosis by controlling leukocyte recruitment. Circulation. 2005;112:U126–U126. [Google Scholar]
- 70. Reference deleted.
- 71.Hand G. M., Muller D. J., Nicholson B. J., Engel A., Sosinsky G. E. Isolation and characterization of gap junctions from tissue culture cells. J. Mol. Biol. 2002;315:587–600. doi: 10.1006/jmbi.2001.5262. [DOI] [PubMed] [Google Scholar]
- 72.Cascio M., Kumar N. M., Safarik R., Gilula N. B. Physical characterization of gap junction membrane connexons (hemi-channels) isolated from rat-liver. J. Biol. Chem. 1995;270:18643–18648. doi: 10.1074/jbc.270.31.18643. [DOI] [PubMed] [Google Scholar]
- 73.Zimmer D. B., Green C. R., Evans W. H., Gilula N. B. Topological analysis of the major protein in isolated intact rat-liver gap-junctions and gap junction-derived single membrane structures. J. Biol. Chem. 1987;262:7751–7763. [PubMed] [Google Scholar]
- 74.Rhee S. K., Bevans C. G., Harris A. L. Channel-forming activity of immunoaffinity-purified connexin32 in single phospholipid membranes. Biochemistry. 1996;35:9212–9223. doi: 10.1021/bi960295m. [DOI] [PubMed] [Google Scholar]
- 75.Harris A. L. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 76.Verselis V. K., Trexler E. B., Bukauskas F. F. Connexin hemichannels and cell–cell channels: comparison of properties. Braz. J. Med. Biol. Res. 2000;33:379–389. doi: 10.1590/s0100-879x2000000400003. [DOI] [PubMed] [Google Scholar]
- 77.Thimm J., Mechler A., Lin H., Rhee S., Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J. Biol. Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 78.Locke D., Liu J., Harris A. L. Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry. 2005;44:13027–13042. doi: 10.1021/bi050495a. [DOI] [PubMed] [Google Scholar]
- 79.Tillman T. S., Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem. Biophys. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- 80.Simons K., Vaz W. L. C. Model systems, lipid rafts, and cell membranes. Ann. Rev. Biophys. Biomol. Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 81.Kronengold J., Trexler E. B., Bukauskas F. F., Bargiello T. A., Verselis V. K. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J. Gen. Physiol. 2003;122:389–405. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez-Hernandez J. M., Miguel M. C., Larrosa B., Gonzalez D., Barrio L. C. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. U.S.A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sosinsky G. E., Nicholson B. J. Structural organization of gap junction channels. Biochim. Biophys. Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Fleishman S. J., Harrington S., Friesner R. A., Honig B., Ben-Tal N. An automatic method for predicting transmembrane protein structures using cryo-EM and evolutionary data. Biophys. J. 2004;87:3448–3459. doi: 10.1529/biophysj.104.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fleishman S. J., Unger V. M., Yeager M., Ben-Tal N. A Cα model for the transmembrane α helices of gap junction intercellular channels. Mol. Cell. 2004;15:879–888. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 86.King T. J., Lampe P. D. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim. Biophys. Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lampe P. D., Lau A. F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lampe P. D., Lau A. F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 89.Contreras J. E., Sanchez H. A., Eugenin E. A., Speidel D., Theis M., Willecke K., Bukauskas F. F., Bennett M. V. L., Saez J. C. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. U.S.A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol. Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 91.Bodin P., Burnstock G. Purinergic signalling: ATP release. Neurochem. Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 92.Bodin P., Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 93.Lazarowski E. R., Boucher R. C., Harden T. K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 94.Faria R. X., Defarias F. P., Alves L. A. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am. J. Physiol. Cell. Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- 95.Evanko D. S., Zhang Q., Zorec R., Haydon P. G. Defining pathways of loss and secretion of chemical messengers from astrocytes. Glia. 2004;47:233–240. doi: 10.1002/glia.20050. [DOI] [PubMed] [Google Scholar]
- 96.Parpura V., Scemes E., Spray D. C. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem. Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 98.Cherian P. P., Siller-Jackson A. J., Gu S. M., Wang X., Bonewald L. F., Sprague E., Jiang J. X. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quist A. P., Rhee S. K., Lin H., Lal R. Physiological role of gap-junctional hemichannels: Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stout C. E., Costantin J. L., Naus C. C. G., Charles A. C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 101.Cotrina M. L., Lin J. H. C., Alves-Rodrigues A., Liu S., Li J., Azmi-Ghadimi H., Kang J., Naus C. C. G., Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Srinivas M., Calderon D. P., Kronengold J., Verselis V. K. Regulation of connexin hemichannels by monovalent cations. J. Gen. Physiol. 2006;127:67–75. doi: 10.1085/jgp.200509397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Contreras J. E., Saez J. C., Bukauskas F. F., Bennett M. V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomes P., Srinivas S. P., Van Driessche W., Vereecke J., Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 105.Romanello M., Veronesi V., D'Andrea P. Mechanosensitivity and intercellular communication in HOBIT osteoblastic cells: a possible role for gap junction hemichannels. Biorheology. 2003;40:119–121. [PubMed] [Google Scholar]
- 106.Bao L., Sachs F., Dahl G. Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 107.Kondo R. P., Wang S. Y., John S. A., Weiss J. N., Goldhaber J. I. Metabolic inhibition activates a non selective current through connexin hemichannels in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 108.John S. A., Kondo R., Wang S. Y., Goldhaber J. I., Weiss J. N. Connexin-43 hemichannels opened by metabolic inhibition. J. Biol. Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 109.Bao X., Reuss L., Altenberg G. A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of serine 368. J. Biol. Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 110.Goodenough D. A., Paul D. L. Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 111.Przyklenk K., Maynard M., Darling C. E., Whittaker P. Pretreatment with D-myo-inositol trisphosphate reduces infarct size in rabbit hearts: role of inositol trisphosphate receptors and gap junctions in triggering protection. J. Pharmacol. Exp. Ther. 2005;314:1386–1392. doi: 10.1124/jpet.105.087742. [DOI] [PubMed] [Google Scholar]
- 112.Beltramello M., Piazza V., Bukauskas F. F., Pozzan T., Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 113.Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 114.Pearson R. A., Dale N., Llaudet E., Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 115.Evans W. H., Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem. Soc. Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 116.Salameh A., Dhein S. Pharmacology of Gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta. 2005;1719:36–58. doi: 10.1016/j.bbamem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 117.Lavado E., Sanchez Abarca L. I., Tabernero A., Bolanos J. P., Medina J. M. Oleic acid inhibits gap junction permeability and increases glucose uptake in cultured rat astrocytes. J. Neurochem. 1997;69:721–728. doi: 10.1046/j.1471-4159.1997.69020721.x. [DOI] [PubMed] [Google Scholar]
- 118.Schmilinsky-Fluri G., Valiunas V., Willi M., Weingart R. Modulation of cardiac gap junctions: the mode of action of arachidonic acid. J. Mol. Cell Cardiol. 1997;29:1703–1713. doi: 10.1006/jmcc.1997.0409. [DOI] [PubMed] [Google Scholar]
- 119.Weingart R., Bukauskas F. F. Long-chain n-alkanols and arachidonic acid interfere with the V-m-sensitive gating mechanism of gap junction channels. Pflugers Arch. Eur. J. Physiol. 1998;435:310–319. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- 120.Guan X. J., Wilson S., Schlender K. K., Ruch R. J. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18β-glycyrrhetinic acid. Mol. Carcinog. 1996;16:157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 121.Srinivas M., Spray D. C. Closure of gap junction channels by arylaminobenzoates. Mol. Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- 122.Harks E. G. A., De Roos A. D. G., Peters P. H. J., De Haan L. H., Brouwer A., Ypey D. L., Van Zoelen E. J. J., Theuvenet A. P. R. Fenamates: a novel class of reversible gap junction blockers. J. Pharmacol. Exp. Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- 123.Verrecchia F., Herve J. C. Reversible inhibition of gap junctional communication by tamoxifen in cultured cardiac myocytes. Pflugers Arch. Eur. J. Physiol. 1997;434:113–116. doi: 10.1007/s004240050370. [DOI] [PubMed] [Google Scholar]
- 124.Shore L., McLean P., Gilmour S. K., Hodgins M. B., Finbow M. E. Polyamines regulate gap junction communication in connexin 43-expressing cells. Biochem. J. 2001;357:489–495. doi: 10.1042/0264-6021:3570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Srinivas M., Hopperstad M. G., Spray D. C. Quinine blocks specific gap junction channel subtypes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Musa H., Fenn E., Crye M., Gemel J., Beyer E. C., Veenstra R. D. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J. Physiol. (London) 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dahl G., Nonner W., Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys. J. 1994;67:1816–1822. doi: 10.1016/S0006-3495(94)80663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Warner A., Clements D. K., Parikh S., Evans W. H., Dehaan R. L. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J. Physiol. (London) 1995;488:721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berthoud V. M., Beyer E. C., Seul K. H. Peptide inhibitors of intercellular communication. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L619–L622. doi: 10.1152/ajplung.2000.279.4.L619. [DOI] [PubMed] [Google Scholar]
- 130.Isakson B. E., Seedorf G. J., Lubman R. L., Evans W. H., Boitano S. Cell–cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;29:552–561. doi: 10.1165/rcmb.2002-0281OC. [DOI] [PubMed] [Google Scholar]
- 131.Chaytor A. T., Martin P. E., Evans W. H., Randall M. D., Griffith T. M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. (London) 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dora K. A., Martin P. E. M., Chaytor A. T., Evans W. H., Garland C. J., Griffith T. M. Role of heterocellular gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem. Biophys. Res. Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- 133.Martin P. E. M., Wall C., Griffith T. M. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br. J. Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boitano S., Evans W. H. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 135.Isakson B. E., Evans W. H., Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L221–L228. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- 136.Chaytor A. T., Evans W. H., Griffith T. M. Peptides homologous to extracellular loop motifs of connexin43 reversibly abolish rhythmic contractile activity in rabbit arteries. J. Physiol. (London) 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Isakson B. E., Duling B. R. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ. Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 138.Isakson B. E., Damon D. N., Day K. H., Liao Y., Duling B. R. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am. J. Physiol. Heart Circ. Physiol. 2005;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 139.Oviedo-Orta E., Evans W. H. Gap junctions and connexin-mediated communication in the immune system. Biochim. Biophys. Acta. 2004;1662:102–112. doi: 10.1016/j.bbamem.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 140.Matsue H., Yao J., Matsue K., Nagasaka A., Sugiyama H., Aoki R., Kitamura M., Shimada S. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 2006;176:181–190. doi: 10.4049/jimmunol.176.1.181. [DOI] [PubMed] [Google Scholar]
- 141.Oviedo-Orta E., Gasque P., Evans W. H. Immunoglobulin and cytokine expression in mixed lymphocyte cultures is reduced by disruption of gap junction intercellular communication. FASEB J. 2001;15:768–774. doi: 10.1096/fj.00-0288com. [DOI] [PubMed] [Google Scholar]
- 142.Braet K., Aspeslagh S., Vandamme W., Willecke K., Martin P. E., Evans W. H., Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell. Physiol. 2003;197:205–213. doi: 10.1002/jcp.10365. [DOI] [PubMed] [Google Scholar]
- 143.Braet K., Vandamme W., Martin P. E., Evans W. H., Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 144.Leybaert L., Braet K., Vandamme W., Cabooter L., Martin P. E., Evans W. H. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun. Adhes. 2003;10:251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- 145.Chaytor A. T., Martin P. E. M., Edwards D. H., Griffith T. M. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- 146.De Vuyst E., Decrock E., Cabooter L., Dubyak G. R., Naus C. C., Evans W. H., Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zorko M., Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 148.Seki A., Duffy H. S., Coombs W., Spray D. C., Taffet S. M., Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ. Res. 2004;95:22–28. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- 149.Gaietta G., Deerinck T. J., Adams S. R., Bouwer J., Tour O., Laird D. W., Sosinsky G. E., Tsien R. Y., Ellisman M. H. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 150.Berman R. S., Martin P. E. M., Evans W. H., Griffith T. M. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc. Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- 151.Clarke T. C., Thomas D., Petersen J. S., Evans W. H., Martin P. E. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin 43. Br. J. Pharmacol. 2006;43:486–495. doi: 10.1038/sj.bjp.0706631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boitano S., Dirksen E. R., Sanderson M. J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 153.Saez J. C., Connor J. A., Spray D. C., Bennett M. V. L. Hepatocyte gap-junctions are permeable to the 2nd messenger, inositol 1,4,5-trisphosphate, and to calcium-ions. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hassinger T. D., Guthrie P. B., Atkinson P. B., Bennett M. V. L., Kater S. B. An extracellular signaling component in propagation of astrocytic calcium waves. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guthrie P. B., Knappenberger J., Segal M., Bennett M. V. L., Charles A. C., Kater S. B. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eugenin E. A., Gonzalez H., Saez C. G., Saez J. C. Gap junctional communication coordinates vasopressin-induced glycogenolysis in rat hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;37:G1109–G1116. doi: 10.1152/ajpgi.1998.274.6.G1109. [DOI] [PubMed] [Google Scholar]
- 157.Trexler E. B., Bennett M. V. L., Bargiello T. A., Verselis V. K. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vandamme W., Braet K., Cabooter L., Leybaert L. Tumour necrosis factor alpha inhibits purinergic calcium signalling in blood–brain barrier endothelial cells. J. Neurochem. 2004;88:411–421. doi: 10.1046/j.1471-4159.2003.02163.x. [DOI] [PubMed] [Google Scholar]
- 159.Arcuino G., Lin J. H. C., Takano T., Liu C., Jiang L., Gao Q., Kang J., Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Locovei S., Wang J., Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 161.De Flora A., Zocchi E., Guida L., Franco L., Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N.Y. Acad. Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- 162.Lee H. C. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol. Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 163.Chin D., Means A. R. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 164.George C. H., Kendall J. M., Campbell A. K., Evans W. H. Connexin–aequorin chimerae report cytoplasmic calcium environments along trafficking pathways leading to gap junction biogenesis in living COS-7 cells. J. Biol. Chem. 1998;273:29822–29829. doi: 10.1074/jbc.273.45.29822. [DOI] [PubMed] [Google Scholar]
- 165.Marsault R., Murgia M., Pozzan T., Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 1997;16:1575–1581. doi: 10.1093/emboj/16.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dakin K., Zhao Y. R., Li W. H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods. 2005;2:55–62. doi: 10.1038/nmeth730. [DOI] [PubMed] [Google Scholar]
- 167.Bennett M. V., Zukin R. S. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 168.Cruikshank S. J., Landisman C. E., Mancilla J. G., Connors B. W. Connexon connexions in the thalamocortical system. Prog. Brain Res. 2005;149:41–57. doi: 10.1016/S0079-6123(05)49004-4. [DOI] [PubMed] [Google Scholar]
- 169.Herve J. C., Phelan P., Bruzzone R., White T. W. Connexins, innexins and pannexins: bridging the communication gap. Biochim. Biophys. Acta. 2005;1719:3–5. doi: 10.1016/j.bbamem.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 170.Panchin Y. V. Evolution of gap junction proteins – the pannexin alternative. J. Exp. Biol. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 171.Baranova A., Ivanova D. V., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 172.Tran Van Nhieu G., Clair C., Bruzzone R., Mesnil M., Sansonetti P., Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 173.Zhu Y., Mao Z., Lou D., Zhang H. The expression of connexin 43 and desmin in viral myocarditis. Zhonghua Bing Li Xue Za Zhi. 2000;29:288–290. in Chinese. [PubMed] [Google Scholar]
- 174.Valiunas V., Polosina Y. Y., Miller H., Potapova I. A., Valiuniene L., Doronin S., Mathias R. T., Robinson R. B., Rosen M. R., Cohen I. S., Brink P. R. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. (London) 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Neijssen J., Herberts C., Drijfhout J. W., Reits E., Janssen L., Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature (London) 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 176.Vinken M., Vanhaecke T., Papeleu P., Snykers S., Henkens T., Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Krysko D. V., Leybaert L., Vandenabeele P., D'Herde K. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis. 2005;10:459–469. doi: 10.1007/s10495-005-1875-2. [DOI] [PubMed] [Google Scholar]
- 178.Braet K., Mabilde C., Cabooter L., Rapp G., Leybaert L. Electroporation loading and photoactivation of caged InsP3: tools to investigate the relation between cellular ATP release in response to intracellular InsP3 elevation. J. Neurosci. Methods. 2004;132:81–89. doi: 10.1016/j.jneumeth.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 179.Nicotera P., Leist M., Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol. Lett. 1998;103:139–142. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- 180.Vergara L., Bao X., Cooper M., Bello-Reuss E., Reuss L. Gap-junctional hemichannels are activated by ATP depletion in human renal proximal tubule cells. J. Membr. Biol. 2003;196:173–184. doi: 10.1007/s00232-003-0636-9. [DOI] [PubMed] [Google Scholar]
- 181.Hur K. C., Shim J. E., Johnson R. G. A potential role for Cx43-hemichannels in staurosporin-induced apoptosis. Cell Commun. Adhes. 2003;10:271–277. doi: 10.1080/cac.10.4-6.271.277. [DOI] [PubMed] [Google Scholar]
- 182.Kalvelyte A., Imbrasaite A., Bukauskiene A., Verselis V. K., Bukauskas F. F. Connexins and apoptotic transformation. Biochem. Pharmacol. 2003;66:1661–1672. doi: 10.1016/s0006-2952(03)00540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Plotkin L. I., Aguirre J. I., Kousteni S., Manolagas S. C., Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J. Biol. Chem. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 184.Plotkin L. I., Manolagas S. C., Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 185.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 186.Headrick J. P., Hack B., Ashton K. J. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- 187.Turner M. S., Haywood G. A., Andreka P., You L., Martin P. E., Evans W. H., Webster K. A., Bishopric N. H. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ. Res. 2004;95:726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- 188.Boengler K., Schulz R., Heusch G. Connexin 43 signaling and cardioprotection. Heart. 2006. in the press. [DOI] [PMC free article] [PubMed]
- 189.Boengler K., Dodoni G., Rodriguez-Sinovas A., Cabestrero A., Ruiz-Meana M., Gres P., Konietzka I., Lopez-Iglesias C., Garcia-Dorado D., Di Lisa, et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 190.Contreras J. E., Sanchez H. A., Veliz L. P., Bukauskas F. F., Bennett M. V. L., Saez J. C. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res. Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kirchhoff F., Dringen R., Giaume C. Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur. Arch. Psychiat. Clin. Neurosci. 2001;251:159–169. doi: 10.1007/s004060170036. [DOI] [PubMed] [Google Scholar]
- 192.de Pina-Benabou M. H., Szostak V., Kyrozis A., Rempe D., Uziel D., Urban-Maldonado M., Benabou S., Spray D. C., Federoff H. J., Stanton P. K., Rozental R. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–2237. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- 193.Takano T., Kang J., Jaiswal J. K., Simon S. M., Lin J. H. C., Yu Y. F., Li Y. X., Yang J., Dienel G., Zielke H. R., Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Caroppo R., Gerbino A., Fistetto G., Colella M., Debellis L., Hofer A. M., Curci S. Extracellular calcium acts as a “third messenger” to regulate enzyme and alkaline secretion. J. Cell Biol. 2004;166:111–119. doi: 10.1083/jcb.200310145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levin M. Isolation and community: A review of the role of gap-junctional communication in embryonic patterning. J. Membr. Biol. 2002;185:177–192. doi: 10.1007/s00232-001-0129-7. [DOI] [PubMed] [Google Scholar]
- 196.Lee S., Gilula N. B., Warner A. E. Gap junctional communication and compaction during preimplantation stages of mouse development. Cell. 1987;51:851–860. doi: 10.1016/0092-8674(87)90108-5. [DOI] [PubMed] [Google Scholar]
- 197.Wilkinson D. G. Multiple roles of Eph receptors and ephrins in neural development. Nat. Rev. Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- 198.Chen Z., Evans W. H., Pflugfelder S. C., Li D. Q. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24:1265–1273. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Phelan P., Starich T. A. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 200.Bauer R., Loer B., Ostrowski K., Martini J., Weimbs A., Lechner H., Hoch M. Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem. Biol. 2005;12:515–526. doi: 10.1016/j.chembiol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 201.Alexopoulos H., Bottger A., Fischer S., Levin A., Wolf A., Fujisawa T., Hayakawa S., Gojobori T., Davies J. A., David C. N., Bacon J. P. Evolution of gap junctions: the missing link? Curr. Biol. 2004;14:R879–R880. doi: 10.1016/j.cub.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 202.Bao L., Locovei S., Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 203.Lai A., Le D. N., Paznekas W. A., Gifford W. D., Jabs E. W., Charles A. C. Oculodentodigital dysplasia connexin43 mutations result in non-functional connexin hemichannels and gap junctions in C6 glioma cells. J. Cell Sci. 2006;119:532–541. doi: 10.1242/jcs.02770. [DOI] [PubMed] [Google Scholar]
- 204.Dahl G., Nonner W., Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys. J. 1994;67:1816–1822. doi: 10.1016/S0006-3495(94)80663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kwak B. R., Jongsma H. J. Selective inhibition of gap junction channel activity by synthetic peptides. J. Physiol. (London) 1999;516:679–685. doi: 10.1111/j.1469-7793.1999.0679u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ye Z. C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]